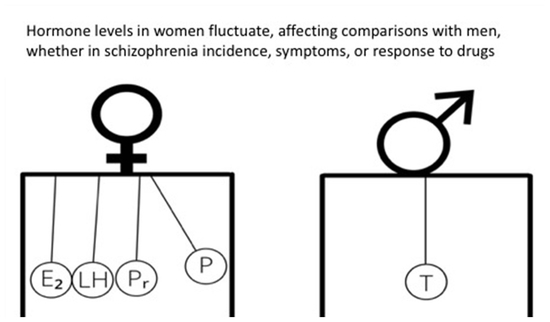
Stratification by Sex and Hormone Level When Contrasting Men and Women in Schizophrenia Trials Will Improve Personalized Treatment
Abstract
:1. Introduction
2. Aims
- Do sex hormones influence the incidence and prevalence of schizophrenia in men and women?
- Do sex hormones influence disease presentation (age at onset, symptoms) in men and women with schizophrenia?
- Do sex hormones influence the pharmacokinetics and pharmacodynamics of antipsychotic drugs?
- Do sex hormones impact disease outcome in men and women diagnosed with schizophrenia?
- Can stratification for sex hormones levels contribute to personalized prevention and treatment in schizophrenia?
3. Methods
4. Results
4.1. Epidemiology
4.1.1. Sex Differences in Incidence
4.1.2. Sex Differences in Prevalence
4.2. Sex Differences in Onset Age
4.3. Sex Differences in Symptoms
4.4. Antipsychotic Response
4.4.1. Genetic Differences
4.4.2. Pharmacokinetic Differences
4.4.3. Pharmacodynamics Differences
4.4.4. Sex Differences in Antipsychotic Response
4.5. Sex Differences in Safety and Adverse Events
4.6. Sex and Outcome
4.7. Personalized Treatment and Prevention of Schizophrenia
5. Discussion
6. Conclusions
Author Contributions
Funding
International Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fernando, P.; Sommer, I.E.C.; Hasan, A. Do we need sex-oriented clinical practice guidelines for the treatment of schizophrenia? Curr. Opin. Psychiatry 2020, 33, 192–199. [Google Scholar] [CrossRef]
- Fineberg, A.M.; Ellman, L.M.; Schaefer, C.A.; Maxwell, S.D.; Shen, L.; Chaudhury, N.H.; Cook, A.L.; Bresnahan, M.A.; Susser, E.S.; Brown, A.S. Fetal exposure to maternal stress and risk for schizophrenia spectrum disorders among offspring: Differential influences of fetal sex. Psychiatry Res. 2016, 236, 91–97. [Google Scholar] [CrossRef] [Green Version]
- Goldstein, J.M.; Lancaster, K.; Longenecker, J.M.; Abbs, B.; Holsen, L.M.; Cherkerzian, S.; Whitfield-Gabrieli, S.; Makris, N.; Tsuang, M.T.; Buka, S.L.; et al. Sex differences, hormones, and fMRI stress response circuitry deficits in psychoses. Psychiatry Res. 2015, 232, 226–236. [Google Scholar] [CrossRef] [Green Version]
- González-Rodríguez, A.; Seeman, M.V. The association between hormones and antipsychotic use: A focus on postpartum and menopausal women. Ther. Adv. Psychopharmacol. 2019, 9, 2045125319859973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldstein, J.M.; Cherkerzian, S.; Seidman, L.J.; Donatelli, J.A.; Remington, A.G.; Tsuang, M.T.; Hornig, M.; Buka, S.L. Prenatal maternal immune disruption and sex-dependent risk for psychoses. Psychol. Med. 2014, 44, 3249–3261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edinoff, A.N.; Silverblatt, N.S.; Vervaeke, H.E.; Horton, C.C.; Girma, E.; Kaye, A.D.; Kaye, A.; Kaye, J.S.; Garcia, A.J.; Neuchat, E.E.; et al. Hyperprolactinemia, Clinical Considerations, and Infertility in Women on Antipsychotic Medications. Psychopharmacol. Bull. 2021, 51, 131–148. [Google Scholar] [PubMed]
- Bhargava, A.; Arnold, A.P.; Bangasser, D.A.; Denton, K.M.; Gupta, A.; Hilliard, K.L.M.; Mayer, E.A.; McCarthy, M.; Miller, W.L.; Raznahan, A.; et al. Considering sex as a biological variable in basic and clinical Studies: An endocrine society scientific statement. Endocr. Rev. 2021, 42, 219–258. [Google Scholar] [CrossRef] [PubMed]
- Beery, A.K.; Zucker, I. Sex bias in neuroscience and biomedical research. Neurosci. Biobehav. Rev. 2011, 35, 565–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Will, T.R.; Proaño, S.B.; Thomas, A.M.; Kunz, L.M.; Thompson, K.C.; Ginnari, L.A.; Jones, C.H.; Lucas, S.C.; Reavis, E.M.; Dorris, D.M.; et al. Problems and progress regarding sex bias and omission in neuroscience research. eNeuro 2017, 4. [Google Scholar] [CrossRef] [Green Version]
- Parekh, A.; Fadiran, E.O.; Uhl, K.; Throckmorton, D.C. Adverse effects in women: Implications for drug development and regulatory policies. Expert Rev. Clin. Pharmacol. 2011, 4, 453–466. [Google Scholar] [CrossRef]
- Farkouh, A.; Riedl, T.; Gottardi, R.; Czejka, M.; Kautzky-Willer, A. Sex-related differences in pharmacokinetics and pharmacodynamics of frequently prescribed drugs: A review of the literature. Adv. Ther. 2020, 37, 644–655. [Google Scholar] [CrossRef]
