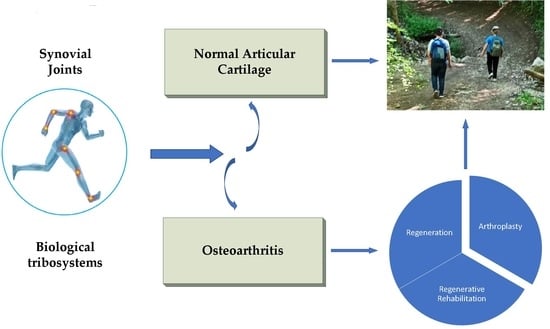
Synovial Joints. Tribology, Regeneration, Regenerative Rehabilitation and Arthroplasty
Abstract
:1. Introduction
2. Materials and Methods
2.1. Articular Cartilage as a Basis of Synovial Joints
2.2. Lubrication and Friction in Synovial Joints
2.2.1. Lubrication and Friction Modes
- articular cartilage is a linear porous-permeable two-phase material filled with a linear viscous (Newtonian) fluid;
- synovial fluid is also a Newtonian fluid;
- articular cartilage is a homogeneous layer of thickness H, and the thickness of the synovial fluid film (h) is significantly less than H; h << H;
- the radius of curvature R of the bearing articular surfaces is much larger than H; R >> H;
- the compression of the synovial fluid film is provided by a stepped load in the form of a Heaviside function applied to both bearing articular surfaces.
- articular cartilage material deforms, while the load transfer area increases;
- articular cartilage deformation leads to a decrease in the synovial fluid velocity, thus increasing the time for the formation of the squeezed film;
- synovial fluid in the gap is forced from the central high-pressure region into articular cartilage, and expelled from the tissue at the low-pressure periphery of the load-bearing region;
- tensile hoop stress exists at the cartilage surface despite the compressive squeeze-film loading condition.
2.2.2. Mathematical Models of Squeeze Film Lubrication
2.3. Regeneration and Regenerative Rehabilitation of Articular Cartilage
3. Results
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| Tibial length | |
| Clearance | |
| Integration constants | |
| Gap between articular surfaces | |
| Rate change in the gap between articular surfaces | |
| Function characterizing the force perceived by articular surfaces | |
| Dimensionless resistance force to the action of an external load | |
| Articular cartilage layer thickness | |
| Thicknes of poro-elastic articular cartilage layers | |
| Total layer thickness of synovial fluid | |
| Synovial film thickness | |
| Dimensionless parameters and | |
| Diffusive drag in articular cartilage | |
| Length of the cylindrical joint model | |
| Synovial fluid constant with the dimension of length: | |
| Dimensionless parameters and | |
| p | Synovial fluid pressure |
| Dimensionless parameter p | |
| Effective radius of curvature of the contact of talus and tibia: | |
| Radius of talus curvature | |
| Radius of tibia curvature | |
| Time | |
| u, v, w | Velocity field components of fluid media |
| Law of change of an external load for unit of length | |
| Cartesian coordinates of the ankle model | |
| Parameter: | |
| Polar angle: | |
| Small parameter: | |
| Small parameter: | |
| Dimensionless parameter: | |
| Couple stress synovial fluid constant | |
| Polar angle of the ankle model | |
| Synovial fluid dynamic viscosity | |
| Apparent viscosity of the interstitial fluid | |
| Φ | Permeability of the cartilage matrix |
| Dimensionless parameter Φ |
Appendix A
| Parameters | Numerical Values | Units |
|---|---|---|
| a | [m] | |
| L | [m] | |
| c | [m] | |
| R | [m] | |
| R1 | [m/s] | |
| Ф | [m2] | |
| [rad] | ||
| [Pa s] |
References
- Pavlova, V.N. Components of the internal environment of the joints and their functional interaction. Adv. Mod. Biol. 1989, 107, 238–242. (In Russian) [Google Scholar]
