A Network Analysis of Depressive Symptoms in the Elderly with Subjective Memory Complaints
Abstract
:1. Introduction
1.1. Subjective Memory Complaints
1.2. Depression and SMCs
1.3. Psychiatric Research and Network Analysis
1.4. Study Purpose
2. Materials and Methods
2.1. Participants
2.2. Measurements
2.2.1. Subjective Memory Complaints Questionnaire (SMCQ)
2.2.2. Patient Health Questionnaire-9 (PHQ-9)
2.3. Network Statistical Analysis
3. Results
3.1. General Characteristics
3.2. Network Analysis
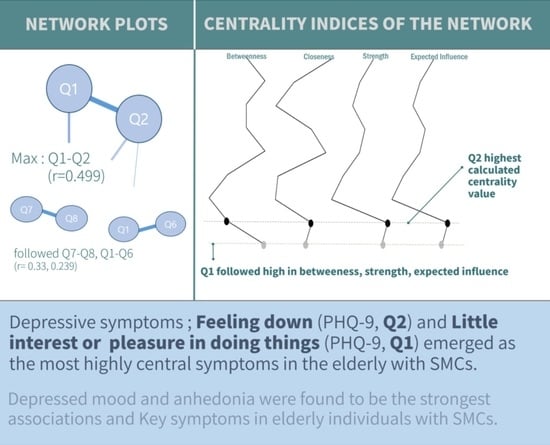
3.2.1. Interaction between Depressive Symptoms of SMCs
3.2.2. Computing Centrality Measures of SMCs
3.2.3. Clustering Coefficient Measures
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Item | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 |
|---|---|---|---|---|---|---|---|---|---|
| Q1 | 0 | ||||||||
| Q2 | 0.499 | 0 | |||||||
| Q3 | 0.017 | 0.105 | 0 | ||||||
| Q4 | 0 | 0.062 | 0.121 | 0 | |||||
| Q5 | 0.074 | 0 | 0.086 | 0.184 | 0 | ||||
| Q6 | 0.239 | 0.134 | 0.123 | 0.101 | 0.17 | 0 | |||
| Q7 | 0.095 | 0.116 | 0.103 | 0.067 | 0.048 | 0 | 0 | ||
| Q8 | 0.006 | 0.063 | 0.108 | 0.037 | 0.156 | 0.06 | 0.33 | 0 | |
| Q9 | 0.132 | 0.134 | 0.021 | 0.2 | 0.178 | 0.112 | 0.087 | 0.14 | 0 |
References
- Balash, Y.; Mordechovich, M.; Shabtai, H.; Merims, D.; Giladi, N. Subjective memory decline in healthy community-dwelling elders. What does this complain mean? Acta Neurol. Scand. 2010, 121, 194–197. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, A.J. The clinical significance of subjective memory complaints in the diagnosis of mild cognitive impairment and dementia: A meta-analysis. Int. J. Geriatr. Psychiatry J. Psychiatry Late Life Allied Sci. 2008, 23, 1191–1202. [Google Scholar] [CrossRef] [PubMed]
- Ponds, R.W.; Commissaris, K.J.; Jolles, J. Prevalence and covariates of subjective forgetfulness in a normal population in The Netherlands. Int. J. Aging Hum. Dev. 1997, 45, 207–221. [Google Scholar] [CrossRef] [PubMed]
- Kaszniak, A.W.; Poon, L.W.; Riege, W.H. Assessing memory deficits: An information-processing approach. In Handbook for Clinical Memory Assessment of Older Adults; Poon, L.W., Crook, T., Davis, K.L., Eisdorfer, C., Gurland, B.J., Kaszniak, A.W., Thompson, L.W., Eds.; American Psychological Association: Washington, DC, USA, 1986; pp. 168–188. [Google Scholar]
- Begum, A.; Morgan, C.; Chiu, C.C.; Tylee, A.; Stewart, R. Subjective memory impairment in older adults: Aetiology, salience and help seeking. Int. J. Geriatr. Psychiatry 2012, 27, 612–620. [Google Scholar] [CrossRef]
- Dickerson, B.C.; Sperling, R.A.; Hyman, B.T.; Albert, M.S.; Blacker, D. Clinical prediction of Alzheimer disease dementia across the spectrum of mild cognitive impairment. Arch. Gen. Psychiatry 2007, 64, 1443–1450. [Google Scholar] [CrossRef] [Green Version]
- Glodzik-Sobanska, L.; Reisberg, B.; De Santi, S.; Babb, J.S.; Pirraglia, E.; Rich, K.E.; Brys, M.; de Leon, M.J. Subjective memory complaints: Presence, severity and future outcome in normal older subjects. Dement. Geriatr. Cogn. Disord. 2007, 24, 177–184. [Google Scholar] [CrossRef]
- Jessen, F.; Wiese, B.; Bachmann, C.; Eifflaender-Gorfer, S.; Haller, F.; Kölsch, H.; Luck, T.; Mösch, E.; van den Bussche, H.; Wagner, M. Prediction of dementia by subjective memory impairment: Effects of severity and temporal association with cognitive impairment. Arch. Gen. Psychiatry 2010, 67, 414–422. [Google Scholar] [CrossRef]