- Palmisano, B.T.; Zhu, L.; Eckel, R.H.; Stafford, J.M. Sex differences in lipid and lipoprotein metabolism. Mol. Metab. 2018, 15, 45–55. [Google Scholar] [CrossRef]
- Jongsma, H.E.; Turner, C.; Kirkbride, J.B.; Jones, P.B. International incidence of psychotic disorders, 2002-17: A systematic review and meta-analysis. Lancet Public Health 2019, 4, e229–e244. [Google Scholar] [CrossRef] [Green Version]
- Stafford, J.; Howard, R.; Dalman, C.; Kirkbride, J.B. The incidence of nonaffective, nonorganic psychotic disorders in older people: A population-based cohort study of 3 million people in Sweden. Schizophr. Bull. 2019, 45, 1152–1160. [Google Scholar] [CrossRef]
- Kirkbride, J.B.; Fearon, P.; Morgan, C.; Dazzan, P.; Morgan, K.; Tarrant, J.; Lloyd, T.; Holloway, J.; Hutchinson, G.; Leff, J.P.; et al. Heterogeneity in incidence rates of schizophrenia and other psychotic syndromes: Findings from the 3-center AeSOP study. Arch. Gen. Psychiatry 2006, 63, 250–258. [Google Scholar] [CrossRef] [Green Version]
- Jackson, D.; Kirkbride, J.; Croudace, T.; Morgan, C.; Boydell, J.; Errazuriz, A.; Murray, R.M.; Jones, P.B. Meta-analytic approaches to determine gender differences in the age-incidence characteristics of schizophrenia and related psychoses. Int. J. Methods Psychiatr. Res. 2013, 22, 36–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalsgaard, S.; Thorsteinsson, E.; Trabjerg, B.B.; Schullehner, J.; Plana-Ripoll, O.; Brikell, I.; Wimberley, T.; Thygesen, M.; Madsen, K.B.; Timmerman, A.; et al. Incidence rates and cumulative incidences of the full spectrum of diagnosed mental disorders in childhood and adolescence. AMA Psychiatry 2020, 77, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Adjorlolo, S.; Setordzi, M. Psychosis in adolescents in Africa: A scoping review for current understanding and future directions. Cogent Psychol. 2021, 8, 1949173. [Google Scholar] [CrossRef]
- Pignon, B.; Eaton, S.; Schürhoff, F.; Szöke, A.; McGorry, P.; O’Donoghue, B. Temporal variation in the incidence of treated psychotic disorders in young people. Schizophr. Res. 2021, 231, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Perälä, J.; Suvisaari, J.J.; Saarni, S.I.; Kuoppasalmi, K.; Isometsa, E.; Pirkola, S.; Partonen, T.; Tuulio-Henriksson, A.; Hintikka, J.J.; Kieseppa, T.; et al. Lifetime prevalence of psychotic and bipolar I disorders in a general population. Arch. Gen. Psychiatry 2007, 64, 19–28. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Küstner, B.; Martin, C.; Pastor, L. Prevalence of psychotic disorders and its association with methodological issues. A systematic review and meta-analyses. PLoS ONE 2018, 13, e0195687. [Google Scholar] [CrossRef]
- Jemli, A.; Inoubli, O.; Trifa, F.; Mechri, A.; Zaafrane, F.; Gaha, L.; Jrad, B.B. IFNGR2 genetic polymorphism associated with sex-specific paranoid schizophrenia risk. Nord. J. Psychiatry 2017, 71, 42–47. [Google Scholar] [CrossRef]
- Wan, L.; Zhang, G.; Liu, M.; Wang, C.; Li, Y.; Li, R. Sex-specific effects of methylenetetrahydrofolate reductase polymorphisms on schizophrenia with methylation changes. Compr. Psychiatry 2019, 94, 152121. [Google Scholar] [CrossRef]
- Sershen, H.; Guidotti, A.; Auta, J.; Drnevich, J.; Grayson, D.R.; Veldic, M.; Meyers, J.; Youseff, M.; Zhubi, A.; Faurot, K.; et al. Gene expression of methylation cycle and related genes in lymphocytes and brain of patients with schizophrenia and non-psychotic controls. Biomark. Neuropsychiatry 2021, 5, 100038. [Google Scholar] [CrossRef]
- Gureje, O.; Olowosegun, O.; Adebayo, K.; Stein, D. The prevalence and profile of non-affective psychosis in the Nigerian Survey of Mental Health and Wellbeing. World J. Psychiatry 2010, 9, 50–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Binbay, T.; Alptekin, K.; Elbi, H.; Binbay, T.; Alptekin, K.; Elbi, H.; Zağli, N.; Drukker, M.; Aksu Tanik, F.; Tanik, F.A.; et al. Lifetime prevalence and correlates of schizophrenia and disorders with psychotic symptoms in the general population of Izmir, Turkey. Turk. J. Psychiatry 2012, 23, 149–160. [Google Scholar]
