Mathematical Models for Blood Flow Quantification in Dialysis Access Using Angiography: A Comparative Study
Abstract
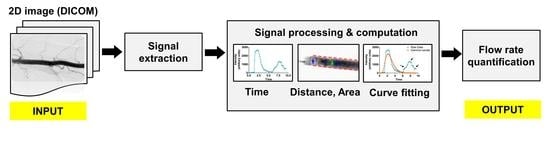
:1. Introduction
2. Materials and Methods
2.1. Transit Time Algorithm
2.1.1. Peak-to-Peak (PP) Algorithm
2.1.2. Cross-Correlation (CC) Algorithm
2.2. Curve-Fitting Models
2.2.1. Gamma-Variate (GV)
2.2.2. Lagged Normal
2.2.3. Polynomial (6th Degree)
2.3. Optimization of Curve-Fitting Parameters
2.4. Distance and Cross-Sectional Area Calculation
2.5. Flow Phantom Set Up
2.6. Image Acquisition Protocol
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bogunović, H.; Loncarić, S. Blood Flow and Velocity Estimation Based on Vessel Transit Time by Combining 2D and 3D X-ray Angiography. Med. Image Comput. Comput. Assist. Interv. 2006, 9, 117–124. [Google Scholar]
- Bonnefous, O.; Pereira, V.M.; Ouared, R.; Brina, O.; Aerts, H.; Hermans, R.; van Nijnatten, F.; Stawiaski, J.; Ruijters, D. Quantification of Arterial Flow Using Digital Subtraction Angiography. Med. Phys. 2012, 39, 6264–6275. [Google Scholar] [CrossRef] [PubMed]
- Waechter, I.; Bredno, J.; Hermans, R.; Weese, J.; Barratt, D.C.; Hawkes, D.J. Model-Based Blood Flow Quantification from Rotational Angiography. Med. Image Anal. 2008, 12, 586–602. [Google Scholar] [CrossRef] [PubMed]
- Koirala, N.; Anvari, E.; McLennan, G. Monitoring and Surveillance of Hemodialysis Access. Semin. Interv. Radiol. 2016, 33, 25–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whittier, W.L. Surveillance of Hemodialysis Vascular Access. Semin. Interv. Radiol. 2009, 26, 130–138. [Google Scholar] [CrossRef] [Green Version]
- Polkinghorne, K. The CARI Guidelines. Vascular Access Surveillance. Nephrology 2008, 13 (Suppl. S2), S1–S11. [Google Scholar] [CrossRef]
- Fitts, M.K.; Pike, D.B.; Anderson, K.; Shiu, Y.-T. Hemodynamic Shear Stress and Endothelial Dysfunction in Hemodialysis Access. Open Urol. Nephrol. J. 2014, 7, 33–44. [Google Scholar] [CrossRef]
- Browne, L.D.; Bashar, K.; Griffin, P.; Kavanagh, E.G.; Walsh, S.R.; Walsh, M.T. The Role of Shear Stress in Arteriovenous Fistula Maturation and Failure: A Systematic Review. PLoS ONE 2015, 10, e0145795. [Google Scholar] [CrossRef] [Green Version]
- Cheung, A.K.; Imrey, P.B.; Alpers, C.E.; Robbin, M.L.; Radeva, M.; Larive, B.; Shiu, Y.-T.; Allon, M.; Dember, L.M.; Greene, T.; et al. Intimal Hyperplasia, Stenosis, and Arteriovenous Fistula Maturation Failure in the Hemodialysis Fistula Maturation Study. J. Am. Soc. Nephrol. 2017, 28, 3005–3013. [Google Scholar] [CrossRef]
- Rothuizen, T.C.; Wong, C.; Quax, P.H.A.; van Zonneveld, A.J.; Rabelink, T.J.; Rotmans, J.I. Arteriovenous Access Failure: More than Just Intimal Hyperplasia? Nephrol. Dial. Transpl. 2013, 28, 1085–1092. [Google Scholar] [CrossRef] [Green Version]
- Stolic, R. Most Important Chronic Complications of Arteriovenous Fistulas for Hemodialysis. Med. Princ. Pr. 2013, 22, 220–228. [Google Scholar] [CrossRef] [Green Version]