- Pawlak, Z.; Pai, R.; Mrela, A.; Kaczmarek, M.; Yusuf, K.Q.; Urbaniak, W. Natural articular cartilage: A smart biointerface. J. Comput. Methods Sci. Eng. 2019, 19, 479–489. [Google Scholar] [CrossRef]
- Chernyakova, Y.M.; Pinchuk, L.S. The synovial joint as a “smart” friction unit. Frict. Wear 2007, 28, 410–417. (In Russian) [Google Scholar]
- Pinchuk, L.S.; Chernyakova, Y.M.; Ermakov, S.F. Tribo Physics of Synovial Fluid, 1st ed.; Belarusian Science: Minsk, Belarus, 2010; pp. 3–381. (In Russian) [Google Scholar]
- Turovskaya, E.F.; Alekseeva, L.I.; Filatova, E.G. Modern ideas about the pathogenetic mechanisms of pain in osteoarthritis. Sci. Pract. Rheumatol. 2014, 52, 438–444. (In Russian) [Google Scholar] [CrossRef]
- Sakovets, T.G. Features of neuropathic pain with joint damage. Pract. Med. 2014, 4, 103–106. (In Russian) [Google Scholar]
- Lim, A.Y.; Doherty, M. What of guidelines for osteoarthritis? Int. J. Rheum. Dis. 2011, 14, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Kraus, V.B.; Kilfoil, M.; Hash, T.W.; McDaniel, G.; Renner, J.B.; Carrino, J.A.; Adams, S. Atlas of radiographic features of osteoarthritis of the ankle and hindfoot. Osteoarthritis Cartilage 2015, 14, 2059–2085. [Google Scholar] [CrossRef] [Green Version]
- Knee Osteoarthritis: Forms, Diseases, Primary Analyzes, Stages. Available online: https://gp195.ru/bolezni/osteoartrit-lechenie.html (accessed on 15 December 2020).
- Kotelkina, A.A.; Struchko, G.Y.; Merkulova, L.M.; Kostrova, O.Y.; Stomenskaya, I.S.; Timofeeva, N.Y. Characteristics of synovial fluid in normal conditions and in some pathological processes. Acta Med. Eurasica 2017, 4, 24–30. [Google Scholar]
- Netyaga, S.V.; Dubrovin, G.M.; Netyaga, A.A. The role of cytological examination of synovial fluid in the diagnosis of degenerative-dystrophic changes in the joints. Man His Health 2005, 1, 45–49. (In Russian) [Google Scholar]
- WHO. Department of Chronic Diseases and Health Promotion. Available online: http://www.who.int/chp/topics/rheumatic/en/ (accessed on 23 September 2020).
- Kaplan, W.; Wirtz, V.J.; Mantel-Teeuwisse, A.; Stolk, P.; Duthey, B.; Laing, R. Priority Medicines for Europe and the World 2013 Update; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- Dabiri, Y.; Li, L.P. Influences of the depth-dependent material inhomogeneity of articular cartilage on the fluid pressurization in the human knee. Med. Eng. Phys. 2013, 35, 1591–1598. [Google Scholar] [CrossRef]
- Mow, V.C.; Gu, W.Y.; Chen, F.H. Structure and Function of Articular Cartilage and Meniscus. In Basic Orthopaedic Biomechanics and Mechano-Biology, 3rd ed.; Mow, V.C., Huiskes, R., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2005; pp. 181–258. [Google Scholar]
- Mow, V.C.; Kuei, S.C.; Lai, W.M.; Armstrong, C.G. Biphasic Creep and Stress Relaxation of Articular Cartilage in Compression: Theory and Experiments. J. Biomech. Eng. 1980, 102, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Pavlova, V.N.; Kopyeva, T.N.; Slutsky, L.I.; Pavlov, G.G. Cartilage, 1st ed.; Medicine: Moscow, Russia, 1988; pp. 3–320. (In Russian) [Google Scholar]
- Brandt, K.D.; Dieppe, P.; Radin, E.L. Etiopathogenesis of Osteoarthritis. Rheum. Dis. Clin. N. Am. 2008, 34, 531–559. [Google Scholar] [CrossRef] [PubMed]