- Kodesh, A.; Goldshtein, I.; Gelkopf, M.; Goren, I.; Chodick, G.; Shalev, V. Epidemiology and comorbidity of severe mental illnesses in the community: Findings from a computerized mental health registry in a large Israeli health organization. Soc. Psychiatry Psychiatr. Epidemiol. 2012, 47, 1775–1782. [Google Scholar] [CrossRef]
- Moreno-Küstner, B.; Mayoral, F.; Navas Campaña, D.; García Herrera, J.M.; Angona, P.; Martín, C.; Rivas, F. Prevalence of schizophrenia and related disorders in Malaga (Spain): Results using multiple clinical databases. Epidemiol. Psychiatr. Sci. 2016, 25, 38–58. [Google Scholar] [CrossRef] [PubMed]
- Orrico-Sánchez, A.; López-Lacort, M.; Muñoz-Quiles, C.; Sanfélix-Gimeno, G.; Díez-Domingo, J. Epidemiology of schizophrenia and its management over 8-years period using real-world data in Spain. BMC Psychiatry 2020, 20, 149. [Google Scholar] [CrossRef] [Green Version]
- Morgan, V.A.; McGrath, J.J.; Jablensky, A.; Badcock, C.; Waterreus, A.; Bush, R.; Carr, V.; Castle, D.; Cohen, M.; Galletly, C.; et al. Psychosis prevalence and physical, metabolic and cognitive co-morbidity: Data from the second Australian national survey of psychosis. Psychol. Med. 2014, 44, 2163–2176. [Google Scholar] [CrossRef] [Green Version]
- Phanthunane, P.; Vos, T.; Whiteford, H.; Bertram, M.; Udomratn, P. Schizophrenia in Thailand: Prevalence and burden of disease. Popul. Health Metr. 2010, 8, 24. [Google Scholar] [CrossRef] [Green Version]
- Jörgensen, L.; Allebeck, P.; Dalman, C. Prevalence of psychoses in Stockholm County: A population-based study using comprehensive healthcare registers. Nord. J. Psychiatry 2014, 68, 60–65. [Google Scholar] [CrossRef] [Green Version]
- Tandon, R.; Keshavan, M.S.; Nasrallah, H.A. Schizophrenia, “Just the Facts”: What we know in 2008. Part 1: Overview. Schizophr. Res. 2007, 100, 4–19. [Google Scholar] [CrossRef]
- Carpenter, W.T. The facts of schizophrenia: A personal commentary. Schizophr. Res. 2011, 128, 3–4. [Google Scholar] [CrossRef]
- Häfner, H. From onset and prodromal stage to a life-long course of schizophrenia and its symptom dimensions: How sex, age, and other risk factors influence incidence and course of illness. Psychiatry J. 2019, 2019, 9804836. [Google Scholar] [CrossRef] [Green Version]
- Abel, K.M.; Drake, R.; Goldstein, J.M. Sex differences in schizophrenia. Int. Rev. Psychiatry 2010, 22, 417–428. [Google Scholar] [CrossRef]
- Gogos, A.; Ney, L.J.; Seymour, N.; Van Rheenen, T.E.; Felmingham, K.L. Sex differences in schizophrenia, bipolar disorder, and post-traumatic stress disorder: Are gonadal hormones the link? Br. J. Psychiatry 2019, 176, 4119–4135. [Google Scholar] [CrossRef]
- Han, M.; Huang, X.F.; Chen, D.C.; Xiu, M.H.; Hui, L.; Liu, H.; Kosten, T.R.; Zhang, X.Y. Gender differences in cognitive function of patients with chronic schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 2012, 39, 358–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, B.; Han, M.; Tan, S.; De Yang, F.; Tan, Y.; Jiang, S.; Zhang, X.; Huang, X.F. Gender differences measured by the MATRICS consensus cognitive battery in chronic schizophrenia patients. Sci. Rep. 2017, 7, 11821. [Google Scholar] [CrossRef] [PubMed]
- Chalkiadaki, K.; Velli, A.; Kyriazidis, E.; Stavroulaki, V.; Vouvoutsis, V.; Chatzaki, E.; Aivaliotis, M.; Sidiropoulou, K. Development of the MAM model of schizophrenia in mice: Sex similarities and differences of hippocampal and prefrontal cortical function. Neuropharmacology 2019, 144, 193–207. [Google Scholar] [CrossRef] [PubMed]
- Gogos, A.; Sbisa, A.M.; Sun, J.; Gibbons, A.; Udawela, M.; Dean, B. A role for estrogen in schizophrenia: Clinical and preclinical findings. Int. J. Endocrinol. 2015, 2015, 615356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wischhof, L.; Irrsack, E.; Osorio, C.; Koch, M. Prenatal LPS-exposure—A neurodevelopmental rat model of schizophrenia—Differentially affects cognitive functions, myelination and parvalbumin expression in male and female offspring. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2015, 57, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Androvičovà, R.; Pfaus, J.G.; Ovsepian, S.V. Estrogen pendulum in schizophrenia and Alzheimer’s disease: Review of therapeutic benefits and outstanding questions. Neurosci. Lett. 2021, 759, 136038. [Google Scholar] [CrossRef]