- MacRae, J.M.; Dipchand, C.; Oliver, M.; Moist, L.; Lok, C.; Clark, E.; Hiremath, S.; Kappel, J.; Kiaii, M.; Luscombe, R.; et al. Arteriovenous Access Failure, Stenosis, and Thrombosis. Can. J. Kidney Health Dis. 2016, 3, 2054358116669126. [Google Scholar] [CrossRef] [Green Version]
- Bountouris, I.; Kritikou, G.; Degermetzoglou, N.; Avgerinos, K.I. A Review of Percutaneous Transluminal Angioplasty in Hemodialysis Fistula. Int. J. Vasc. Med. 2018, 2018, 1420136. [Google Scholar] [CrossRef]
- Vascular Access 2006 Work Group. Clinical Practice Guidelines for Vascular Access. Am. J. Kidney Dis. 2006, 48 (Suppl. S1), S176–S247. [Google Scholar] [CrossRef]
- Forkert, N.D.; Fiehler, J.; Ries, T.; Illies, T.; Möller, D.; Handels, H.; Säring, D. Reference-Based Linear Curve Fitting for Bolus Arrival Time Estimation in 4D MRA and MR Perfusion-Weighted Image Sequences. Magn. Reson. Med. 2011, 65, 289–294. [Google Scholar] [CrossRef]
- Muir, E.R.; Watts, L.T.; Tiwari, Y.V.; Bresnen, A.; Shen, Q.; Duong, T.Q. Quantitative Cerebral Blood Flow Measurements Using MRI. Methods Mol. Biol. 2014, 1135, 205–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prevrhal, S.; Forsythe, C.H.; Harnish, R.J.; Saeed, M.; Yeh, B.M. CT Angiographic Measurement of Vascular Blood Flow Velocity by Using Projection Data. Radiology 2011, 261, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Holland, C.K.; Clancy, M.J.; Taylor, K.J.; Alderman, J.L.; Purushothaman, K.; McCauley, T.R. Volumetric Flow Estimation In Vivo and In Vitro Using Pulsed-Doppler Ultrasound. Ultrasound Med. Biol. 1996, 22, 591–603. [Google Scholar] [CrossRef]
- Strouthos, C.; Lampaskis, M.; Sboros, V.; McNeilly, A.; Averkiou, M. Indicator Dilution Models for the Quantification of Microvascular Blood Flow with Bolus Administration of Ultrasound Contrast Agents. IEEE Trans. Ultrason Ferroelectr. Freq. Control 2010, 57, 1296–1310. [Google Scholar] [CrossRef] [PubMed]
- Grant, E.G.; Benson, C.B.; Moneta, G.L.; Alexandrov, A.V.; Baker, J.D.; Bluth, E.I.; Carroll, B.A.; Eliasziw, M.; Gocke, J.; Hertzberg, B.S.; et al. Carotid Artery Stenosis: Gray-Scale and Doppler US Diagnosis—Society of Radiologists in Ultrasound Consensus Conference. Radiology 2003, 229, 340–346. [Google Scholar] [CrossRef]
- Oglat, A.A.; Matjafri, M.Z.; Suardi, N.; Oqlat, M.A.; Abdelrahman, M.A.; Oqlat, A.A. A Review of Medical Doppler Ultrasonography of Blood Flow in General and Especially in Common Carotid Artery. J. Med. Ultrasound 2018, 26, 3–13. [Google Scholar] [CrossRef]
- Winkler, P.; Helmke, K.; Mahl, M. Major Pitfalls in Doppler Investigations. Part II. Low Flow Velocities and Colour Doppler Applications. Pediatr. Radiol. 1990, 20, 304–310. [Google Scholar] [CrossRef]
- O’Brien, W.D.J. Ultrasound-Biophysics Mechanisms. Prog. Biophys. Mol. Biol. 2007, 93, 212–255. [Google Scholar] [CrossRef] [Green Version]
- Ziskin, M.C. Fundamental Physics of Ultrasound and Its Propagation in Tissue. Radiographics 1993, 13, 705–709. [Google Scholar] [CrossRef] [Green Version]
- Pinto, A.; Pinto, F.; Faggian, A.; Rubini, G.; Caranci, F.; Macarini, L.; Genovese, E.A.; Brunese, L. Sources of Error in Emergency Ultrasonography. Crit. Ultrasound J. 2013, 5 (Suppl. S1), S1. [Google Scholar] [CrossRef] [Green Version]