- Stewart, R.; Godin, O.; Crivello, F.; Maillard, P.; Mazoyer, B.; Tzourio, C.; Dufouil, C. Longitudinal neuroimaging correlates of subjective memory impairment: 4-year prospective community study. Br. J. Psychiatry 2011, 198, 199–205. [Google Scholar] [CrossRef]
- Steinberg, S.I.; Negash, S.; Sammel, M.D.; Bogner, H.; Harel, B.T.; Livney, M.G.; McCoubrey, H.; Wolk, D.A.; Kling, M.A.; Arnold, S.E. Subjective Memory Complaints, Cognitive Performance, and Psychological Factors in Healthy Older Adults. Am. J. Alzheimers Dis. Other Dement.® 2013, 28, 776–783. [Google Scholar] [CrossRef]
- Jorm, A.F.; Butterworth, P.; Anstey, K.J.; Christensen, H.; Easteal, S.; Maller, J.; Mather, K.; Turakulov, R.; Wen, W.; Sachdev, P. Memory complaints in a community sample aged 60–64 years: Associations with cognitive functioning, psychiatric symptoms, medical conditions, APOE genotype, hippocampus and amygdala volumes, and white-matter hyperintensities. Psychol. Med. 2004, 34, 1495–1506. [Google Scholar] [CrossRef]
- Waldorff, F.B.; Rishoj, S.; Waldemar, G. If you don’t ask (about memory), they probably won’t tell: If elders do self-report memory problems, their quality of life is probably suffering. J. Fam. Pract. 2008, 57, 41–45. [Google Scholar] [PubMed]
- Geerlings, M.I.; Jonker, C.; Bouter, L.M.; Adèr, H.J.; Schmand, B. Association between memory complaints and incident Alzheimer’s disease in elderly people with normal baseline cognition. Am. J. Psychiatry 1999, 156, 531–537. [Google Scholar] [PubMed]
- Schmand, B.; Jonker, C.; Geerlings, M.I.; Lindeboom, J. Subjective memory complaints in the elderly: Depressive symptoms and future dementia. Br. J. Psychiatry 1997, 171, 373–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kral, V.A. Senescent forgetfulness: Benign and malignant. Can. Med. Assoc. J. 1962, 86, 257. [Google Scholar]
- Crook, T.; Bartus, R.T.; Ferris, S.H.; Whitehouse, P.; Cohen, G.D.; Gershon, S. Age-associated memory impairment: Proposed diagnostic criteria and measures of clinical change—report of a national institute of mental health work group. Dev. Neuropsychol. 1986, 2, 261–276. [Google Scholar] [CrossRef]
- Dixon, R.A.; Hultsch, D.F. Metamemory and memory for text relationships in adulthood: A cross-validation study. J. Gerontol. 1983, 38, 689–694. [Google Scholar] [CrossRef]
- Larrabee, G.J.; West, R.L.; Crook, T.H. The association of memory complaint with computer-simulated everyday memory performance. J. Clin. Exp. Neuropsychol. 1991, 13, 466–478. [Google Scholar] [CrossRef]
- Riege, W.H. Self-report and tests of memory aging. Clin. Gerontol. 1983, 1, 23–36. [Google Scholar] [CrossRef]
- Zelinski, E.M.; Gilewski, M.J.; Thompson, L.W. Do laboratory tests relate to self-assessment of memory ability in the young and old. New Dir. Mem. Aging 1980, 1, 519–544. [Google Scholar]
- Bolla, K.I.; Lindgren, K.N.; Bonaccorsy, C.; Bleecker, M.L. Memory complaints in older adults: Fact or fiction? Arch. Neurol. 1991, 48, 61–64. [Google Scholar] [CrossRef]
- Kahn, R.L.; Zarit, S.H.; Hilbert, N.M.; Niederehe, G. Memory complaint and impairment in the aged: The effect of depression and altered brain function. Arch. Gen. Psychiatry 1975, 32, 1569–1573. [Google Scholar] [CrossRef] [PubMed]
