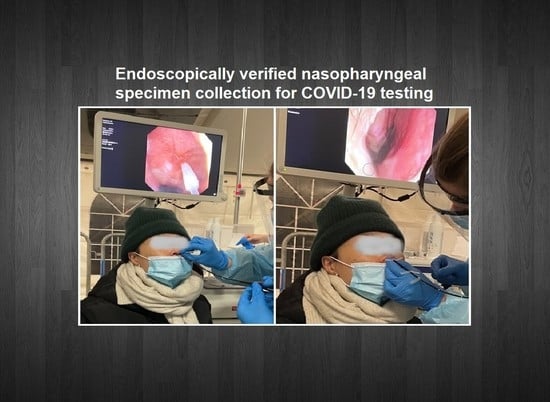
Optimal Insertion Depth for Nasal Mid-Turbinate and Nasopharyngeal Swabs
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hasell, J.; Mathieu, E.; Beltekian, D.; Macdonald, B.; Giattino, C.; Ortiz-Ospina, E.; Roser, M.; Ritchie, H. A cross-country database of COVID-19 testing. Sci. Data 2020, 7, 345. Available online: https://ourworldindata.org/coronavirus-testing#how-many-tests-are-performed-each-day (accessed on 23 March 2021). [CrossRef]
- Lee, R.A.; Herigon, J.C.; Benedetti, A.; Pollock, N.R.; Denkinger, C.M. Performance of Saliva, Oropharyngeal Swabs, and Nasal Swabs for SARS-CoV-2 Molecular Detection: A Systematic Review and Meta-analysis. J. Clin. Microbiol. 2021, 59, e02881-20. [Google Scholar] [CrossRef]
- Tsang, N.N.Y.; So, H.C.; Ng, K.Y.; Cowling, B.J.; Leung, G.M.; Ming, D.K. Diagnostic performance of different sampling approaches for SARS-CoV-2 RT-PCR testing: A systematic review and meta-analysis. Lancet Infect. Diseases 2021. [Google Scholar] [CrossRef]
- Jakobsen, K.; Jensen, J.S.; Todsen, T.; Tolsaard, M.G.; Kirkby, N.; Lippert, F.; Vangsted, A.; Martel, C.J.; Klokker, M.; von Buchwald, C. Accuracy and cost description of rapid antigen test compared with reverse transcriptase-polymerase chain reaction for SARS-CoV-2 detection. Dan. Med. J. 2021, 68, A03210217. [Google Scholar] [PubMed]
- Hiebert, N.M.; Chen, B.A.; Sowerby, L.J. Variability in instructions for performance of nasopharyngeal swabs across Canada in the era of COVID-19—What type of swab is actually being performed? J. Otolaryngol. Head Neck Surg. 2021, 50, 5. [Google Scholar] [CrossRef] [PubMed]
- Higgins, T.S.; Wu, A.W.; Ting, J.Y. SARS-CoV-2 Nasopharyngeal Swab Testing—False-Negative Results from a Pervasive Anatomical Misconception. JAMA Otolaryngol. Head Neck Surg. 2020, 146, 993–994. [Google Scholar] [CrossRef] [PubMed]
- Website with Testing Guide. Available online: https://www.albertahealthservices.ca/assets/wf/plab/wf-provlab-collection-of-nasopharyngeal-and-throat-swab.pdf (accessed on 19 June 2021).
- Website with Testing Guide. Available online: https://www.amboss.com/us/knowledge/COVID-19_(coronavirus_disease_2019) (accessed on 19 June 2021).
- Website with Testing Guide. Available online: https://www.ottawapublichealth.ca/en/professionals-and-partners/how-to-collect-a-nasopharyngeal--np--swab.aspx (accessed on 19 June 2021).
- Interim Guidelines for Collecting and Handling of Clinical Specimens for COVID-19 Testing. Center for Disease Control and Prevention. Updated 26 February 2021. Available online: https://www.cdc.gov/coronavirus/2019-ncov/lab/guidelines-clinical-specimens.html (accessed on 23 March 2021).
- Lindner, A.K.; Nikolai, O.; Kausch, F.; Wintel, M.; Hommes, F.; Gertler, M.; Krüger, L.J.; Gaeddert, M.; Tobian, F.; Lainati, F.; et al. Head-to-head comparison of SARS-CoV-2 antigen-detecting rapid test with self-collected nasal swab versus professional-collected nasopharyngeal swab. Eur. Respir. J. 2021. Epub ahead of print. [Google Scholar] [CrossRef]
- West, C.P.; Montori, V.M.; Sampathkumar, P. COVID-19 Testing: The Threat of False-Negative Results. Mayo Clin Proc. 2020, 95, 1127–1129. [Google Scholar] [CrossRef] [PubMed]
- Arevalo-Rodriguez, I.; Buitrago-Garcia, D.; Simancas-Racines, D.; Zambrano-Achig, P.; Del Campo, R.; Ciapponi, A.; Sued, O.; Martinez-García, L.; Rutjes, A.W.; Low, N.; et al. False-negative results of initial RT-PCR assays for COVID-19: A systematic review. PLoS ONE 2020, 15, e0242958. [Google Scholar] [CrossRef] [PubMed]
- İşlek, A.; Balcı, M.K. Analysis of Factors Causing False-Negative Real-Time Polymerase Chain Reaction Results in Oropharyngeal and Nasopharyngeal Swabs of Patients With COVID-19. Ear Nose Throat J. 2021. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.r-project.org/ (accessed on 23 March 2021).
