Association Analysis in Young and Middle-Aged Mothers—Relation between Expression of Cardiovascular Disease Associated MicroRNAs and Abnormal Clinical Findings
Abstract
:1. Introduction
2. Results
2.1. Substantially Altered Expression Profile of Diabetes/Cardiovascular/Cerebrovascular Disease Associated Micrornas in Mothers after Complicated Pregnancies
2.2. No Association between Postpartal Expression of Diabetes/Cardiovascular/Cerebrovascular Disease Associated Micrornas and Actual Hormonal Contraceptive Use, Active Smoking of Cigarettes, Total Serum Cholesterol Levels, Serum HDL Cholesterol Levels, Serum LDL Cholesterol Levels, Serum Triglycerides Levels, Serum Lipoprotein A levels, Serum CRP Levels, Serum Uric Acid Levels, and Plasma Homocysteine Levels
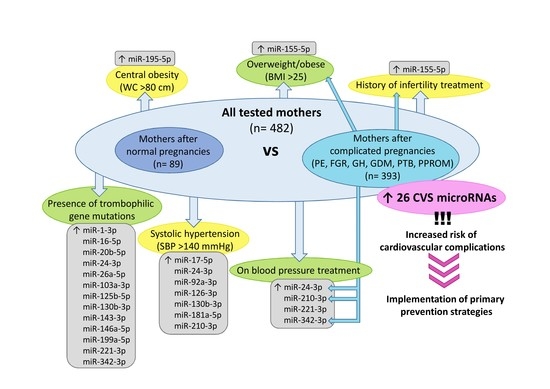
2.3. Postpartal Expression Profile of miR-155-5p Differentiates between Overweight/Obese Mothers and Mothers with Normal BMI Values
2.4. Postpartal Expression Profile of miR-195-5p Differentiates between Mothers with Central Obesity and Mothers with Normal Values of Waist Circumference
2.5. Postpartal Expression Profile of miR-17-5p, miR-24-3p, miR-92a-3p, miR-126-3p, miR-130b-3p, miR-181a-5p, and miR-210-3p Differentiates between Mothers with Systolic Hypertension and Mothers with Normal SBP Values
2.6. Postpartal Expression Profile of miR-24-3p, miR-210-3p, miR-221-3p, and miR-342-3p Differentiates between Mothers on Blood Pressure Treatment and Mothers without Medication
2.7. Postpartal Expression Profile of miR-155-5p Differentiates between Mothers with Respect to Infertility Treatment
2.8. Postpartal Expression Profile of miR-1-3p, miR-16-5p, miR-20b-5p, miR-24-3p, miR-26a-5p, miR-103a-3p, miR-125b-5p, miR-130b-3p, miR-143-3p, miR-146a-5p, miR-199a-5p, miR-221-3p, and miR-342-3p Differentiates between Mothers with and without the Presence of Trombophilic Gene Mutations
2.9. The Effect of Physical Activity on Postpartal microRNA Expression Profile, BMI Values, SBP and DBP Values
3. Discussion
4. Materials and Methods
4.1. Participants
4.2. Blood Pressure, BMI and Waist Circumference Measurements
4.3. Biological Sampling and Data Collection
4.4. Processing of Samples, Reverse Transcription, and Relative Quantification of microRNAS
4.5. Statistical Analysis
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GDM | Gestational diabetes mellitus |
| PE | Preeclampsia |
| FGR | Fetal growth restriction |
| GH | Gestational hypertension |
| AUC | Area under the Curve |
| ROC | Receive Operating Characteristic |
| FPR | False Positive Rate |
| CI | Confidence Interval |
| LR+ | Positive Likelihood Ratio |
| LR‒ | Negative Likelihood Ratio |
| NP | Normal Pregnancies |
| BP | Blood Pressure |
| BMI | Body Mass Index |
| SBP | Systolic Blood Pressure |
