Antibody Arrays Identified Cycle-Dependent Plasma Biomarker Candidates of Peritoneal Endometriosis
Abstract
:1. Introduction
2. Materials and Methods
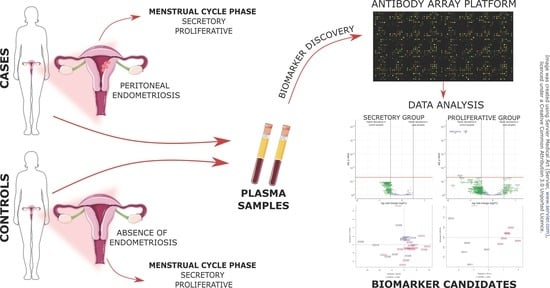
2.1. Patient Enrollment and Study Design
2.2. Sample and Data Collection
2.3. Preparation of Samples for Antibody Microarray Analysis
2.4. Data Acquisition and Statistical Analysis of Microarray Data
2.5. Statistical Analysis of Patients’ Clinical Data
3. Results
3.1. Clinical Characteristics of Patients
3.2. Differentially Expressed Proteins Were Identified Only in the Proliferative Group of Patients
3.3. The Levels of Identified Proteins Allowed for the Separation of Endometriosis Patients from Control Patients
3.4. Studying Protein Interactions Revealed That the Differential Proteins Play Roles in Inflammation, the Immune System, Cell Adhesion, Platelet Aggregation, and Angiogenesis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anastasiu, C.V.; Moga, M.A.; Neculau, A.E.; Bălan, A.; Scârneciu, I.; Dragomir, R.M.; Dull, A.-M.; Chicea, L.-M. Biomarkers for the Noninvasive Diagnosis of Endometriosis: State of the Art and Future Perspectives. Int. J. Mol. Sci. 2020, 21, 1750. [Google Scholar] [CrossRef] [Green Version]
- Bulun, S.E. Endometriosis. N. Engl. J. Med. 2009, 360, 268–279. [Google Scholar] [CrossRef]
- Brosens, I.; Benagiano, G. Endometriosis, a modern syndrome. Indian J. Med. Res. 2011, 133, 581–593. [Google Scholar]
- A Pritts, E.; Taylor, R.N. An evidence-based evaluation of endometriosis-associated infertility. Endocrinol. Metab. Clin. North. Am. 2003, 32, 653–667. [Google Scholar] [CrossRef]
- Berkkanoglu, M.; Arici, A. Immunology and Endometriosis. Am. J. Reprod. Immunol. 2003, 50, 48–59. [Google Scholar] [CrossRef] [Green Version]
- Nicolaus, K.; Reckenbeil, L.; Bräuer, D.; Sczesny, R.; Diebolder, H.; Runnebaum, I.B. Cycle-related Diarrhea and Dysmenorrhea are Independent Predictors of Peritoneal Endometriosis, Cycle-related Dyschezia is an Independent Predictor of Rectal Involvement. Geburtshilfe Frauenheilkd 2020, 80, 307–315. [Google Scholar] [CrossRef] [Green Version]
- Elbiss, H.M.; Abu-Zidan, F.M. Bowel injury following gynecological laparoscopic surgery. Afr. Heal. Sci. 2018, 17, 1237–1245. [Google Scholar] [CrossRef] [Green Version]
- Sourial, S.; Tempest, N.; Hapangama, D.K. Theories on the Pathogenesis of Endometriosis. Int. J. Reprod. Med. 2014, 2014, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Ferrero, S.; Vellone, V.G.; Barra, F. Pathophysiology of pain in patients with peritoneal endometriosis. Ann. Transl. Med. 2019, 7, S8. [Google Scholar] [CrossRef]
- Ballard, K.D.; Seaman, H.E.; De Vries, C.S.; Wright, J.T. Can symptomatology help in the diagnosis of endometriosis? Findings from a national case-control study-Part 1. BJOG Int. J. Obstet. Gynaecol. 2008, 115, 1382–1391. [Google Scholar] [CrossRef]
- Fassbender, A.; Vodolazkaia, A.; Saunders, P.; Lebovic, D.; Waelkens, E.; De Moor, B.; D’Hooghe, T. Biomarkers of endometriosis. Fertil. Steril. 2013, 99, 1135–1145. [Google Scholar] [CrossRef] [PubMed]
- Burney, R.O. Biomarker development in endometriosis. Scand. J. Clin. Lab. Investig. 2014, 74, 75–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharpe-Timms, K.L. Defining endometrial cells: The need for improved identification at ectopic sites and characterization in eutopic sites for developing novel methods of management for endometriosis. Fertil. Steril. 2005, 84, 35–37. [Google Scholar] [CrossRef] [PubMed]