- Stasi, G.; Ruoti, E.M. A Critical Evaluation in the Delivery of the Ultrasound Practice: The Point of View of the Radiologist. Ital. J. Med. 2015, 9, 5. [Google Scholar] [CrossRef] [Green Version]
- Piola, M.; Ruiter, M.; Vismara, R.; Mastrullo, V.; Agrifoglio, M.; Zanobini, M.; Pesce, M.; Soncini, M.; Fiore, G.B. Full Mimicking of Coronary Hemodynamics for Ex-Vivo Stimulation of Human Saphenous Veins. Ann. Biomed. Eng. 2017, 45, 884–897. [Google Scholar] [CrossRef]
- Bihari, P.; Shelke, A.; Nwe, T.H.; Mularczyk, M.; Nelson, K.; Schmandra, T.; Knez, P.; Schmitz-Rixen, T. Strain Measurement of Abdominal Aortic Aneurysm with Real-Time 3D Ultrasound Speckle Tracking. Eur. J. Vasc. Endovasc. Surg. 2013, 45, 315–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polanczyk, A.; Klinger, M.; Nanobachvili, J.; Huk, I.; Neumayer, C. Artificial Circulatory Model for Analysis of Human and Artificial Vessels. Appl. Sci. 2018, 8, 1017. [Google Scholar] [CrossRef] [Green Version]
- Polanczyk, A.; Podgorski, M.; Polanczyk, M.; Piechota-Polanczyk, A.; Stefanczyk, L.; Strzelecki, M. A Novel Vision-Based System for Quantitative Analysis of Abdominal Aortic Aneurysm Deformation. Biomed. Eng. Online 2019, 18, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xaplanteris, P.; Fournier, S.; Keulards, D.C.J.; Adjedj, J.; Ciccarelli, G.; Milkas, A.; Pellicano, M.; van’t Veer, M.; Barbato, E.; Pijls, N.H.J.; et al. Catheter-Based Measurements of Absolute Coronary Blood Flow and Microvascular Resistance: Feasibility, Safety, and Reproducibility in Humans. Circ. Cardiovasc. Interv. 2018, 11, 6194. [Google Scholar] [CrossRef]
- Khanmohammadi, M.; Engan, K.; Sæland, C.; Eftestøl, T.; Larsen, A.I. Automatic Estimation of Coronary Blood Flow Velocity Step 1 for Developing a Tool to Diagnose Patients with Micro-Vascular Angina Pectoris. Front. Cardiovasc. Med. 2019, 6, 1. [Google Scholar] [CrossRef]
- Zafar, H.; Sharif, F.; Leahy, M.J. Measurement of the Blood Flow Rate and Velocity in Coronary Artery Stenosis Using Intracoronary Frequency Domain Optical Coherence Tomography: Validation against Fractional Flow Reserve. IJC Heart Vasc. 2014, 5, 68–71. [Google Scholar] [CrossRef] [Green Version]
- Koirala, N.; Setser, R.M.; Bullen, J.; McLennan, G. Blood Flow Measurement Using Digital Subtraction Angiography for Assessing Hemodialysis Access Function. In Proceedings of the SPIE 10137, Medical Imaging 2017: Biomedical Applications in Molecular, Structural, and Functional Imaging, Orlando, FL, USA, 13 March 2017; Volume 10137, p. 101370J. [Google Scholar]
- Koirala, N.; Setser, R.; Bullen, J.; McLennan, G. Determination of Dialysis Access Patency Using 2D Angiographic Images. In 2017 IEEE 17th International Conference on Bioinformatics and Bioengineering (BIBE), Washington, DC, USA, 23–25 October 2017; IEEE: Washington, DC, USA, 2017; pp. 321–326. [Google Scholar]
- Blomley, M.J.; Coulden, R.; Bufkin, C.; Lipton, M.J.; Dawson, P. Contrast Bolus Dynamic Computed Tomography for the Measurement of Solid Organ Perfusion. Investig. Radiol. 1993, 28 (Suppl. S5), S72–S77; discussion S78. [Google Scholar] [CrossRef]