| DBP | Diastolic Blood Pressure |
| LDL | Low-density Lipoprotein |
| GA | Gestational age |
| CS | Caesarean Section |
References
- Lykke, J.A.; Langhoff-Roos, J.; Sibai, B.M.; Funai, E.F.; Triche, E.W.; Paidas, M.J. Hypertensive pregnancy disorders and subsequent cardiovascular morbidity and type 2 diabetes mellitus in the mother. Hypertension 2009, 53, 944–951. [Google Scholar] [CrossRef] [PubMed]
- Männistö, T.; Mendla, P.; Vääräsmäki, M.; Järvelin, M.R.; Hartikainen, A.L.; Pouta, A.; Suvanto, E. Elevated blood pressure in pregnancy and subsequent chronic disease risk. Circulation 2013, 127, 681–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thilaganathan, B. Association of Higher Maternal Blood Pressure with Lower Infant Birthweight: Placental Cause or Cardiovascular Effect? Hypertension 2016, 67, 499–500. [Google Scholar] [CrossRef] [PubMed]
- Thilaganathan, B. Placental syndromes: Getting to the heart of the matter. Ultrasound Obs. Gynecol. 2017, 49, 7–9. [Google Scholar] [CrossRef]
- Bellamy, L.; Casas, J.P.; Hingorani, A.D.; Williams, D.J. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: Systematic review and meta-analysis. BMJ 2007, 335, 974. [Google Scholar] [CrossRef] [Green Version]
- Craici, I.M.; Wagner, S.J.; Hayman, S.R.; Garovic, V.D. Pre-eclamptic pregnancies: An opportunity to identify women at risk for future cardiovascular disease. Womens Health 2008, 4, 133–135. [Google Scholar] [CrossRef] [Green Version]
- American College of Obstetricians and Gynecologists; Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet. Gynecol. 2013, 122, 1122–1131. [Google Scholar]
- Veerbeek, J.H.; Hermes, W.; Breimer, A.Y.; van Rijn, B.B.; Koenen, S.V.; Mol, B.W.; Franx, A.; de Groot, C.J.; Koster, M.P. Cardiovascular disease risk factors after early-onset preeclampsia, late-onset preeclampsia, and pregnancy-induced hypertension. Hypertension 2015, 65, 600–606. [Google Scholar] [CrossRef] [Green Version]
- Ray, J.G.; Vermeulen, M.J.; Schull, M.J.; Redelmeier, D.A. Cardiovascular health after maternal placental syndromes (CHAMPS): Population-based retrospective cohort study. Lancet 2005, 366, 1797–1803. [Google Scholar] [CrossRef]
- Libby, G.; Murphy, D.J.; McEwan, N.F.; Greene, S.A.; Forsyth, J.S.; Chien, P.W.; Morris, A.D.; DARTS/MEMO Collaboration. Pre-eclampsia and the later development of type 2 diabetes in mothers and their children: An intergenerational study from the Walker cohort. Diabetologia 2007, 50, 523–530. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.J.; Lee, S.A.; Choi, J.Y.; Song, M.; Han, S.; Yoon, H.S.; Lee, Y.; Oh, J.; Lee, J.K.; Kang, D. Subsequent risk of metabolic syndrome in women with a history of preeclampsia: Data from the Health Examinees Study. J. Epidemiol. 