- Rižner, T.L. Noninvasive biomarkers of endometriosis: Myth or reality? Expert Rev. Mol. Diagn. 2014, 14, 365–385. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, S.; Bergqvist, A.; Chapron, C.; D’Hooghe, T.; Dunselman, G.; Greb, R.; Hummelshoj, L.; Prentice, A.; Saridogan, E.; on behalf of the ESHRE Special Interest Group for Endometriosis and Endometrium Guideline Development Group. ESHRE guideline for the diagnosis and treatment of endometriosis. Hum. Reprod. 2005, 20, 2698–2704. [Google Scholar] [CrossRef] [PubMed]
- May, K.E.; Conduit-Hulbert, S.A.; Villar, J.; Kirtley, S.; Kennedy, S.H.; Becker, C.M. Peripheral biomarkers of endometriosis: A systematic review. Hum. Reprod. Updat. 2010, 16, 651–674. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, L.M.; Ferreira, M.C.; Rocha, A.L.L.; Carneiro, M.M.; Reis, F.M. New biomarkers in endometriosis. Adv. Clin. Chem. 2019, 89, 59–77. [Google Scholar] [CrossRef]
- Ahn, S.H.; Singh, V.; Tayade, C. Biomarkers in endometriosis: Challenges and opportunities. Fertil. Steril. 2017, 107, 523–532. [Google Scholar] [CrossRef] [Green Version]
- Nisenblat, V.; Bossuyt, P.M.; Shaikh, R.; Farquhar, C.; Jordan, V.; Scheffers, C.S.; Mol, B.W.J.; Johnson, N.; Hull, M.L. Blood biomarkers for the non-invasive diagnosis of endometriosis. Cochrane Database Syst. Rev. 2016, 2016, CD012179. [Google Scholar] [CrossRef] [Green Version]
- Knific, T.; Fishman, D.; Vogler, A.; Gstöttner, M.; Wenzl, R.; Peterson, H.; Rižner, T.L. Multiplex analysis of 40 cytokines do not allow separation between endometriosis patients and controls. Sci. Rep. 2019, 9, 16738. [Google Scholar] [CrossRef]
- Knific, T.; Vouk, K.; Vogler, A.; Osredkar, J.; Gstöttner, M.; Wenzl, R.; Rižner, T.L. Models including serum CA-125, BMI, cyst pathology, dysmenorrhea or dyspareunia for diagnosis of endometriosis. Biomarkers Med. 2018, 12, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Kocbek, V.; Vouk, K.; Bersinger, N.A.; Mueller, M.D.; Rižner, T.L. Panels of Cytokines and Other Secretory Proteins as Potential Biomarkers of Ovarian Endometriosis. J. Mol. Diagn. 2015, 17, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Vouk, K.; Ribič-Pucelj, M.; Adamski, J.; Rižner, T.L. Altered levels of acylcarnitines, phosphatidylcholines, and sphingomyelins in peritoneal fluid from ovarian endometriosis patients. J. Steroid Biochem. Mol. Biol. 2016, 159, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Vouk, K.; Hevir, N.; Ribič-Pucelj, M.; Haarpaintner, G.; Scherb, H.; Osredkar, J.; Möller, G.; Prehn, C.; Rižner, T.L.; Adamski, J. Discovery of phosphatidylcholines and sphingomyelins as biomarkers for ovarian endometriosis. Hum. Reprod. 2012, 27, 2955–2965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizner, T.L.; Adamski, J. Paramount importance of sample quality in pre-clinical and clinical research—Need for standard operating procedures (SOPs). J. Steroid Biochem. Mol. Biol. 2018, 186, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Janša, V.; Klančič, T.; Pušić, M.; Klein, M.; Bokal, E.V.; Frangež, H.B.; Rižner, T.L. Proteomic analysis of peritoneal fluid identified COMP and TGFBI as new candidate biomarkers for endometriosis. Sci. Rep. 2021, 11, 20870. [Google Scholar] [CrossRef]