- Larrabee, G.J.; Levin, H.S. Memory self-ratings and objective test performance in a normal elderly sample. J. Clin. Exp. Neuropsychol. 1986, 8, 275–284. [Google Scholar] [CrossRef] [PubMed]
- McGlone, J.; Gupta, S.; Humphrey, D.; Oppenheimer, S.; Mirsen, T.; Evans, D.R. Screening for early dementia using memory complaints from patients and relatives. Arch. Neurol. 1990, 47, 1189–1193. [Google Scholar] [CrossRef] [PubMed]
- Plotkin, D.A.; Mintz, J.; Jarvik, L.F. Subjective memory complaints in geriatric depression. Am. J. Psychiatry 1985, 142, 1103–1105. [Google Scholar] [PubMed]
- Broadbent, D.E.; Cooper, P.F.; FitzGerald, P.; Parkes, K.R. The cognitive failures questionnaire (CFQ) and its correlates. Br. J. Clin. Psychol. 1982, 21, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Derouesné, C.; Alperovitch, A.; Arvay, N.; Migeon, P. Memory complaints in the elderly: A study of 367 community-dwelling individuals from 50 to 80 years old. Arch. Gerontol. Geriatr. 1989, 1, 151–163. [Google Scholar]
- O’Connor, D.W.; Pollitt, P.A.; Roth, M.; Brook, C.P.B.; Reiss, B.B. Memory complaints and impairment in normal, depressed, and demented elderly persons identified in a community survey. Arch. Gen. Psychiatry 1990, 47, 224–227. [Google Scholar] [CrossRef]
- Poitrenaud, J.; Malbezin, M.; Guez, D. Self-rating and psychometric assessment of age-related changes in memory among young-elderly managers. Dev. Neuropsychol. 1989, 5, 285–294. [Google Scholar] [CrossRef]
- Zonderman, A.; Costa, P.; Kawas, C. Personality predicts complaints of benign memory loss. Neurology 1989, 39, 194. [Google Scholar]
- Barker, A.; Prior, J.; Jones, R. Memory complaint in attenders at a self-referral memory clinic: The role of cognitive factors, affective symptoms and personality. Int. J. Geriatr. Psychiatry 1995, 10, 777–781. [Google Scholar] [CrossRef]
- Derouesné, C.; Lacomblez, L.; Thibault, S.; Leponcin, M. Memory complaints in young and elderly subjects. Int. J. Geriatr. Psychiatry 1999, 14, 291–301. [Google Scholar] [CrossRef]
- Minett, T.S.; Dean, J.L.; Firbank, M.; English, P.; O’Brien, J.T. Subjective memory complaints, white-matter lesions, depressive symptoms, and cognition in elderly patients. Am. J. Geriatr. Psychiatry 2005, 13, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Stewart, R.; Russ, C.; Richards, M.; Brayne, C.; Lovestone, S.; Mann, A. Depression, APOE genotype and subjective memory impairment: A cross-sectional study in an African-Caribbean population. Psychol. Med. 2001, 31, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Zarit, S.H.; Cole, K.D.; Guider, R.L. Memory training strategies and subjective complaints of memory in the aged. Gerontologist 1981, 21, 158–164. [Google Scholar] [CrossRef]
- Blazer, D.G.; Hays, J.C.; Fillenbaum, G.G.; Gold, D.T. Memory complaint as a predictor of cognitive decline: A comparison of African American and White elders. J. Aging Health 1997, 9, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Clarnette, R.M.; Almeida, O.P.; Forstl, H.; Paton, A.; Martins, R.N. Clinical characteristics of individuals with subjective memory loss in Western Australia: Results from a cross-sectional survey. Int. J. Geriatr. Psychiatry 2001, 16, 168–174. [Google Scholar] [CrossRef]