2015, 25, 281–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Udenze, I.C. Association of pre-eclampsia with metabolic syndrome and increased risk of cardiovascular disease in women: A systemic review. Niger J. Clin. Pract. 2016, 19, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Haukkamaa, L.; Moilanen, L.; Kattainen, A.; Luoto, R.; Kahonen, M.; Leinonen, M.; Jula, A.; Kesäniemi, Y.A.; Kaaja, R. Pre-eclampsia is a risk factor of carotid artery atherosclerosis. Cereb. Dis. 2009, 27, 599–607. [Google Scholar] [CrossRef] [PubMed]
- McDonald, S.D.; Ray, J.; Teo, K.; Jung, H.; Salehian, O.; Yusuf, S.; Lonn, E. Measures of cardiovascular risk and subclinical atherosclerosis in a cohort of women with a remote history of preeclampsia. Atherosclerosis 2013, 229, 234–239. [Google Scholar] [CrossRef]
- Irgens, H.U.; Reisaeter, L.; Irgens, L.M.; Lie, R.T. Long term mortality of mothers and fathers after pre-eclampsia: Population based cohort study. BMJ 2001, 323, 1213–1217. [Google Scholar] [CrossRef] [Green Version]
- Garovic, V.D.; Hayman, S.R. Hypertension in pregnancy: An emerging risk factor for cardiovascular disease. Nat. Clin. Pr. Nephrol. 2007, 3, 613–622. [Google Scholar] [CrossRef]
- Mongraw-Chaffin, M.L.; Cirillo, P.M.; John, B.A. Preeclampsia and cardiovascular disease death: Prospective evidence from the child health and development studies cohort. Hypertension 2010, 56, 166–171. [Google Scholar] [CrossRef] [Green Version]
- Borna, S.; Neamatipoor, E.; Radman, N. Risk of coronary artery disease in women with history of pregnancies complicated by preeclampsia and LBW. J. Matern Fetal Neonatal Med. 2012, 25, 1114–1116. [Google Scholar] [CrossRef]
- Berks, D.; Hoedjes, M.; Raat, H.; Duvekot, J.J.; Steegers, E.A.; Habbema, J.D. Risk of cardiovascular disease after pre-eclampsia and the effect of lifestyle interventions: A literature-based study. BJOG 2013, 120, 924–931. [Google Scholar] [CrossRef]
- Hromadnikova, I.; Kotlabova, K.; Dvorakova, L.; Krofta, L. Postpartum profiling of microRNAs involved in pathogenesis of cardiovascular/cerebrovascular diseases in women exposed to pregnancy-related complications. Int. J. Cardiol. 2019, 291, 158–167. [Google Scholar] [CrossRef]
- Hromadnikova, I.; Kotlabova, K.; Dvorakova, L.; Krofta, L. Diabetes Mellitus and Cardiovascular Risk Assessment in Mothers with a History of Gestational Diabetes Mellitus Based on Postpartal Expression Profile of MicroRNAs Associated with Diabetes Mellitus and Cardiovascular and Cerebrovascular Diseases. Int. J. Mol. Sci. 2020, 21, 2437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- International Association of Diabetes and Pregnancy Study Groups Consensus Panel; Metzger, B.E.; Gabbe, S.G.; Persson, B.; Buchanan, T.A.; Catalano, P.A.; Damm, P.; Dyer, A.R.; Leiva, A.D.; Hod, M.; et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010, 33, 676–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ACOG Practice Bulletin. Diagnosis and management of preeclampsia and eclampsia. Obstet. Gynecol. 2002, 99, 159–167. [Google Scholar]