- Sill, M.; Schröder, C.; Hoheisel, J.D.; Benner, A.; Zucknick, M. Assessment and optimisation of normalisation methods for dual-colour antibody microarrays. BMC Bioinform. 2010, 11, 556. [Google Scholar] [CrossRef] [Green Version]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein–protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef] [Green Version]
- Aghajanova, L.; Burney, R.O.; Tran, N.D.; Giudice, L.C. mRNA and miRNA biomarkers for endometriosis. In Biomarkers for Endometriosis: State of the Art; Springer International Publishing AG: Berlin, Germany, 2017; pp. 165–183. ISBN 9783319598567. [Google Scholar]
- Simoens, S.; Dunselman, G.; Dirksen, C.; Hummelshoj, L.; Bokor, A.; Brandes, I.; Brodszky, V.; Canis, M.; Colombo, G.L.; DeLeire, T.; et al. The burden of endometriosis: Costs and quality of life of women with endometriosis and treated in referral centres. Hum. Reprod. 2012, 27, 1292–1299. [Google Scholar] [CrossRef] [Green Version]
- O, D.; Waelkens, E.; Vanhie, A.; Peterse, D.; Fassbender, A.; D’Hooghe, T. The Use of Antibody Arrays in the Discovery of New Plasma Biomarkers for Endometriosis. Reprod. Sci. 2020, 27, 751–762. [Google Scholar] [CrossRef]
- Watson, C.N.; Begum, G.; Ashman, E.; Thorn, D.; Yakoub, K.M.; Al Hariri, M.; Nehme, A.; Mondello, S.; Kobeissy, F.; Belli, A.; et al. Co-Expression Analysis of microRNAs and Proteins in Brain of Alzheimer’s Disease Patients. Cells 2022, 11, 163. [Google Scholar] [CrossRef] [PubMed]
- Nijaguna, M.B.; Schröder, C.; Patil, V.; Shwetha, S.D.; Hegde, A.S.; Chandramouli, B.A.; Arivazhagan, A.; Santosh, V.; Hoheisel, J.D.; Somasundaram, K. Definition of a serum marker panel for glioblastoma discrimination and identification of Interleukin 1β in the microglial secretome as a novel mediator of endothelial cell survival induced by C-reactive protein. J. Proteom. 2015, 128, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Schröder, C.; Srinivasan, H.; Sill, M.; Linseisen, J.; Fellenberg, K.; Becker, N.; Nieters, A.; Hoheisel, J.D. Plasma protein analysis of patients with different B-cell lymphomas using high-content antibody microarrays. Proteom. Clin. Appl. 2013, 7, 802–812. [Google Scholar] [CrossRef] [PubMed]
- Hudson, Q.J.; Perricos, A.; Wenzl, R.; Yotova, I. Challenges in uncovering non-invasive biomarkers of endometriosis. Exp. Biol. Med. 2020, 245, 437–447. [Google Scholar] [CrossRef]
- Buchweitz, O.; Poel, T.; Diedrich, K.; Malik, E. The Diagnostic Dilemma of Minimal and Mild Endometriosis under Routine Conditions. J. Am. Assoc. Gynecol. Laparosc. 2003, 10, 85–89. [Google Scholar] [CrossRef]
- Hsu, A.L.; Khachikyan, I.; Stratton, P. Invasive and Noninvasive Methods for the Diagnosis of Endometriosis. Clin. Obstet. Gynecol. 2010, 53, 413–419. [Google Scholar] [CrossRef]
- Taylor, H.S.; Adamson, G.D.; Diamond, M.P.; Goldstein, S.R.; Horne, A.W.; Missmer, S.A.; Snabes, M.C.; Surrey, E.; Taylor, R.N. An evidence-based approach to assessing surgical versus clinical diagnosis of symptomatic endometriosis. Int. J. Gynecol. Obstet. 2018, 142, 131–142. [Google Scholar] [CrossRef]
- Kazanegra, R.; Zaritsky, E.; Lathi, R.B.; Clopton, P.; Nezhat, C. Diagnosis of Stage I Endometriosis: Comparing Visual Inspection to Histologic Biopsy Specimen. J. Minim. Invasive Gynecol. 2008, 15, 176–180. [Google Scholar] [CrossRef]