- Lim, H.; Lee, J.H.; Son, K.K.; Han, Y.J.; Ko, S. A method for optimal depth of the nasopharyngeal temperature probe: The philtrum to tragus distance. Korean J. Anesthesiol. 2014, 66, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Marty, F.M.; Chen, K.; Verrill, K.A. How to Obtain a Nasopharyngeal Swab Specimen. N. Engl. J. Med. 2020, 382, e76. [Google Scholar] [CrossRef] [PubMed]
- Piras, A.; Rizzo, D.; Longoni, E.; Turra, N.; Urru, S.; Saba, P.P.; Musumano, L.; Bussu, F. Nasopharyngeal swab collection in the suspicion of Covid-19. Am. J. Otolaryngol. 2020, 41, 102551. [Google Scholar] [CrossRef] [PubMed]
- Koskinen, A.; Tolvi, M.; Jauhiainen, M.; Kekäläinen, E.; Laulajainen-Hongisto, A.; Lamminmäki, S. Complications of COVID-19 Nasopharyngeal Swab Test. JAMA Otolaryngol. Head Neck Surg. 2021, 147, 672–674. [Google Scholar] [CrossRef] [PubMed]
- Smieja, M.; Castriciano, S.; Carruthers, S.; So, G.; Chong, S.; Luinstra, K.; Mahony, J.B.; Petrich, A.; Chernesky, M.; Savarese, M.; et al. Development and evaluation of a flocked nasal midturbinate swab for self-collection in respiratory virus infection diagnostic testing. J. Clin. Microbiol. 2010, 48, 3340–3342. [Google Scholar] [CrossRef] [Green Version]
- Ramos, K.J.; Kapnadak, S.G.; Collins, B.F.; Pottinger, P.S.; Wall, R.; Mays, J.A.; Perchetti, G.A.; Jerome, K.R.; Khot, S.; Limaye, A.P.; et al. Detection of SARS-CoV-2 by bronchoscopy after negative nasopharyngeal testing: Stay vigilant for COVID-19. Respir. Med. Case Rep. 2020, 30, 101120. [Google Scholar] [CrossRef] [PubMed]
- Nazerian, P.; Sacco, R.M.; Solbiati, M.; Targetti, E.; Marta, C.; Blasi, F.; Casazza, G.; Colao, M.G.; Tomassetti, S.; Grifoni, S.; et al. Laryngotracheal aspiration test reduce the false negative rate in patients with suspected SARS-COV-2 pneumonia despite a negative nasopharyngeal swab. Eur. J. Intern. Med. 2021. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]


| Mean Insertion Depth (SD) to the Posterior Nasopharyngeal Wall | Mean Insertion Depth (SD) to the Anterior Part of the Inferior Turbinate | Mean Insertion Depth (SD) to the Posterior Part of the Inferior Turbinate | Mean Insertion Depth (SD) to the Nasal Mid-Turbinate | Mean Length (SD) from the Nose Tip to Vestibulum Nasi | |
|---|---|---|---|---|---|
| All | 9.40 (0.64) | 1.95 (0.61) | 6.39 (0.62) | 4.17 (0.48) | 1.42 (0.36) |
| Women | 9.04 (0.55) | 1.79 (0.47) | 6.13 (0.50) | 3.96 (0.39) | 1.27 (0.29) |
| Men | 9.75 (0.53) | 2.09 (0.68) | 6.63 (0.61) | 4.36 (0.47) | 1.56 (0.36) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Callesen, R.E.; Kiel, C.M.; Hovgaard, L.H.; Jakobsen, K.K.; Papesch, M.; von Buchwald, C.; Todsen, T. Optimal Insertion Depth for Nasal Mid-Turbinate and Nasopharyngeal Swabs. Diagnostics 2021, 11, 1257. https://0-doi-org.brum.beds.ac.uk/10.3390/diagnostics11071257
Callesen RE, Kiel CM, Hovgaard LH, Jakobsen KK, Papesch M, von Buchwald C, Todsen T. Optimal Insertion Depth for Nasal Mid-Turbinate and Nasopharyngeal Swabs. Diagnostics. 2021; 11(7):1257. https://0-doi-org.brum.beds.ac.uk/10.3390/diagnostics11071257
Chicago/Turabian StyleCallesen, Rasmus Eið, Cecilie Mullerup Kiel, Lisette Hvid Hovgaard, Kathrine Kronberg Jakobsen, Michael Papesch, Christian von Buchwald, and Tobias Todsen. 2021. "Optimal Insertion Depth for Nasal Mid-Turbinate and Nasopharyngeal Swabs" Diagnostics 11, no. 7: 1257. https://0-doi-org.brum.beds.ac.uk/10.3390/diagnostics11071257