- Ionita, C.N.; Wang, W.; Bednarek, D.R.; Rudin, S. Assessment of Contrast Flow Modification in Aneurysms Treated with Closed-Cell Self-Deploying Asymmetric Vascular Stents (SAVS). Proc. SPIE Int. Soc. Opt. Eng. 2010, 7626, 844327. [Google Scholar] [CrossRef] [Green Version]
- Shpilfoygel, S.D.; Close, R.A.; Valentino, D.J.; Duckwiler, G.R. X-ray Videodensitometric Methods for Blood Flow and Velocity Measurement: A Critical Review of Literature. Med. Phys. 2000, 27, 2008–2023. [Google Scholar] [CrossRef]
- Argueta, E.E.; Paniagua, D. Thermodilution Cardiac Output: A Concept over 250 Years in the Making. Cardiol. Rev. 2019, 27, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Zierler, K. Indicator Dilution Methods for Measuring Blood Flow, Volume, and Other Properties of Biological Systems: A Brief History and Memoir. Ann. Biomed. Eng. 2000, 28, 836–848. [Google Scholar] [CrossRef] [PubMed]
- Rosen, L.; Silverman, N.R. Videodensitometric Measurements of Blood Flow Using Crosscorrelation Techniques. Radiology 1973, 109, 305–310. [Google Scholar] [CrossRef]
- Kalisz, K.; Buethe, J.; Saboo, S.S.; Abbara, S.; Halliburton, S.; Rajiah, P. Artifacts at Cardiac CT: Physics and Solutions. Radiographics 2016, 36, 2064–2083. [Google Scholar] [CrossRef] [PubMed]
- Lieber, B.B.; Sadasivan, C.; Hao, Q.; Seong, J.; Cesar, L. The Mixability of Angiographic Contrast with Arterial Blood. Med. Phys. 2009, 36, 5064–5078. [Google Scholar] [CrossRef] [Green Version]
- Brands, J.; Vink, H.; Van Teeffelen, J.W.G.E. Comparison of Four Mathematical Models to Analyze Indicator-Dilution Curves in the Coronary Circulation. Med. Biol. Eng. Comput. 2011, 49, 1471–1479. [Google Scholar] [CrossRef] [Green Version]
- Thompson, H.K.J.; Starmer, C.F.; Whalen, R.E.; McIntosh, H.D. Indicator Transit Time Considered as a Gamma Variate. Circ. Res. 1964, 14, 502–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bassingthwaighte, J.B.; Ackerman, F.H.; Wood, E.H. Applications of the Lagged Normal Density Curve as a Model for Arterial Dilution Curves. Circ. Res. 1966, 18, 398–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stohanzlova, P.; Kolar, R. Tissue Perfusion Modelling in Optical Coherence Tomography. Biomed. Eng. Online 2017, 16, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harabis, V.; Kolar, R.; Mezl, M.; Jirik, R. Comparison and Evaluation of Indicator Dilution Models for Bolus of Ultrasound Contrast Agents. Physiol. Meas. 2013, 34, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Koirala, N.; McLennan, G. Analysis of Noisy 2D Angiographic Images for Improved Blood Flow Rate Quantification in Dialysis Access. In 2017 IEEE Western New York Image and Signal Processing Workshop (WNYISPW), Rochester, NY, USA, 17 Novvember 2017; IEEE: Rochester, NY, USA, 2017; pp. 1–5. [Google Scholar]
- Bland, J.M.; Altman, D.G. Statistical Methods for Assessing Agreement between Two Methods of Clinical Measurement. Lancet 1986, 1, 307–310. [Google Scholar] [CrossRef]