- Figueras, F.; Gratacos, E. Stage-based approach to the management of fetal growth restriction. Prenat. Diagn. 2014, 34, 655–659. [Google Scholar] [CrossRef]
- Baschat, A.A. Neurodevelopment following fetal growth restriction and its relationship with antepartum parameters of placental dysfunction. Ultrasound Obstet Gynecol. 2011, 37, 501–514. [Google Scholar] [CrossRef]
- Nardozza, L.M.; Caetano, A.C.; Zamarian, A.C.; Mazzola, J.B.; Silva, C.P.; Marçal, V.M.; Lobo, T.F.; Peixoto, A.B.; Araujo Júnior, E. Fetal growth restriction: Current knowledge. Arch. Gynecol. Obstet. 2017, 295, 1061–1077. [Google Scholar] [CrossRef]
- Goldenberg, R.L.; Culhane, J.F.; Iams, J.D.; Romero, R. Epidemiology and causes of preterm birth. Lancet 2008, 371, 75–84. [Google Scholar] [CrossRef]
- Moutquin, J.M.; Milot Roy, V.; Irion, O. Preterm prevention: Effectivenss of current strategies. J. Soc. Obstet. Gynaecol. Can. 1996, 18, 571–588. [Google Scholar] [CrossRef]
- Romero, R.; Espinoza, J.; Kusanovic, J.P.; Gotsch, F.; Hassan, S.; Erez, O.; Chaiworapongsa, T.; Mazor, M. The preterm parturition syndrome. BJOG 2006, 113, 17–42. [Google Scholar] [CrossRef]
- Hromadnikova, I.; Kotlabova, K.; Dvorakova, L.; Krofta, L. Maternal Cardiovascular Risk Assessment 3-to-11 Years Postpartum in Relation to Previous Occurrence of Pregnancy-Related Complications. J. Clin. Med. 2019, 8, E544. [Google Scholar] [CrossRef] [Green Version]
- Hromadnikova, I.; Kotlabova, K.; Dvorakova, L.; Krofta, L. Evaluation of Vascular Endothelial Function in Young and Middle-Aged Women with Respect to a History of Pregnancy, Pregnancy-Related Complications, Classical Cardiovascular Risk Factors, and Epigenetics. Int. J. Mol. Sci. 2020, 21, E430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Vandesompele, J.; de Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; de Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, research0034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shapiro, S.S.; Wilk, M.B. An Analysis of Variance Test for Normality (Complete Samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]



| NP (n = 89) | PE (n = 123) | FGR (n = 38) | GH (n = 47) | GDM (n = 112) | PTB (n = 34) | PPROM (n = 39) | p1 | p2 | p3 | p4 | p5 | p6 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| At Follow-Up | |||||||||||||
| Age (years) | 38 (29–50) | 38 (28–52) | 38 (26–45) | 38 (31–58) | 39 (31–50) | 37 (25–43) | 37 (26–47) | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 |