- Davies-Tuck, M.L.; Wluka, A.E.; Wang, Y.; Teichtahl, A.J.; Jones, G.; Ding, C.; Cicuttini, F.M. The natural history of cartilage defects in people with knee osteoarthritis. Osteoarthr. Cartil. 2008, 16, 337–342. [Google Scholar] [CrossRef] [Green Version]
- Scott, D.; Coleman, P.J.; Mason, R.M.; Levick, J.R. Concentration Dependence of Interstitial Flow Buffering by Hyaluronan in Synovial Joints. Microvasc. Res. 2000, 59, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.; Coleman, P.J.; Mason, R.M.; Levick, J.R. Interaction of intraarticular hyaluronan and albumin in the attenuation of fluid drainage from joints. Arthritis Rheum. 2000, 43, 1175–1182. [Google Scholar] [CrossRef]
- Hunter, W. Of the structure and diseases of articulating cartilages, by William Hunter, surgeon. Philos. Trans. R. Soc. Lond. 1743, 42, 514–521. [Google Scholar] [CrossRef]
- Dedinaite, A. Biomimetic lubrication. Soft Matter 2012, 8, 273–284. [Google Scholar] [CrossRef]
- Klein, J. Molecular mechanisms of synovial joint lubrication. J. Eng. Tribol. 2006, 220, 691–710. [Google Scholar] [CrossRef]
- Wang, M.; Liu, C.; Thormann, E.; Dėdinaitė, A. Hyaluronan and Phospholipid Association in Biolubrication. Biomacromolecules 2013, 14, 4198–4206. [Google Scholar] [CrossRef]
- Dėdinaitė, A.; Wieland, D.C.F.; Bełdowski, P.; Claesson, P.M. Biolubrication synergy: Hyaluronan—Phospholipid interactions at interfaces. Adv. Colloid Interface Sci. 2019, 274, 102050. [Google Scholar] [CrossRef]
- Pradal, C.; Yakubov, G.E.; Williams, M.A.K.; McGuckin, M.A.; Stokes, J.R. Lubrication by biomacromolecules: Mechanisms and biomimetic strategies. Bioinspiration Biomim. 2019, 14, 051001. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Smith, D.W.; Miramini, S.; Gardiner, B.S.; Zhang, L. A coupled contact model of cartilage lubrication in the mixed-mode regime under static compression. Tribol. Int. 2020, 1–11, 106185. [Google Scholar] [CrossRef]
- Dowson, D.; Unsworth, A.; Wright, V. Analysis of ‘boosted lubrication’ in human joints (reprinted from vol 1, 1959). Proc. Inst. Mech. Eng. Part C J. Mech. Eng. Sci. 2009, 223, 71–76. [Google Scholar]
- Dowson, D. Paper 12: Modes of Lubrication in Human Joints. Proc. Inst. Mech. Eng. Conf. Proc. 1966, 181, 45–54. [Google Scholar] [CrossRef]
- Dowson, D.; Wright, V.; Longfield, M.D. Human joint lubrication. Biomed. Eng. 1969, 4, 160–165. [Google Scholar] [PubMed]
- Wright, V.; Dowson, D.; Unsworth, A. The lubrication and stiffness of joints. Mod. Trends Rheumatol. 1971, 2, 30–45. [Google Scholar] [PubMed]
- Unsworth, A.; Dowson, D.; Wright, V. Some new evidence on human joint lubrication. Ann. Rheum. Dis. 1973, 32, 587–588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, Z.M.; Dowson, D.; Fisher, J. The Effect of Porosity of Articular Cartilage on the Lubrication of a Normal Human Hip Joint. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 1992, 206, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Jalali-Vahid, D.; Jagatia, M.; Jin, Z.M.; Dowson, D. Prediction of lubricating film thickness in a ball-in-socket model with a soft lining representing human natural and artificial hip joints. Proc. Inst. Mech. Eng. Part J J. Eng. Tribol. 2001, 215, 363–372. [Google Scholar] [CrossRef]