- Cahill, L.; Aswad, D. Sex influences on the brain: An issue whose time has come. Neuron 2015, 88, 1084–1085. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Huang, J.; Zhou, Q.X.; Yang, C.X.; Yang, C.P.; Mei, W.Y.; Zhang, L.; Zhang, Q.; Hu, L.; Hu, Y.Q.; et al. ZFP804A mutant mice display sex-dependent schizophrenia-like behaviors. Mol. Psychiatry 2021, 26, 2514–2532. [Google Scholar] [CrossRef] [PubMed]
- Grech, A.M.; Du, X.; Murray, S.S.; Xiao, J.; Hill, R.A. Sex-specific spatial memory deficits in mice with a conditional TrkB deletion on parvalbumin interneurons. Behav. Brain Res. 2019, 372, 111984. [Google Scholar] [CrossRef]
- Krysta, K.; Krzystanek, M.; Jakuszkowiak-Wojten, K.; Wegielnik-Galuszko, M.; Wilkowska, A.; Wiglusz, M.; Cubala, W. Influence of sex hormones and inflammatory processes on cognition in schizophrenia. Psychiatr. Danub. 2019, 31 (Suppl. 3), 517–519. [Google Scholar]
- Mendrek, A.; Mancini-Marie, A. Sex/gender differences in the brain and cognition in schizophrenia. Neurosci. Biobehav. Rev. 2016, 67, 57–78. [Google Scholar] [CrossRef] [PubMed]
- Mu, L.; Liang, J.; Wang, H.; Chen, D.; Xiu, M.; Zhang, X.Y. Sex differences in association between clinical correlates and cognitive impairment in patients with chronic schizophrenia. J. Psychiatr. Res. 2020, 131, 194–202. [Google Scholar] [CrossRef]
- Madzarac, Z.; Tudor, L.; Sagud, M.; Nedic Erjavec, G.; Mihaljevic Peles, A.; Pivac, N. The associations between COMT and MAO-B genetic variants with negative symptoms in patients with schizophrenia. Curr. Issues Mol. Biol. 2021, 43, 45. [Google Scholar] [CrossRef]
- Papaleo, F.; Sannino, S.; Piras, F.; Spalletta, G. Sex-dichotomous effects of functional COMT genetic variations on cognitive functions disappear after menopause in both health and schizophrenia. Eur. Neuropsychopharmacol. 2015, 25, 2349–2363. [Google Scholar] [CrossRef]
- Bristow, G.C.; Bostrom, J.A.; Haroutunian, V.; Sodhi, M.S. Sex differences in GABAergic gene expression occur in the anterior cingulate cortex in schizophrenia. Schizophr. Res. 2015, 167, 57–63. [Google Scholar] [CrossRef] [Green Version]
- Seeman, M.V. Men and women respond differently to antipsychotic drugs. Neuropharmacology 2020, 163, 107631. [Google Scholar] [CrossRef]
- Franconi, F.; Campesi, I. Sex impacts on biomarkers, pharmacokinetics and pharmacodynamics. Curr. Med. Chem. 2017, 24, 2561–2575. [Google Scholar] [CrossRef]
- Marazziti, D.; Baroni, S.; Picchetti, M.; Piccini, A.; Carlini, M.; Vatteroni, E.; Falaschi, V.; Lombardi, A.; Dell’Osso, L. Pharmacokinetics and pharmacodinamics of psychotropic drugs: Effect of sex. CNS Spectrums. 2013, 18, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Soldin, O.P.; Mattison, D.R. Sex differences in pharmacokinetics and pharmacodynamics. Clin. Pharmacokinet. 2009, 48, 143–157. [Google Scholar] [CrossRef] [Green Version]
- Seeman, M.V. The pharmacodynamics of antipsychotic drugs in women and men. Front. Psychiatry 2021, 12, 650904. [Google Scholar] [CrossRef] [PubMed]
- Spoletini, I.; Vitale, C.; Malorni, W.; Rosano, G.M.C. Sex differences in drug effects: Interaction with sex hormones in adult life. In Sex and Gender Differences in Pharmacology; Regitz-Zagrosek, V., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 91–106. [Google Scholar]
- Arad, M.; Weiner, I. Sex-dependent antipsychotic capacity of 17β-estradiol in the latent inhibition model: A typical antipsychotic drug in both sexes, atypical antipsychotic drug in males. Neuropsychopharmacology 2010, 35, 2179–2192. [Google Scholar] [CrossRef] [Green Version]
- Barth, C.; Villringer, A.; Sacher, J. Sex hormones affect neurotransmitters and shape the adult female brain during hormonal transition periods. Front. Neurosci. 2015, 9, 37. [Google Scholar] [CrossRef] [Green Version]
- Deems, N.P.; Leuner, B. Pregnancy, postpartum and parity: Resilience and vulnerability in brain health and disease. Front. Neuroendocrinol. 2020, 5, 100820. [Google Scholar] [CrossRef] [PubMed]