- Gagnon, M.; Dartigues, J.F.; Mazaux, J.M.; Dequae, L.; Letenneur, L.; Giroire, J.M.; Barberger-Gateau, P. Self-reported memory complaints and memory performance in elderly French community residents: Results of the PAQUID Research Program. Neuroepidemiology 1994, 13, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Jonker, C.; Launer, L.J.; Hooijer, C.; Lindeboom, J. Memory complaints and memory impairment in older individuals. J. Am. Geriatr. Soc. 1996, 44, 44–49. [Google Scholar] [CrossRef]
- Kim, J.-M.; Stewart, R.; Shin, I.-S.; Choi, S.-K.; Yoon, J.-S. Subjective memory impairment, cognitive function and depression–a community study in older Koreans. Dement. Geriatr. Cogn. Disord. 2003, 15, 218–225. [Google Scholar] [CrossRef]
- Riedel-Heller, S.G.; Matschinger, H.; Schork, A.; Angermeyer, M.C. Do memory complaints indicate the presence of cognitive impairment?—Results of a field study. Eur. Arch. Psychiatry Clin. Neurosci. 1999, 249, 197–204. [Google Scholar] [CrossRef]
- Baldwin, R.; Jacoby, R.; Oppenheimer, C.; Dening, T.; Thomas, A. Oxford textbook of old age psychiatry. Mood Disord. Depress. Disord. 2008, 1, 529–556. [Google Scholar]
- Friedman, B.; Heisel, M.J.; Delavan, R.L. Psychometric properties of the 15-item geriatric depression scale in functionally impaired, cognitively intact, community-dwelling elderly primary care patients. J. Am. Geriatr. Soc. 2005, 53, 1570–1576. [Google Scholar] [CrossRef] [PubMed]
- Mills, T.L.; Alea, N.L.; Cheong, J.A. Differences in the indicators of depressive symptoms among a community sample of African–American and Caucasian older adults. Community Ment. Health J. 2004, 40, 309–331. [Google Scholar] [CrossRef]
- Shahpesandy, H. Different manifestation of depressive disorder in the elderly. Neuroendocrinol. Lett. 2005, 26, 691–695. [Google Scholar]
- Jung, I.-K.; Kwak, D.-I.; Joe, S.-H.; Lee, H.-S. A preliminary study on standardization of Korean form of geriatric depression scale (KGDS). J. Korean Neuropsychiatr. Assoc. 1998, 37, 340–351. [Google Scholar]
- Zakzanis, K.K.; Leach, L.; Kaplan, E. On the nature and pattern of neurocognitive function in major depressive disorder. Neuropsychiatry Neuropsychol. Behav. Neurol. 1998, 11, 111–119. [Google Scholar]
- Wilson, R.S.; Evans, D.A. How clearly do we see our memories? J. Am. Geriatr. Soc. 1996, 44, 93–94. [Google Scholar] [CrossRef]
- Borsboom, D.; Cramer, A.O. Network analysis: An integrative approach to the structure of psychopathology. Annu. Rev. Clin. Psychol. 2013, 9, 91–121. [Google Scholar] [CrossRef] [Green Version]
- Borgatti, S.P.; Mehra, A.; Brass, D.J.; Labianca, G. Network analysis in the social sciences. Science 2009, 323, 892–895. [Google Scholar] [CrossRef] [Green Version]
- Antikainen, R.; Hänninen, T.; Honkalampi, K.; Hintikka, J.; Koivumaa-Honkanen, H.; Tanskanen, A.; Viinamäki, H. Mood improvement reduces memory complaints in depressed patients. Eur. Arch. Psychiatry Clin. Neurosci. 2001, 251, 6–11. [Google Scholar] [CrossRef]
- Dentone, M.J.; Insua, A.M. Sanos y depp imidos. Medicina 1997, 57, 535–540. [Google Scholar] [PubMed]
- Lahr, D.; Beblo, T.; Hartje, W. Cognitive performance and subjective complaints before and after remission of major depression. Cogn. Neuropsychiatry 2007, 12, 25–45. [Google Scholar] [CrossRef] [PubMed]
- Youn, J.C.; Kim, K.W.; Lee, D.Y.; Jhoo, J.H.; Lee, S.B.; Park, J.H.; Choi, E.A.; Choe, J.Y.; Jeong, J.W.; Choo, I.H. Development of the subjective memory complaints questionnaire. Dement. Geriatr. Cogn. Disord. 2009, 27, 310–317. [Google Scholar] [CrossRef]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B. The PHQ-9: Validity of a brief depression severity measure. J. Gen. Intern. Med. 2001, 16, 606–613. [Google Scholar] [CrossRef]