- Chen, M.-C.; Tsai, W.-L.; Tsai, I.-C.; Chan, S.-W.; Liao, W.-C.; Lin, P.-C.; Yang, S.J. Arteriovenous Fistula and Graft Evaluation in Hemodialysis Patients Using MDCT: A Primer. AJR Am. J. Roentgenol. 2010, 194, 838–847. [Google Scholar] [CrossRef]
- Murphy, E.A.; Ross, R.A.; Jones, R.G.; Gandy, S.J.; Aristokleous, N.; Salsano, M.; Weir-McCall, J.R.; Matthew, S.; Houston, J.G. Imaging in Vascular Access. Cardiovasc. Eng. Technol. 2017, 8, 255–272. [Google Scholar] [CrossRef] [Green Version]
- Rt, S.A.; Brehmer, K.; Torkel, B.B. Evaluation of Arteriovenous Fistula-Bilateral Computed Tomography Venography Combining Diluted and Undiluted Contrast Media Injection: A Case Study. Radiol. Case Rep. 2020, 15, 85–88. [Google Scholar] [CrossRef]
- Huda, W.; Abrahams, R.B. Radiographic Techniques, Contrast, and Noise in X-ray Imaging. AJR Am. J. Roentgenol. 2015, 204, W126–W131. [Google Scholar] [CrossRef]
- Crowhurst, J.; Whitby, M. Lowering Fluoroscopy Pulse Rates to Reduce Radiation Dose during Cardiac Procedures. J. Med. Radiat. Sci. 2018, 65, 247–249. [Google Scholar] [CrossRef]
- Shin, J.H. Recent Radiation Reduction Strategies for Neurointerventionists. Neurointervention 2020, 15, 167–170. [Google Scholar] [CrossRef]
- Bratschitsch, G.; Leitner, L.; Stücklschweiger, G.; Guss, H.; Sadoghi, P.; Puchwein, P.; Leithner, A.; Radl, R. Radiation Exposure of Patient and Operating Room Personnel by Fluoroscopy and Navigation during Spinal Surgery. Sci. Rep. 2019, 9, 17652. [Google Scholar] [CrossRef] [PubMed]
- Pereira, V.M.; Bonnefous, O.; Ouared, R.; Brina, O.; Stawiaski, J.; Aerts, H.; Ruijters, D.; Narata, A.P.; Bijlenga, P.; Schaller, K.; et al. A DSA-Based Method Using Contrast-Motion Estimation for the Assessment of the Intra-Aneurysmal Flow Changes Induced by Flow-Diverter Stents. AJNR Am. J. Neuroradiol. 2013, 34, 808–815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seifalian, A.M.; Hawkes, D.J.; Hardingham, C.R.; Colchester, A.C.; Reidy, J.F. Validation of a Quantitative Radiographic Technique to Estimate Pulsatile Blood Flow Waveforms Using Digital Subtraction Angiographic Data. J. Biomed. Eng. 1991, 13, 225–233. [Google Scholar] [CrossRef]
- Mulder, G.; Bogaerds, A.C.B.; Rongen, P.; van de Vosse, F.N. The Influence of Contrast Agent Injection on Physiological Flow in the Circle of Willis. Med. Eng. Phys. 2011, 33, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.-H.; Lin, C.-J.; Lin, Y.-H.; Guo, W.-Y.; Huang, T.-C. Quantitative Analysis of Digital Subtraction Angiography Using Optical Flow Method on Occlusive Cerebrovascular Disease. Comput. Methods Programs Biomed. 2013, 111, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Pereira, V.M.; Ouared, R.; Brina, O.; Bonnefous, O.; Satwiaski, J.; Aerts, H.; Ruijters, D.; van Nijnatten, F.; Perren, F.; Bijlenga, P.; et al. Quantification of Internal Carotid Artery Flow with Digital Subtraction Angiography: Validation of an Optical Flow Approach with Doppler Ultrasound. AJNR Am. J. Neuroradiol. 2014, 35, 156–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tenjin, H.; Asakura, F.; Nakahara, Y.; Matsumoto, K.; Matsuo, T.; Urano, F.; Ueda, S. Evaluation of Intraaneurysmal Blood Velocity by Time-Density Curve Analysis and Digital Subtraction Angiography. AJNR Am. J. Neuroradiol. 1998, 19, 1303–1307. [Google Scholar]