- Madla, C.M.; Gavins, F.K.H.; Merchant, H.A.; Orlu, M.; Murdan, S.; Basit, A.W. Let’s talk about sex: Differences in drug therapy in males and females. Adv. Drug Deliv. Rev. 2021, 175, 113804. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, J.; Gurvich, C.; Lee, S.; Gilbert, H.; Gavrilidis, E.; de Castella, A.; Berk, M.; Dodd, S.; Fitzgerald, P.; Davis, S.R. Piloting the effective therapeutic dose of adjunctive selective estrogen receptor modulator treatment in postmenopausal women with schizophrenia. Psychoneuroendocrinology 2010, 35, 1142–1147. [Google Scholar] [CrossRef]
- Kulkarni, J.; Butler, S.; Riecher-Rössler, A.; Alfred, M.; Maprc, C. Estrogens and SERMS as adjunctive treatments for schizophrenia. Front. Neuroendocrinol. 2019, 53, 100743. [Google Scholar] [CrossRef]
- Weiser, M.; Levi, L.; Burshtein, S.; Hagin, M.; Matei, V.P.; Podea, D.; Micluţia, I.; Tiugan, A.; Păcală, B.; Grecu, I.G.; et al. Raloxifene plus antipsychotics versus placebo plus antipsychotics in severely ill decompensated postmenopausal women with schizophrenia or schizoaffective disorder: A randomized controlled trial. J. Clin. Psychiatr. 2017, 78, e758–e765. [Google Scholar] [CrossRef]
- Brand, B.A.; de Boer, J.N.; Oude Ophuis, S.B.J.; Slot, M.I.E.; De Wilde, B.; Catthoor, K.C.E.E.R.; Goverde, A.J.; Bakker, P.R.; Marcelis, M.C.; Grootens, K.P.; et al. Raloxifene augmentation in men and women with a schizophrenia spectrum disorder: A study protocol. Contemp. Clin. Trials Commun. 2020, 20, 100681. [Google Scholar] [CrossRef] [PubMed]
- Inman, W.; Theriot, R.; Briggs, R.; Heaston, A. The plight of aging women in Arab nations. J. Pub. Health Issues Pract. 2019, 3, 151. [Google Scholar]
- Gogos, A.; Kwek, P.; van den Buuse, M. The role of estrogen and testosterone in female rats in behavioral models of relevance to schizophrenia. Psychopharmacology 2012, 219, 213–224. [Google Scholar] [CrossRef]
- Georgiu, P.; Zanos, P.; Jenne, C.E.; Gould, T.D. Sex-specific involvement of estrogen receptors in behavioural responses to stress and psychomotor activation. Front. Psychiatry 2019, 10, 81. [Google Scholar] [CrossRef] [Green Version]
- Ceskova, E.; Prikryl, R.; Libiger, J.; Svancara, J.; Jarkovsky, J. Gender differences in the treatment of first-episode schizophrenia: Results from the European First Episode Schizophrenia Trial. Schizophr. Res. 2015, 169, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Ismail, Z.; Wessels, A.M.; Uchida, H.; Ng, W.; Mamo, D.C.; Rajji, T.K.; Pollock, B.G.; Mulsant, B.H.; Bies, R.R. Age and sex impact clozapine plasma concentrations in inpatients and outpatients with schizophrenia. Am. J. Geriatr. Psychiatry 2012, 20, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Jönsson, A.K.; Spigset, O.; Reis, M. A compilation of serum concentrations of 12 antipsychotic drugs in a therapeutic drug monitoring setting. Ther. Drug Monit. 2019, 41, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Hoekstra, S.; Bartz-Johannessen, C.; Sinkeviciute, I.; Reitan, S.K.; Kroken, R.A.; Løberg, E.-M.; Larsen, T.K.; Rettenbacher, M.; Johnsen, E.; Sommer, I.E. Sex differences in antipsychotic efficacy and side effects in schizophrenia spectrum disorder: Results from the BeSt InTro study. NPJ Schizophr. 2021, 7, 39. [Google Scholar] [CrossRef] [PubMed]
- Rahman, T.; Sahrmann, J.M.; Olsen, M.A.; Nickel, K.B.; Miller, J.P.; Ma, C.; Grucza, R.A. Antipsychotic drugs and the risk of breast cancer. medRxiv 2021. [Google Scholar] [CrossRef]
- Wang, M.T.; Liou, J.T.; Huang, Y.W.; Lin, C.W.; Wu, G.J.; Chu, C.L.; Yeh, C.B.; Wang, Y.H. Use of antipsychotics and risk of venous thromboembolism in postmenopausal women. Thromb. Haemost. 2016, 115, 1209–1219. [Google Scholar]
- González-Rodríguez, A.; Labad, J.; Seeman, M.V. Antipsychotic-induced Hyperprolactinemia in aging populations: Prevalence, implications, prevention and management. Prog. Neuropsychopharmacol. Biol. Psychiatry 2020, 101, 109941. [Google Scholar] [CrossRef]
- Suzuki, Y.; Sugai, T.; Fukui, N.; Watanabe, J.; Ono, S.; Tsuneyama, N.; Saito, M.; Someya, T. Sex differences in the effect of four second-generation antipsychotics on QTc interval in patients with schizophrenia. Hum. Psychopharmacol. 2013, 28, 215–219. [Google Scholar] [CrossRef]