- Audebert, A.; Lecointre, L.; Afors, K.; Koch, A.; Wattiez, A.; Akladios, C. Adolescent Endometriosis: Report of a Series of 55 Cases With a Focus on Clinical Presentation and Long-Term Issues. J. Minim. Invasive Gynecol. 2015, 22, 834–840. [Google Scholar] [CrossRef]
- D’Hooghe, T.; Mihalyi, A.; Simsa, P.; Kyama, C.; Peeraer, K.; De Loecker, P.; Meeuwis, L.; Segal, L.; Meuleman, C. Why We Need a Noninvasive Diagnostic Test for Minimal to Mild Endometriosis with a High Sensitivity. Gynecol. Obstet. Investig. 2006, 62, 136–138. [Google Scholar] [CrossRef]
- Jackson, D.H.; Banks, R.E. Banking of clinical samples for proteomic biomarker studies: A consideration of logistical issues with a focus on pre-analytical variation. Proteom. Clin. Appl. 2010, 4, 250–270. [Google Scholar] [CrossRef] [PubMed]
- Zondervan, K.; Cardon, L.R.; Kennedy, S.H. What makes a good case–control study? Hum. Reprod. 2002, 17, 1415–1423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bell, S.C.; Patel, S.R.; Kirwan, P.H.; Drife, J.O. Protein synthesis and secretion by the human endometrium during the menstrual cycle and the effect of progesterone in vitro. Reproduction 1986, 77, 221–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prašnikar, E.; Knez, J.; Kovačič, B.; Kunej, T. Molecular signature of eutopic endometrium in endometriosis based on the multi-omics integrative synthesis. J. Assist. Reprod. Genet. 2020, 37, 1593–1611. [Google Scholar] [CrossRef]
- Daniilidis, A.; Giannoulis, H.; Tantanasis, T.; Papathanasiou, K.; Loufopoulos, A.; Tzafettas, J. Diagnostic laparoscopy, infertility, and endometriosis—5 years experience. Gynecol. Surg. 2007, 5, 231–234. [Google Scholar] [CrossRef] [Green Version]
- Darwish, I.A. Immunoassay Methods and their Applications in Pharmaceutical Analysis: Basic Methodology and Recent Advances. Int. J. Biomed. Sci. IJBS 2006, 2, 217–235. [Google Scholar]
- Prassas, I.; Diamandis, E.P. Translational researchers beware! Unreliable commercial immunoassays (ELISAs) can jeopardize your research. Clin. Chem. Lab. Med. (CCLM) 2014, 52, 765–766. [Google Scholar] [CrossRef]
- Pan, L.; Zhao, Y.; Yuan, Z.; Qin, G. Research advances on structure and biological functions of integrins. SpringerPlus 2016, 5, 1094. [Google Scholar] [CrossRef] [Green Version]
- Bennett, J.S. Structure and function of the platelet integrin IIb 3. J. Clin. Investig. 2005, 115, 3363–3369. [Google Scholar] [CrossRef] [Green Version]
- Mahabeleshwar, G.H.; Feng, W.; Phillips, D.R.; Byzova, T.V. Integrin signaling is critical for pathological angiogenesis. J. Exp. Med. 2006, 203, 2495–2507. [Google Scholar] [CrossRef] [Green Version]
- Ding, D.; Liu, X.; Duan, J.; Guo, S.-W. Platelets are an unindicted culprit in the development of endometriosis: Clinical and experimental evidence. Hum. Reprod. 2015, 30, 812–832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lessey, B.A.; Castelbaum, A.J.; Sawin, S.W.; Buck, C.A.; Schinnar, R.; Bilker, W.; Strom, B.L. Aberrant integrin expression in the endometrium of women with endometriosis. J. Clin. Endocrinol. Metab. 1994, 79, 643–649. [Google Scholar] [CrossRef] [PubMed]