- Park, S.-J.; Choi, H.-R.; Choi, J.-H.; Kim, K.-W.; Hong, J.-P. Reliability and validity of the Korean version of the Patient Health Questionnaire-9 (PHQ-9). Anxiety Mood 2010, 6, 119–124. [Google Scholar]
- Lauritzen, S.L.; Wermuth, N. Graphical models for associations between variables, some of which are qualitative and some quantitative. Ann. Stat. 1989, 17, 31–57. [Google Scholar] [CrossRef]
- Costantini, G.; Epskamp, S.; Borsboom, D.; Perugini, M.; Mõttus, R.; Waldorp, L.J.; Cramer, A.O. State of the aRt personality research: A tutorial on network analysis of personality data in R. J. Res. Personal. 2015, 54, 13–29. [Google Scholar] [CrossRef]
- Epskamp, S.; Borsboom, D.; Fried, E.I. Estimating psychological networks and their accuracy: A tutorial paper. Behav. Res. Methods 2018, 50, 195–212. [Google Scholar] [CrossRef] [Green Version]
- Hosseini, S.H.; Hoeft, F.; Kesler, S.R. GAT: A graph-theoretical analysis toolbox for analyzing between-group differences in large-scale structural and functional brain networks. PLoS ONE 2012, 7, e40709. [Google Scholar] [CrossRef] [Green Version]
- Watts, D.J.; Strogatz, S.H. Collective dynamics of ‘small-world’networks. Nature 1998, 393, 440–442. [Google Scholar] [CrossRef]
- Barrat, A.; Barthelemy, M.; Pastor-Satorras, R.; Vespignani, A. The architecture of complex weighted networks. Proc. Natl. Acad. Sci. USA 2004, 101, 3747–3752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onnela, J.-P.; Saramäki, J.; Kertész, J.; Kaski, K. Intensity and coherence of motifs in weighted complex networks. Phys. Rev. E 2005, 71, 065103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, B.; Horvath, S. A general framework for weighted gene co-expression network analysis. Stat. Appl. Genet. Mol. Biol. 2005, 4, 1–45. [Google Scholar] [CrossRef] [PubMed]
- Hasler, G.; Drevets, W.C.; Manji, H.K.; Charney, D.S. Discovering Endophenotypes for Major Depression. Neuropsychopharmacology 2004, 29, 1765–1781. [Google Scholar] [CrossRef] [Green Version]
- APA. Diagnostic and Statistical Manual of Mental Disorders, 4th ed.; APA: Washington, DC, USA, 2000. [Google Scholar]
- Klein, D.F. Endogenomorphic depression: A conceptual and terminological revision. Arch. Gen. Psychiatry 1974, 31, 447–454. [Google Scholar] [CrossRef]
- Lemke, M.R.; Brecht, H.M.; Koester, J.; Kraus, P.H.; Reichmann, H. Anhedonia, depression, and motor functioning in Parkinson’s disease during treatment with pramipexole. J. Neuropsychiatry Clin. Neurosci. 2005, 17, 214–220. [Google Scholar] [CrossRef]
- Santangelo, G.; Morgante, L.; Savica, R.; Marconi, R.; Grasso, L.; Antonini, A.; De Gaspari, D.; Ottaviani, D.; Tiple, D.; Simoni, L. Anhedonia and cognitive impairment in Parkinson’s disease: Italian validation of the Snaith–Hamilton Pleasure Scale and its application in the clinical routine practice during the PRIAMO study. Parkinsonism Relat. Disord. 2009, 15, 576–581. [Google Scholar] [CrossRef]
- Zimmerman, M.; McGlinchey, J.B.; Young, D.; Chelminski, I. Diagnosing major depressive disorder: II: Is there justification for compound symptom criteria? J. Nerv. Ment. Dis. 2006, 194, 235–240. [Google Scholar] [CrossRef]