- Kurokawa, J.; Kodama, M.; Clancy, C.E.; Furukawa, T. Sex hormonal regulation of cardiac ion channels in drug-induced QT syndromes. Pharmacol. Ther. 2016, 168, 23–28. [Google Scholar] [CrossRef] [Green Version]
- Dhindsa, R.S.; Goldstein, D.B. Schizophrenia: From genetics to physiology at last. Nature 2016, 530, 162–163. [Google Scholar] [CrossRef] [Green Version]
- Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature 2014, 511, 421–427. [Google Scholar] [CrossRef] [Green Version]
- Sekar, A.; Bialas, A.R.; de Rivera, H.; Davis, A.; Hammond, T.R.; Kamitaki, N.; Tooley, K.; Presumey, J.; Baum, M.; Van Doren, V.; et al. Schizophrenia risk from complex variation of complement component 4. Nature 2016, 530, 177–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrientos, R.M.; Brunton, P.J.; Lenz, K.M.; Pyter, L.; Spencer, S.J. Neuroimmunology of the female brain across the lifespan: Plasticity to psychopathology. Brain Behav. Immun. 2019, 79, 39–55. [Google Scholar] [CrossRef]
- Taneja, V. Sex hormones determine immune response. Front. Immunol. 2018, 9, 1931. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.; Seger, D.L.; Lai, K.H.; Goss, F.R.; Blumenthal, K.G.; Zhou, L. Drug hypersensitivity reactions documented in electronic health records within a large health system. J. Allergy Clin. Immunol. Pract. 2019, 7, 1253–1260. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Song, X.; Guo, Y.; Lang, X.; Li, Z.; Zhang, X.Y. Sex differences in metabolic disorder patterns of first-episode drug-naive patients with schizophrenia. Psychoneuroendocrinology 2021, 124, 105061. [Google Scholar] [CrossRef] [PubMed]
- Goossens, G.H.; Jocken, J.W.E.; Blaak, E.E. Sexual dimorphism in cardiometabolic health: The role of adipose tissue, muscle and liver. Nat. Rev. Endocrinol. 2021, 17, 47–66. [Google Scholar] [CrossRef] [PubMed]
- Kraal, A.Z.; Ward, K.M.; Ellingrod, V.L. Sex differences in antipsychotic related metabolic functioning in schizophrenia spectrum disorders. Psychopharmacol. Bull. 2017, 47, 8–21. [Google Scholar] [PubMed]
- Li, Q.; Chen, D.; Liu, T.; Walss-Bass, C.; de Quevedo, J.L.; Soares, J.C.; Zhao, J.; Zhang, X.Y. Sex differences in body mass index and obesity in Chinese patients with chronic schizophrenia. J. Clin. Psychopharmacol. 2016, 36, 643–648. [Google Scholar] [CrossRef]
- Chen, S.; Broqueres-You, D.; Yang, G.; Wang, Z.; Li, Y.; Yang, F.; Tan, Y. Male sex may be associated with higher metabolic risk in first-episode schizophrenia patients: A preliminary study. Asian J. Psychiatr. 2016, 21, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Carli, M.; Kolachalam, S.; Longoni, B.; Pintaudi, A.; Baldini, M.; Aringhieri, S.; Fasciani, I.; Annibale, P.; Maggio, R.; Scarselli, M. Atypical antipsychotics and metabolic syndrome: From molecular mechanisms to clinical differences. Pharmaceuticals 2021, 14, 238. [Google Scholar] [CrossRef]
- Lau, S.L.; Muir, C.; Assur, Y.; Beach, R.; Tran, B.; Bartrop, R.; McLean, M.; Caetano, D. Predicting weight gain in patients treated with clozapine: The role of sex, body mass index, and smoking. J. Clin. Psychopharmacol. 2016, 36, 120–124. [Google Scholar] [CrossRef]
- Uguz, F. Antipsychotic use during pregnancy and the risk of gestational diabetes mellitus: A systematic review. J. Clin. Psychopharmacol. 2019, 39, 162–167. [Google Scholar] [CrossRef]
- Wang, Z.; Brauer, R.; Man, K.K.C.; Alfageh, B.; Mongkhon, P.; Wong, I.C.K. Prenatal exposure to antipsychotic agents and the risk of congenital malformations in children: A systematic review and meta-analysis. Br. J. Clin. Pharmacol. 2021. [Google Scholar] [CrossRef]
- Poels, E.M.P.; Schrijver, L.; Kamperman, A.M.; Hillegers, M.H.J.; Hoogendijk, W.J.G.; Kushner, S.A.; Roza, S.J. Long-term neurodevelopmental consequences of intrauterine exposure to lithium and antipsychotics: A systematic review and meta-analysis. Eur. Child Adolesc. Psychiatry 2018, 27, 1209–1230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uguz, F. A new safety scoring system for the use of psychotropic drugs during lactation. Am. J. Ther. 2021, 28, e118–e126. [Google Scholar] [CrossRef] [PubMed]