| Time since index pregnancy (years) | 5 (3–11) | 4 (3–11) | 4 (3–10) | 4 (3–10) | 5 (4–10) | 5 (3–8) | 5 (3–9) | 1.000 | 0.274 | 0.318 | 1.000 | 1.000 | 1.000 |
| Height (cm) | 167.0 (153–181) | 166.0 (152–183) | 166.5 (156–173) | 167.5 (157.5–180) | 166.0 (151–178.5) | 168.5 (155–182) | 164.0 (146–176.5) | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 |
| Weight (kg) | 62.7 (46–109) | 70.0 (41–121) | 62.5 (46.7–107) | 72.3 (47–128) | 64.5 (43.4–98.7) | 61.7 (49.9–87.4) | 68.8 (43.5–103.4) | 0.004 | 1.000 | 0.002 | 1.000 | 1.000 | 1.000 |
| BMI (kg/m2) | 22.23 (17.7–39.08) | 24.31 (16.22–40.9) | 22.25 (17.06–36.76) | 25.92 (17.91–46.45) | 23.51 (17.39–34.37) | 21.09 (18.25–29.9) | 24.49 (18.25–34.35) | <0.001 | 1.000 | <0.001 | 0.233 | 1.000 | 0.344 |
| Waist circumference (cm) | 74 (63–117) | 79 (64–120) | 75 (63–115) | 84 (65–131) | 80 (63–105) | 74 (65–98) | 77 (63–105) | <0.001 | 1.000 | <0.001 | 0.008 | 1.000 | 1.000 |
| Systolic BP (mmHg) | 112 (87–138) | 123 (95–161) | 117 (96–163) | 126 (109–166) | 112.5 (92–158) | 110.5 (98–144) | 113 (99–189) | <0.001 | 0.246 | <0.001 | 1.000 | 1.000 | 1.000 |
| Diastolic BP (mmHg) | 71 (55–91) | 77 (54–105) | 77 (59–110) | 80 (61–104) | 75 (55–109) | 72 (61–83) | 74 (64–112) | <0.001 | 0.098 | <0.001 | 0.877 | 1.000 | 1.000 |
| Heart rate at rest (n/min) | 70 (44–107) | 73 (56–92) | 73 (57–94) | 74 (51–115) | 74 (53–102) | 74.5 (59–90) | 74 (59–94) | 1.000 | 1.000 | 1.000 | 0.311 | 0.828 | 1.000 |
| Serum total cholesterol (mmol/L) | 5.04 (3.49–7.22) | 5.07 (3.33–7.33) | 4.92 (3.59–7.54) | 5.04 (3.76–7.52) | 4.90 (3.02–8.52) | 4.86 (3.75–6.8) | 4.97 (3.11–6.86) | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 |
| Serum HDL cholesterol (mmol/L) | 1.60 (0.93–2.55) | 1.46 (0.92–2.61) | 1.56 (0.97–2.19) | 1.41 (1.07–2.49) | 1.54 (0.83–2.71) | 1.61 (1.02–2.18) | 1.58 (1.01–2.27) | 0.292 | 1.000 | 0.042 | 1.000 | 1.000 | 1.000 |
| Serum LDL cholesterol (mmol/L) | 3.07 (1.87–5.1) | 3.16 (1.68–4.99) | 3.19 (1.97–5.55) | 3.23 (2.34–5.44) | 3.11 (1.54–6.25) | 2.98 (1.93–4.22) | 3.16 (1.71–4.68) | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 |
| Serum triglycerides (mmol/L) | 0.74 (0.35–2.66) | 0.85 (0.39–4.69) | 0.80 (0.41–2.80) | 0.87 (0.39–2.13) | 0.76 (0.36–4.76) | 0.71 (0.43–2.55) | 0.74 (0.42–2.81) | 0.516 | 1.000 | 0.532 | 1.000 | 1.000 | 1.000 |
| Serum Lp(a) (nmol/L) | 1.0 (0.2–105.5) | 1.8 (0.2–16.6) | 1.15 (0.2–65) | 1.4 (0.2–14.2) | 1.2 (0.2–12.9) | 0.8 (0.4–4.0) | 1.6 (0.2–13.5) | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 |
| Serum CRP (mg/L) | 10.4 (5.7–18.85) | 10.8 (6.1–29.1) | 11.1 (7.7–21.2) | 10.6 (6.96–20.2) | 10.8 (5.16–16.22) | 9.03 (4.89–15.29) | 9.56 (4.89–21.39) | 0.364 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 |