- Wacker, J.; Dillon, D.G.; Pizzagalli, D.A. The role of the nucleus accumbens and rostral anterior cingulate cortex in anhedonia: Integration of resting EEG, fMRI, and volumetric techniques. Neuroimage 2009, 46, 327–337. [Google Scholar] [CrossRef] [Green Version]
- Alexopoulos, G.S.; Kiosses, D.N.; Heo, M.; Murphy, C.F.; Shanmugham, B.; Gunning-Dixon, F. Executive dysfunction and the course of geriatric depression. Biol. Psychiatry 2005, 58, 204–210. [Google Scholar] [CrossRef]
- Gallagher, D.; Savva, G.M.; Kenny, R.; Lawlor, B.A. What predicts persistent depression in older adults across Europe? Utility of clinical and neuropsychological predictors from the SHARE study. J. Affect. Disord. 2013, 147, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Miranda, B.; Madureira, S.; Verdelho, A.; Ferro, J.; Pantoni, L.; Salvadori, E.; Chabriat, H.; Erkinjuntti, T.; Fazekas, F.; Hennerici, M. Self-perceived memory impairment and cognitive performance in an elderly independent population with age-related white matter changes. J. Neurol. Neurosurg. Psychiatry 2008, 79, 869–873. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Kang, Y. Characteristics of Memory Complaints, Cognitive Functions, and Emotion in Subjective Memory Impairment. Dement. Neurocognitive Disord. 2011, 10, 125–136. [Google Scholar]
- Dux, M.C.; Woodard, J.L.; Calamari, J.E.; Messina, M.; Arora, S.; Chik, H.; Pontarelli, N. The moderating role of negative affect on objective verbal memory performance and subjective memory complaints in healthy older adults. J. Int. Neuropsychol. Soc. 2008, 14, 327–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischer, C.; Schweizer, T.A.; Atkins, J.H.; Bozanovic, R.; Norris, M.; Herrmann, N.; Nisenbaum, R.; Rourke, S.B. Neurocognitive profiles in older adults with and without major depression. Int. J. Geriatr. Psychiatry 2008, 23, 851–856. [Google Scholar] [CrossRef]
- Jorm, A.; Christensen, H.; Henderson, A.; Korten, A.; Mackinnon, A.; Scott, R. Complaints of cognitive decline in the elderly: A comparison of reports by subjects and informants in a community survey. Psychol. Med. 1994, 24, 365–374. [Google Scholar] [CrossRef]
- Herrmann, D.J. Know thy memory: The use of questionnaires to assess and study memory. Psychol. Bull. 1982, 92, 434. [Google Scholar] [CrossRef]
- Crook, T.H.; Larrabee, G.J. A self-rating scale for evaluating memory in everyday life. Psychol. Aging 1990, 5, 48. [Google Scholar] [CrossRef]
- Gilewski, M.J.; Zelinski, E.M.; Schaie, K.W. The Memory Functioning Questionnaire for assessment of memory complaints in adulthood and old age. Psychol. Aging 1990, 5, 482. [Google Scholar] [CrossRef]
- Hertzog, C.; Hultsch, D.F.; Dixon, R.A. Evidence for the convergent validity of two self-report metamemory questionnaires. Dev. Psychol. 1989, 25, 687. [Google Scholar] [CrossRef]
- McNally, R.J. Can network analysis transform psychopathology? Behav. Res. Ther. 2016, 86, 95–104. [Google Scholar] [CrossRef] [PubMed]
- van Borkulo, C.; Boschloo, L.; Borsboom, D.; Penninx, B.; Waldorp, L.; Schoevers, R. Package ‘NetworkComparisonTest’. JAMA Psychiatry 2015, 72, 1219–1226. [Google Scholar] [CrossRef]
- Sinoff, G.; Werner, P. Anxiety disorder and accompanying subjective memory loss in the elderly as a predictor of future cognitive decline. Int. J. Geriatr. Psychiatry 2003, 18, 951–959. [Google Scholar] [CrossRef] [PubMed]