- Sommer, I.E.; Tiihonen, J.; van Mourik, A.; Tanskanen, A.; Taipale, H. The clinical course of schizophrenia in women and men —A nation-wide cohort study. NPJ Schizophr. 2020, 6, 12. [Google Scholar] [CrossRef]
- Seeman, M.V. Does gender influence outcome in schizophrenia? Psychiatr. Q. 2019, 90, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Lamsma, J.; Harte, J.M. Violence in psychosis: Conceptualizing its causal relationship with risk factors. Aggress. Violent Behav. 2015, 24, 75–82. [Google Scholar] [CrossRef] [Green Version]
- Cho, W.; Shin, W.S.; An, I.; Bang, M.; Cho, D.Y.; Lee, S.H. Biological aspects of aggression and violence in schizophrenia. Clin. Psychopharmacol. Neurosci. 2019, 17, 475–486. [Google Scholar] [CrossRef]
- Popovic, D.; Benabarre, A.; Crespo, J.M.; Goikolea, J.M.; González-Pinto, A.; Gutiérrez-Rojas, L.; Montes, J.M.; Vieta, E. Risk factors for suicide in schizophrenia: Systematic review and clinical recommendations. Acta Psychiatr. Scand. 2014, 130, 418–426. [Google Scholar] [CrossRef]
- Fuller-Thomson, E.; Hollister, B. Schizophrenia and suicide attempts: Findings from a representative community-based Canadian sample. Schizophr. Res. Treat. 2016, 2016, 3165243. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Preventing Suicide: A Global Imperative; WHO: Geneva, Switzerland, 2014; Available online: https://www.who.int/mental_health/suicide-prevention/exe_summary_english.pdf (accessed on 17 September 2021).
- Bagalkot, T.R.; Park, J.I.; Kim, H.T.; Kim, H.M.; Kim, M.S.; Yoon, M.S.; Ko, S.H.; Cho, H.C.; Chung, Y.C. Lifetime prevalence of and risk factors for suicidal ideation and suicide attempts in a Korean community sample. Psychiatry 2014, 77, 360–373. [Google Scholar] [CrossRef]
- Plana-Ripoll, O.; Musliner, K.L.; Dalsgaard, S.; Momen, N.C.; Weye, N.; Christensen, M.K.; Agerbo, E.; Iburg, K.M.; Laursen, T.M.; Mortensen, P.B.; et al. Nature and prevalence of combinations of mental disorders and their association with excess mortality in a population-based cohort study. World Psychiatry 2020, 19, 339–349. [Google Scholar] [CrossRef]
- Olfson, M.; Gerhard, T.; Huang, C.; Crystal, S.; Stroup, T.S. Premature mortality among adults with schizophrenia in the United States. JAMA Psychiatry 2015, 72, 1172–1181. [Google Scholar] [CrossRef] [Green Version]
- Appelman, Y.; van Rijn, B.B.; Ten Haaf, M.E.; Boersma, E.; Peters, S.A. Sex differences in cardiovascular risk factors and disease prevention. Atherosclerosis 2015, 241, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Aarestrup Hellemose, A.; Munk Larsen, T.; Tiselbak Larsen, J.; Toender, A. Accidental deaths among persons with schizophrenia: A nationwide population-based cohort study. Schizophr. Res. 2018, 199, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Andersson, H.W.; Lilleeng, S.E.; Ruud, T.; Osborg Ose, S. Substance use among patients in specialized mental health services in Norway: Prevalence and patient characteristics based on a national census. Nord. J. Psychiatry 2021, 75, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Bachman, S. Epidemiology of suicide and the psychiatric perspective. Int. J. Environ. Res. Public Health 2018, 15, 1425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Silva, T.L.; Ravindran, A.V. Contribution of sex hormones to gender differences in schizophrenia: A review. Asian J. Psychiatry 2015, 18, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Falkenburg, J.; Tracy, D.K. Sex and schizophrenia: A review of gender differences. Psychosis 2014, 6, 61–69. [Google Scholar] [CrossRef]
- Remberk, B.; Bażyńska, A.K.; Krempa-Kowalewska, A.; Rybakowski, F. Adolescent insanity revisited: Course and outcome in early-onset schizophrenia spectrum psychoses in an 8-year follow-up study. Compr. Psychiatry 2014, 55, 1174–1181. [Google Scholar] [CrossRef]