- Jin, Z.M.; Dowson, D. Elastohydrodynamic Lubrication in Biological Systems. Proc. Inst. Mech. Eng. Part J J. Eng. Tribol. 2005, 219, 367–380. [Google Scholar] [CrossRef]
- Dowson, D. Bio-tribology. Faraday Discuss. 2009, 156, 9–30. [Google Scholar] [CrossRef] [PubMed]
- Ghanbarzadeh, A.; Wilson, M.; Morina, A.; Dowson, D.; Neville, A. Development of a new mechano-chemical model in boundary lubrication. Tribol. Int. 2016, 93, 573–582. [Google Scholar] [CrossRef]
- Medley, J.B.; Dowson, D.; Wright, V. Transient Elastohydrodynamic Lubrication Models for the Human Ankle Joint. Eng. Med. 1984, 13, 137–151. [Google Scholar] [CrossRef] [PubMed]
- Dowson, D.; Jin, Z.-M. Micro-Elastohydrodynamic Lubrication of Synovial Joints. Eng. Med. 1986, 15, 63–65. [Google Scholar] [CrossRef]
- Dowson, D. Elastohydrodynamic and micro-elastohydrodynamic lubrication. Wear 1995, 190, 125–138. [Google Scholar] [CrossRef]
- Fein, R.S. Research Report 3: Are Synovial Joints Squeeze-Film Lubricated? Proc. Inst. Mech. Eng. Conf. Proc. 1966, 181, 125–128. [Google Scholar] [CrossRef]
- Hou, J.S.; Mow, V.C.; Lai, W.M.; Holmes, M.H. An analysis of the squeeze-film lubrication mechanism for articular cartilage. J. Biomech. 1992, 25, 247–259. [Google Scholar] [CrossRef]
- Hlaváček, M. The role of synovial fluid filtration by cartilage in lubrication of synovial joints—II. Squeeze-film lubrication: Homogeneous filtration. J. Biomech. 1993, 26, 1151–1160. [Google Scholar] [CrossRef]
- Hlavachek, M. Squeeze-film lubrication of the human ankle joint with synovial fluid filtrated by articular cartilage with the superficial zone worn out. J. Biomech. 2000, 33, 1415–1422. [Google Scholar]
- Hlavachek, M. The thixotropic effect of the synovial fluid in squeeze-film lubrication of the human hip joint. Biorheology 2001, 38, 319–334. [Google Scholar]
- Ruggiero, A.; Gòmez, E.; D’Amato, R. Approximate Analytical Model for the Squeeze-Film Lubrication of the Human Ankle Joint with Synovial Fluid Filtrated by Articular Cartilage. Tribol. Lett. 2011, 41, 337–343. [Google Scholar] [CrossRef] [Green Version]
- Naduvinamani, N.B.; Savitramma, G.K. Squeeze Film Lubrication between Rough Poroelastic Rectangular Plates with Micropolar Fluid: A Special Reference to the Study of Synovial Joint Lubrication. ISRN Tribol. 2013, 2013, 431508. [Google Scholar] [CrossRef] [Green Version]
- De Boer, G.N.; Raske, N.; Soltanahmadi, S.; Dowson, D.; Bryant, M.G.; Hewson, R.W. A porohyperelastic lubrication model for articular cartilage in the natural synovial joint. Tribol. Int. 2020, 149, 105760. [Google Scholar] [CrossRef]
- Ruggiero, A. Milestones in Natural Lubrication of Synovial Joints. Front. Mech. Eng. 2020, 6, 52. [Google Scholar] [CrossRef]
- Medley, J.B.; Dowson, D.; Wright, V. Surface Geometry of the Human Ankle Joint. Eng. Med. 1983, 12, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, A.; Gomez, E.; Roberto, D. Approximate closed-form solution of the synovial fluid film force in the human ankle joint with non-Newtonian lubricant. Tribol. Int. 2013, 57, 156–161. [Google Scholar] [CrossRef]
- Stokes, V.K. Couple Stresses in Fluids. Phys. Fluids 1966, 9, 1709. [Google Scholar] [CrossRef]
- Ankle X-ray: Indications, Conduct, Results. Available online: https://ocrb.ru/lechenie/rentgenografiya-golenostopnogo-sustava.html (accessed on 15 December 2020).