| Plasma homocysteine (μmol/L) | 19.0 (2.39–248.4) | 16.8 (2.39–443.72) | 16.55 (2.4–307.2) | 13.7 (2.4–226.5) | 20.64 (2.39–493.16) | 16.08 (4.0–264.94) | 18.96 (2.39–182.62) | 1.000 | 1.000 | 1.000 | 1.000 | 0.043 | 1.000 |
| Serum uric acid (μmol/L) | 246 (143–376) | 272 (133–414) | 252 (158–431) | 279 (163–470) | 265.5 (149–477) | 254 (170–330) | 248 (145–396) | 0.063 | 1.000 | 0.046 | 0.565 | 1.000 | 1.000 |
| Smoking | |||||||||||||
| Never smoker | 54 (60.67%) | 76 (61.79%) | 26 (68.42%) | 32 (68.08%) | 95 (84.82%) | 24 (70.59%) | 21 (53.85%) | 0.153 | 0.601 | 0.634 | <0.001 | 0.515 | 0.732 |
| Past smoker | 21 (23.60%) | 30 (24.39%) | 6 (15.79%) | 10 (21.28%) | 8 (7.14%) | 7 (20.59%) | 10 (25.64%) | ||||||
| Current smoker | 14 (15.73%) | 17 (13.82%) | 6 (15.79%) | 5 (10.64%) | 9 (8.04%) | 3 (8.82%) | 8 (20.51%) | ||||||
| Contraceptive use | |||||||||||||
| Never | 37 (41.57%) | 32 (26.02%) | 8 (21.05%) | 15 (31.91%) | 7 (6.25%) | 4 (11.76%) | 2 (5.13%) | 0.045 | 0.060 | 0.141 | <0.001 | <0.001 | <0.001 |
| In the past | 30 (33.71%) | 58 (47.15%) | 20 (52.63%) | 24 (51.06%) | 96 (85.71%) | 24 (70.59%) | 33 (84.61%) | ||||||
| Current | 22 (24.72%) | 33 (26.83%) | 10 (26.32%) | 8 (17.02%) | 9 (8.04%) | 6 (17.65%) | 4 (10.26%) | ||||||
| Trombophilic gene mutations | 1 (1.12%) | 11 (8.94%) | 5 (13.16%) | 4 (8.51%) | 8 (7.14%) | 4 (11.76%) | 4 (10.26%) | 0.015 | 0.003 | 0.029 | 0.040 | 0.007 | 0.014 |
| During Gestation | |||||||||||||
| Maternal age at delivery (years) | 32 (25–43) | 32 (21–44) | 32 (21–41) | 33 (27–51) | 34 (26–45) | 32 (20–39) | 32 (22–42) | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 |
| GA at delivery (weeks) | 39.86 (37.71–41.86) | 36.43 (26–41.72) | 35.50 (28–41) | 39 (35–41.57) | 39.64 (37–41.29) | 31.15 (24–36.43) | 34.0 (24.71–36.86) | <0.001 | <0.001 | 0.014 | 1.000 | <0.001 | <0.001 |
| Mode of delivery | |||||||||||||
| Vaginal | 82 (92.13%) | 20 (16.26%) | 7 (18.42%) | 21 (44.68%) | 71 (63.39%) | 24 (70.59%) | 18 (46.15%) | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| CS | 7 (7.87%) | 103 (83.74%) | 31 (81.58%) | 26 (55.32%) | 41 (36.61%) | 10 (38.24%) | 21 (53.85%) | ||||||
| Fetal birth weight (g) | 3410 (2530–4450) | 2430 (610–4490) | 1765 (650–3010) | 3170 (2275–4440) | 3450 (2660–4400) | 1575 (542–2820) | 2100 (600–2710) | <0.001 | <0.001 | 1.000 | 1.000 | <0.001 | <0.001 |
| Fetal sex | |||||||||||||
| Boy | 47 (52.81%) | 54 (43.90%) | 21 (55.26%) | 24 (51.06%) | 67 (59.82%) | 21 (61.76%) | 17 (43.59%) | 0.200 | 0.800 | 0.846 | 0.319 | 0.372 | 0.337 |
| Girl | 42 (47.19%) | 69 (56.10%) | 17 (44.74%) | 23 (48.94%) | 45 (40.18%) | 13 (38.24%) | 22 (56.41%) | ||||||
| Primiparity at index pregnancy | |||||||||||||
| Yes | 43 (48.31%) | 99 (80.49%) | 37 (97.37%) | 31 (65.96%) | 50 (44.64%) | 23 (67.65%) | 29 (74.36%) | <0.001 | <0.001 | 0.049 | 0.604 | 0.054 | 0.006 |