- Thorup, A.; Albert, N.; Bertelsen, M.; Petersen, L.; Jeppesen, P.; Le Quack, P.; Krarup, G.; Jørgensen, P.; Nordentoft, M. Gender differences in first-episode psychosis at 5-year follow-up—Two different courses of disease? Results from the OPUS study at 5-year follow-up. Eur. Psychiatry 2014, 29, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Jääskeläinen, E.; Juola, P.; Hirvonen, N.; McGrath, J.J.; Saha, S.; Isohanni, M.; Veijola, J.; Miettunen, J. A systematic review and meta-analysis of recovery in schizophrenia. Schizophr. Bull. 2013, 39, 1296–1306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayesa-Arriola, R.; de la Foz, V.O.; Setién-Suero, E.; Ramirez-Bonilla, M.L.; Suárez-Pinilla, P.; Mayoral-van Son, J.; Vázquez-Bourgon, J.; Juncal-Ruiz, M.; Gómez-Revuelta, M.; Tordesillas-Gutiérrez, D.; et al. Understanding sex differences in long-term outcomes after a first episode of psychosis. NPJ Schizophr. 2020, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Thomas, N.; Gurvich, C.; Hudaib, A.R.; Gavrilidis, E.; Kulkarni, J. Dissecting the syndrome of schizophrenia: Associations between symptomatology and hormone levels in women with schizophrenia. Psychiatry Res. 2019, 280, 112510. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.; Graf, E.; Brooks Jackson, J. A hot flash about menopause hormone therapy. Clin. Rev. Cases 2021, 3, 1–4. [Google Scholar]
- Reinehr, T.; Roth, C.L. Is there a causal relationship between obesity and puberty? Lancet Child Adolesc. Health 2019, 3, 44–54. [Google Scholar] [CrossRef]
- Damme, K.S.F.; Ristanovic, I.; Vargas, T.; Mittal, V.A. Timing of menarche and abnormal hippocampal connectivity in youth at clinical-high risk for psychosis. Psychoneuroendocrinology 2020, 117, 104672. [Google Scholar] [CrossRef]
- Moyer, A.M.; Matey, E.T.; Miller, V.M. Individualized medicine: Sex, hormones, genetics, and adverse drug reactions. Pharmacol. Res. Perspect. 2019, 7, e00541. [Google Scholar] [CrossRef]
- Seeman, M.V. Gendering psychosis: The illness of Zelda Fitzgerald. Med. Humanit. 2016, 42, 65–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Animal Models | |
|---|---|
| Global findings | Ovariectomized rats show low levels of estrogens with hyperfunction of the dopaminergic system (an animal model of menopausal psychosis) [41,45]. |
| Findings by hormonal status | 17-betaestradiol combined with antipsychotics reversed amphetamine-induced latent inhibition disruption in rats [46]. |
| Human Models | |
| Global findings | Men with schizophrenia show worse immediate and delayed memory and worse social cognition, processing speed, and verbal and visual learning than women [37,43]. The proportion of psychopathological symptoms may differ by sex but their variety shows no sex difference [43]. |
| Findings by hormonal status | Estradiol has a positive effect on psychopathological and cognitive symptoms [43]. |
| Pharmacokinetic Differences | |
|---|---|
| Absorption [55,56] | Women absorb antipsychotic drugs better than men during their reproductive years. |
| Distribution [57] | Women’s bodies have more adipose tissue within which antipsychotic drugs can accumulate. |
| Metabolism [57] | Estrogen levels impact some, but not all liver and intestinal metabolic enzymes. |
| Elimination [57] | Hepatic clearance is lower in premenopausal women than in postmenopausal women or men. |
| Pharmacodynamic Differences | |
| Efficacy (wanted effects) [57,58] | Premenopausal women show a better response to antipsychotics compared to men and postmenopausal women. |
| Side-effects, safety concerns (unwanted effects) [58] | Women suffer more frequently than men from cardiac arrhythmia, obesity, the effects of hyperprolactinemia, immune reactions. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seeman, M.V.; González-Rodríguez, A. Stratification by Sex and Hormone Level When Contrasting Men and Women in Schizophrenia Trials Will Improve Personalized Treatment. J. Pers. Med. 2021, 11, 929. https://0-doi-org.brum.beds.ac.uk/10.3390/jpm11090929
Seeman MV, González-Rodríguez A. Stratification by Sex and Hormone Level When Contrasting Men and Women in Schizophrenia Trials Will Improve Personalized Treatment. Journal of Personalized Medicine. 2021; 11(9):929. https://0-doi-org.brum.beds.ac.uk/10.3390/jpm11090929
Chicago/Turabian StyleSeeman, Mary V., and Alexandre González-Rodríguez. 2021. "Stratification by Sex and Hormone Level When Contrasting Men and Women in Schizophrenia Trials Will Improve Personalized Treatment" Journal of Personalized Medicine 11, no. 9: 929. https://0-doi-org.brum.beds.ac.uk/10.3390/jpm11090929