- Fried, E.I.; Epskamp, S.; Nesse, R.M.; Tuerlinckx, F.; Borsboom, D. What are ‘good’ depression symptoms? Comparing the centrality of DSM and non-DSM symptoms of depression in a network analysis. J. Affect. Disord. 2016, 189, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Heaton, R.K.; Pendleton, M.G. Use of Neuropsychological tests to predict adult patients’ everyday functioning. J. Consult. Clin. Psychol. 1981, 49, 807. [Google Scholar] [CrossRef]
- Brigola, A.G.; Manzini, C.S.S.; Oliveira, G.B.S.; Ottaviani, A.C.; Sako, M.P.; Vale, F.A.C. Subjective memory complaints associated with depression and cognitive impairment in the elderly: A systematic review. Dement. Neuropsychol. 2015, 9, 51–57. [Google Scholar] [CrossRef] [Green Version]



| Variable | No SMC (n= 2867) | SMC (n = 622) | SE Difference | Cohen’s d | t | p | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | SE | Mean | SD | SE | ||||||
| Q1 | Anhedonia | 0.797 | 0.735 | 0.014 | 1.302 | 1.052 | 0.042 | 0.035 | −0.631 | −14.268 | <0.001 |
| Q2 | Feeling depressed | 0.81 | 0.767 | 0.014 | 1.357 | 1.102 | 0.044 | 0.037 | −0.654 | −14.777 | <0.001 |
| Q3 | Sleep problems | 1.028 | 0.98 | 0.018 | 1.608 | 1.25 | 0.05 | 0.046 | −0.561 | −12.69 | <0.001 |
| Q4 | Low energy | 0.786 | 0.763 | 0.014 | 1.278 | 1.121 | 0.045 | 0.037 | −0.587 | −13.28 | <0.001 |
| Q5 | Appetite change | 0.67 | 0.669 | 0.013 | 1.172 | 1.079 | 0.043 | 0.034 | −0.662 | −14.97 | <0.001 |
| Q6 | Low self-esteem | 1.004 | 0.887 | 0.017 | 1.698 | 1.194 | 0.048 | 0.042 | −0.731 | −16.526 | <0.001 |
| Q7 | Concentration problems | 0.727 | 0.666 | 0.012 | 1.185 | 1.02 | 0.041 | 0.033 | −0.617 | −13.949 | <0.001 |
| Q8 | Agitation/Retardation | 0.657 | 0.628 | 0.012 | 1.14 | 1.025 | 0.041 | 0.032 | −0.675 | −15.254 | <0.001 |
| Q9 | Suicidal ideation | 0.646 | 0.607 | 0.011 | 1.002 | 0.867 | 0.035 | 0.029 | −0.539 | −12.178 | <0.001 |
| Item | Betweenness | Closeness | Strength | Expected Influence |
|---|---|---|---|---|
| Q1 | 1.054 | 0.174 | 1.101 | 1.101 |
| Q2 | −0.843 | 0.504 | 1.464 | 1.464 |
| Q3 | −1.792 | −1.195 | −1.676 | −1.676 |
| Q4 | −0.843 | −1.075 | −1.040 | −1.040 |
| Q5 | 1.054 | 1.169 | −0.113 | −0.113 |
| Q6 | 0.105 | 0.489 | 0.189 | 0.189 |
| Q7 | 0.105 | −1.170 | −0.496 | −0.496 |
| Q8 | 1.054 | −0.328 | −0.101 | −0.101 |
| Q9 | 0.105 | 1.432 | 0.671 | 0.671 |
| Item | Barrat | Onnela | WS | Zhang |
|---|---|---|---|---|
| Q1 | −1.550 | −0.644 | 0.667 | 0.279 |
| Q2 | −0.645 | 1.071 | 0.667 | −0.399 |
| Q3 | −0.400 | −1.662 | −1.333 | 0.137 |
| Q4 | 0.751 | −0.001 | 0.667 | 0.245 |
| Q5 | 0.783 | 0.761 | 0.667 | −0.163 |
| Q6 | −0.334 | 1.207 | 0.667 | 1.808 |
| Q7 | 1.838 | 0.001 | 0.667 | −0.689 |
| Q8 | 0.137 | −1.194 | −1.333 | −1.869 |
| Q9 | −0.579 | 0.462 | −1.333 | 0.651 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Lee, K. A Network Analysis of Depressive Symptoms in the Elderly with Subjective Memory Complaints. J. Pers. Med. 2022, 12, 821. https://0-doi-org.brum.beds.ac.uk/10.3390/jpm12050821
Kim S, Lee K. A Network Analysis of Depressive Symptoms in the Elderly with Subjective Memory Complaints. Journal of Personalized Medicine. 2022; 12(5):821. https://0-doi-org.brum.beds.ac.uk/10.3390/jpm12050821
Chicago/Turabian StyleKim, Sunhae, and Kounseok Lee. 2022. "A Network Analysis of Depressive Symptoms in the Elderly with Subjective Memory Complaints" Journal of Personalized Medicine 12, no. 5: 821. https://0-doi-org.brum.beds.ac.uk/10.3390/jpm12050821