- Brockow, K. Reduced Iodinated Contrast Media Dose and Injection Speed for CT: How Much Does This Decrease the Risk of a Hypersensitivity Reactions? Quant. Imaging Med. Surg. 2020, 10, 537–540. [Google Scholar] [CrossRef] [PubMed]
- Fåhræus, R.; Lindqvist, T. The Viscosity of the Blood in Narrow Capillary Tubes. Am. J. Physiol.-Leg. Content 1931, 96, 562–568. [Google Scholar] [CrossRef]
- Brindise, M.C.; Busse, M.M.; Vlachos, P.P. Density and Viscosity Matched Newtonian and Non-Newtonian Blood-Analog Solutions with PDMS Refractive Index. Exp. Fluids 2018, 59, 173. [Google Scholar] [CrossRef]
- Huang, T.-C.; Chang, C.-K.; Liao, C.-H.; Ho, Y.-J. Quantification of Blood Flow in Internal Cerebral Artery by Optical Flow Method on Digital Subtraction Angiography in Comparison with Time-of-Flight Magnetic Resonance Angiography. PLoS ONE 2013, 8, e54678. [Google Scholar] [CrossRef] [Green Version]
- Cai, J.; Wu, D.; Mo, Y.; Wang, A.; Hu, S.; Ren, L. Comparison of Extracranial Artery Stenosis and Cerebral Blood Flow, Assessed by Quantitative Magnetic Resonance, Using Digital Subtraction Angiography as the Reference Standard. Medicine 2016, 95, e5370. [Google Scholar] [CrossRef]
- Wen, W.-L.; Fang, Y.-B.; Yang, P.-F.; Zhang, Y.-W.; Wu, Y.-N.; Shen, H.; Ge, J.-J.; Xu, Y.; Hong, B.; Huang, Q.-H.; et al. Parametric Digital Subtraction Angiography Imaging for the Objective Grading of Collateral Flow in Acute Middle Cerebral Artery Occlusion. World Neurosurg. 2016, 88, 119–125. [Google Scholar] [CrossRef]






| Imaging Mode, Sampling Rate | Algorithm | Without Curve Fit | With GV Curve Fit | p Value † | ||||
|---|---|---|---|---|---|---|---|---|
| Bias [Lower Limit, Upper Limit] (mL/min) | 95% CI |MQE| (%) | Corr.* (r) | Bias [Lower Limit, Upper Limit] (mL/min) | 95% CI |MQE| (%) | Corr.* (r) | |||
| DSA acquisition, 6 F/s | PP | −176 [−842, 490] | [29, 43] | 0.574 | −246 [−676, 184] | [21, 32] | 0.773 | 0.175 ‡ |
| CC | −234 [−691, 223] | [25, 35] | 0.731 | −145 [−484, 195] | [18, 25] | 0.865 | 0.017 § | |
| Fluoroscopic, 10 P/s | PP | −374 [−1098, 351] | [42, 67] | 0.545 | −339 [−891, 213] | [34, 51] | 0.515 | 0.027 ‡ |
| CC | −205 [−930, 519] | [43, 59] | 0.534 | −198 [−722, 327] | [30, 44] | 0.576 | 0.031 § | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koirala, N.; McLennan, G. Mathematical Models for Blood Flow Quantification in Dialysis Access Using Angiography: A Comparative Study. Diagnostics 2021, 11, 1771. https://0-doi-org.brum.beds.ac.uk/10.3390/diagnostics11101771
Koirala N, McLennan G. Mathematical Models for Blood Flow Quantification in Dialysis Access Using Angiography: A Comparative Study. Diagnostics. 2021; 11(10):1771. https://0-doi-org.brum.beds.ac.uk/10.3390/diagnostics11101771
Chicago/Turabian StyleKoirala, Nischal, and Gordon McLennan. 2021. "Mathematical Models for Blood Flow Quantification in Dialysis Access Using Angiography: A Comparative Study" Diagnostics 11, no. 10: 1771. https://0-doi-org.brum.beds.ac.uk/10.3390/diagnostics11101771