- González-Núñez, M.; Muñoz-Félix, J.M.; López-Novoa, J.M. The ALK-1/Smad1 pathway in cardiovascular physiopathology. A new target for therapy? Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2013, 1832, 1492–1510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makker, V.; Filiaci, V.L.; Chen, L.-M.; Darus, C.J.; Kendrick, J.E.; Sutton, G.; Moxley, K.; Aghajanian, C. Phase II evaluation of dalantercept, a soluble recombinant activin receptor-like kinase 1 (ALK1) receptor fusion protein, for the treatment of recurrent or persistent endometrial cancer: An NRG Oncology/Gynecologic Oncology Group Study 0229N. Gynecol. Oncol. 2015, 138, 24–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geumann, C.; Grønborg, M.; Hellwig, M.; Martens, H.; Jahn, R. A sandwich enzyme-linked immunosorbent assay for the quantification of insoluble membrane and scaffold proteins. Anal. Biochem. 2010, 402, 161–169. [Google Scholar] [CrossRef] [Green Version]
- Harada, T.; Iwabe, T.; Terakawa, N. Role of cytokines in endometriosis. Fertil. Steril. 2001, 76, 1–10. [Google Scholar] [CrossRef]
- Narahara, H. Role of chemokines in the pathogenesis of endometriosis. Front. Biosci. 2011, S3, 1196–1204. [Google Scholar] [CrossRef] [Green Version]
- Borrelli, G.M.; Abrao, M.S.; Mechsner, S. Can chemokines be used as biomarkers for endometriosis? A systematic review. Hum. Reprod. 2013, 29, 253–266. [Google Scholar] [CrossRef]
- Wu, M.-H.; Hsiao, K.-Y.; Tsai, S.-J. Endometriosis and possible inflammation markers. Gynecol. Minim. Invasive Ther. 2015, 4, 61–67. [Google Scholar] [CrossRef] [Green Version]
- Rižner, T.L. Diagnostic potential of peritoneal fluid biomarkers of endometriosis. Expert Rev. Mol. Diagn. 2015, 15, 557–580. [Google Scholar] [CrossRef]





| Characteristics | Unit | Menstrual Phase | Subgroup | Patients with Peritoneal Endometriosis | Controls | p-Value |
|---|---|---|---|---|---|---|
| Total number of patients | n | Secretory | - | 14 | 14 | - |
| Proliferative | - | 6 | 6 | - | ||
| Age (mean ± SD) | years | Secretory | - | 31.93 ± 4.10 | 30.14 ± 4.15 | Mann-Whitney test (p = 0.2114) |
| Proliferative | - | 30 ± 3.46 | 28 ± 2.19 | unpaired t-test (p = 0.2596) | ||
| BMI (mean ± SD) | kg/m2 | Secretory | - | 22.55 ± 2.39 | 22.31 ± 2.72 | unpaired t-test (p = 0.8062) |
| Proliferative | - | 21.27 ± 1.02 | 22.35 ± 2.42 | unpaired t-test (p = 0.3389) | ||
| rAFS score | n (%) | Secretory | I | 13 (92.86) | 0 (0) | - |
| II | 1 (7.14) | 0 (0) | - | |||
| Proliferative | I | 5 (83.30) | 0 (0) | - | ||
| II | 1 (16.70) | 0 (0) | - | |||
| Oral contraception 3 months before surgery | n (%) | Secretory | Yes | 0 (0) | 0 (0) | Fisher’s exact test (p > 0.9999) |
| No | 14 (100) | 14 (100) | ||||
| Proliferative | Yes | 1 (16.70) | 0 (0) | Fisher’s exact test (p > 0.9999) | ||
| No | 5 (83.30) | 6 (100) | ||||
| Hormonal therapy 3 months before surgery | n (%) | Secretory | Yes | 4 (28.57) | 4 (28.57) | Fisher’s exact test (p > 0.9999) |
| No | 10 (71.43) | 10 (71.43) | ||||
| Proliferative | Yes | 0 (0) | 1 (16.70) | Fisher’s exact test (p > 0.9999) | ||
| No | 6 (100) | 5 (83.30) | ||||
| Medication 1 week before surgery | n (%) | Secretory | Yes | 4 (28.57) | 4 (28.57) | Fisher’s exact test (p > 0.9999) |
| No | 10 (71.43) | 10 (71.43) | ||||
| Proliferative | Yes | 4 (66.7) | 2 (33.30) | Fisher’s exact test (p = 0.5671) | ||
| No | 2 (33.30) | 4 (66.70) | ||||
| Smoking status | n (%) | Secretory | Non-smoker | 7 (50) | 5 (35.71) | Chi-squared test for trend (p = 0.1222) |
| Smoker | 4 (28.57) | 4 (28.57) | ||||
| Occasionally (weekly) | 3 (21.43) | 1 (7.14) | ||||
| Occasionally (monthly) | 0 (0) | 2 (14.29) | ||||
| Former smoker | 0 (0) | 2 (14.29) | ||||
| n (%) | Proliferative | Non-smoker | 4 (66.70) | 3 (50) | Chi-squared test for trend (p = 0.5582) | |