| No | 46 (51.69%) | 24 (19.51%) | 1 (2.63%) | 16 (34.04%) | 62 (55.36%) | 11 (32.35%) | 10 (25.64%) | ||||||
| Birth order of index pregnancy | |||||||||||||
| 1st | 35 (39.32%) | 82 (66.67%) | 30 (78.95%) | 25 (53.19%) | 38 (33.93%) | 14 (41.18%) | 18 (46.15%) | <0.001 | <0.001 | 0.191 | 0.170 | 0.343 | 0.792 |
| 2nd | 33 (37.08%) | 20 (16.26%) | 3 (7.89%) | 9 (19.15%) | 44 (39.29%) | 9 (26.47%) | 13 (33.34%) | ||||||
| 3rd | 16 (17.98%) | 14 (11.38%) | 3 (7.89%) | 10 (21.28%) | 14 (12.50%) | 6 (17.65%) | 5 (12.82%) | ||||||
| 4th+ | 5 (5.62%) | 7 (5.69%) | 2 (5.26%) | 3 (6.38%) | 16 (14.28%) | 5 (14.70%) | 3 (7.69%) | ||||||
| Total number of pregnancies per patient | |||||||||||||
| 1 | 8 (8.99%) | 38 (30.89%) | 12 (31.58%) | 10 (21.28%) | 10 (8.93%) | 2 (5.88%) | 9 (23.08%) | <0.001 | 0.004 | 0.121 | 0.940 | 0.738 | 0.085 |
| 2 | 45 (50.56%) | 52 (42.28%) | 17 (44.74%) | 19 (40.42%) | 54 (48.21%) | 16 (47.06%) | 15 (38.46%) | ||||||
| 3+ | 36 (40.45%) | 33 (26.83%) | 9 (23.68%) | 18 (38.30%) | 48 (42.86%) | 16 (47.06%) | 15 (38.46%) | ||||||
| Final parity per patient | |||||||||||||
| 1 | 13 (14.61%) | 40 (32.52%) | 15 (38.47%) | 16 (34.04%) | 15 (13.39%) | 7 (20.59%) | 15 (38.46%) | 0.011 | 0.005 | 0.030 | 0.767 | 0.297 | 0.006 |
| 2 | 62 (69.66%) | 66 (53.66%) | 21 (55.26%) | 26 (55.32%) | 75 (66.96%) | 25 (73.53%) | 22 (56.41%) | ||||||
| 3+ | 14 (15.73%) | 17 (13.82%) | 2 (5.26%) | 5 (10.64%) | 22 (19.64%) | 2 (5.88%) | 2 (5.13%) | ||||||
| Infertility treatment | |||||||||||||
| Yes | 4 (4.49%) | 25 (20.33%) | 8 (21.05%) | 6 (12.77%) | 17 (15.18%) | 4 (11.76%) | 6 (15.38%) | <0.001 | 0.003 | 0.079 | 0.014 | 0.144 | 0.034 |
| No | 85 (95.51%) | 98 (79.67%) | 30 (78.95%) | 41 (87.23%) | 95 (84.82%) | 30 (88.24%) | 33 (84.62%) | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hromadnikova, I.; Kotlabova, K.; Krofta, L. Association Analysis in Young and Middle-Aged Mothers—Relation between Expression of Cardiovascular Disease Associated MicroRNAs and Abnormal Clinical Findings. J. Pers. Med. 2021, 11, 39. https://0-doi-org.brum.beds.ac.uk/10.3390/jpm11010039
Hromadnikova I, Kotlabova K, Krofta L. Association Analysis in Young and Middle-Aged Mothers—Relation between Expression of Cardiovascular Disease Associated MicroRNAs and Abnormal Clinical Findings. Journal of Personalized Medicine. 2021; 11(1):39. https://0-doi-org.brum.beds.ac.uk/10.3390/jpm11010039
Chicago/Turabian StyleHromadnikova, Ilona, Katerina Kotlabova, and Ladislav Krofta. 2021. "Association Analysis in Young and Middle-Aged Mothers—Relation between Expression of Cardiovascular Disease Associated MicroRNAs and Abnormal Clinical Findings" Journal of Personalized Medicine 11, no. 1: 39. https://0-doi-org.brum.beds.ac.uk/10.3390/jpm11010039