| Smoker | 2 (33.30) | 3 (50) | ||||
| Occasionally (weekly) | 0 (0) | 0 (0) | ||||
| Occasionally (monthly) | 0 (0) | 0 (0) | ||||
| Former smoker | 0 (0) | 0 (0) | ||||
| Sport/recreation | n (%) | Secretory | Regularly | 8 (57.14) | 9 (64.29) | Chi-squared test for trend (p = 0.5016) |
| Occasionally | 5 (35.71) | 5 (35.71) | ||||
| No | 1 (7.15) | 0 (0) | ||||
| n (%) | Proliferative | Regularly | 3 (50) | 1 (16.70) | Chi-squared test for trend (p = 0.0929) | |
| Occasionally | 3 (50) | 3 (50) | ||||
| No | 0 (0) | 2 (33.30) |
| Protein Abbreviation (Uniprot) | Full Protein Name | Uniprot ID | logFC | FC | P |
|---|---|---|---|---|---|
| ITB3 | Integrin beta-3 | P05106 | −1.20 | 0.44 | 2.8 × 10−5 |
| ACVL-1 | Serine threonine-protein kinase receptor R3 | P37023 | −1.43 | 0.37 | 4.7 × 10−2 |
| ITA2B | Integrin alpha-IIb | P08514 | −1.66 | 0.32 | 2.9 × 10−5 |
| BIOLOGICAL PROCESSES | ||
| PROLIFERATIVE GROUP | ||
| Gene ontology (GO) term | Description | Protein count in the network |
| GO:0034097 | Response to cytokine | 34 of 1101 |
| GO:0007166 | Cell surface receptor signaling pathway | 45 of 2325 |
| GO:0050896 | Response to stimulus | 70 of 8046 |
| GO:0071345 | Cellular response to cytokine stimulus | 31 of 1013 |
| GO:0051716 | Cellular response to stimulus | 64 of 6489 |
| SECRETORY GROUP | ||
| Gene ontology (GO) term | Description | Protein count in the network |
| GO:0006950 | Response to stress | 31 of 3485 |
| GO:0050900 | Leukocyte migration | 12 of 316 |
| GO:0002376 | Immune system process | 26 of 2481 |
| GO:0071345 | Cellular response to cytokine stimulus | 17 of 1013 |
| GO:0019221 | Cytokine-mediated signaling pathway | 14 of 678 |
| MOLECULAR FUNCTIONS | ||
| PROLIFERATIVE GROUP | ||
| Gene ontology (GO) term | Description | Protein count in the network |
| GO:0005102 | Signaling receptor binding | 37 of 1581 |
| GO:0030545 | Receptor regulator activity | 23 of 536 |
| GO:0048018 | Receptor ligand activity | 22 of 490 |
| GO:0005126 | Cytokine receptor binding | 15 of 264 |
| GO:0005515 | Protein binding | 58 of 7026 |
| SECRETORY GROUP | ||
| Gene ontology (GO) term | Description | Protein count in the network |
| GO:0005125 | Cytokine activity | 8 of 233 |
| GO:0048018 | Receptor ligand activity | 10 of 490 |
| GO:0005102 | Signaling receptor binding | 16 of 1581 |
| GO:0005126 | Cytokine receptor binding | 7 of 264 |
| GO:0005515 | Protein binding | 34 of 7026 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pušić, M.; Klančič, T.; Knific, T.; Vogler, A.; Schmidt, R.; Schröder, C.; Lanišnik Rižner, T. Antibody Arrays Identified Cycle-Dependent Plasma Biomarker Candidates of Peritoneal Endometriosis. J. Pers. Med. 2022, 12, 852. https://0-doi-org.brum.beds.ac.uk/10.3390/jpm12060852
Pušić M, Klančič T, Knific T, Vogler A, Schmidt R, Schröder C, Lanišnik Rižner T. Antibody Arrays Identified Cycle-Dependent Plasma Biomarker Candidates of Peritoneal Endometriosis. Journal of Personalized Medicine. 2022; 12(6):852. https://0-doi-org.brum.beds.ac.uk/10.3390/jpm12060852
Chicago/Turabian StylePušić, Maja, Teja Klančič, Tamara Knific, Andrej Vogler, Ronny Schmidt, Christoph Schröder, and Tea Lanišnik Rižner. 2022. "Antibody Arrays Identified Cycle-Dependent Plasma Biomarker Candidates of Peritoneal Endometriosis" Journal of Personalized Medicine 12, no. 6: 852. https://0-doi-org.brum.beds.ac.uk/10.3390/jpm12060852