- Schmidt, T.A.; Gastelum, N.S.; Nguyen, Q.T.; Schumacher, B.L.; Sah, R.L. Boundary lubrication of articular cartilage: Role of synovial fluid constituents. Arthritis Rheum. 2007, 56, 882–891. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Dipankar, C.; Nabangshu, S.; Belinda, P.-M. Tribological role of synovial fluid compositions on artificial joints—A systematic review of the last 10 years. Lubr. Sci. 2014, 26, 387–410. [Google Scholar] [CrossRef]
- Lin, J.R. Couple-stress effect on the squeeze film characteristics of hemi-spherical bearings with reference to synovial joints. Appl. Mech. Eng. 1996, 1, 317–332. [Google Scholar]
- Meziane, A.; Bou-Saïd, B.; Tichy, J. Modelling human hip joint lubrication subject to walking cycle. Lubr. Sci. 2008, 20, 205–222. [Google Scholar] [CrossRef]
- Yousfi, M.; Bou-Saïd, B.; Tichy, J. An analytical study of the squeezing flow of synovial fluid. Mech. Ind. 2013, 14, 59–69. [Google Scholar] [CrossRef]
- De Boer, G.N.; Raske, N.; Soltanahmadi, S.; Bryant, M.G.; Hewson, R.W. Compliant-poroelastic lubrication in cartilage-on-cartilage line contacts. Tribol. Mater. Surf. Interfaces 2020, 14, 151–165. [Google Scholar] [CrossRef]
- Ruggiero, A.; Sicilia, A. A Mixed Elasto-Hydrodynamic Lubrication Model for Wear Calculation in Artificial Hip Joints. Lubricants 2020, 8, 72. [Google Scholar] [CrossRef]
- Naduvinamani, N.B.; Hiremath, P.S.; Fathima, S.T. On the squeeze film lubrication of long porous journal bearings with couple stress fluids. Ind. Lubr. Tribol. 2005, 57, 12–20. [Google Scholar] [CrossRef]
- Ateshian, G.A.; Hung, C.T. The natural synovial joint: Properties of cartilage. Proc. Inst. Mech. Eng. Part J J. Eng. Tribol. 2006, 220, 657–670. [Google Scholar] [CrossRef]
- Gill, T.J.; Asnis, P.D.; Berkson, E.M.; Gill, T.J. The Treatment of Articular Cartilage Defects Using the Microfracture Techniques. J. Orthop. Sports Phys. Ther. 2006, 10, 728–738. [Google Scholar] [CrossRef] [Green Version]
- Julkunen, P.; Livarinen, J.; Brama, P.A.; Arokoski, J.; Jurvelin, S.; Helminen, H.J. Maturation of collagen fibril network structure in tibial and femoral cartilage of rabbits. Osteoarthr. Cartil. 2010, 18, 406–415. [Google Scholar] [CrossRef] [Green Version]
- Steward, A.J.; Kelly, D.J. Mechanical regulation of mesenchymal stem cell differentiation. J. Anat. 2015, 227, 717–731. [Google Scholar] [CrossRef] [Green Version]
- Wong, M.; Siegrist, M.; Cao, X. Cyclic compression of articular cartilage explants is associated with progressive consolidation and altered expression pattern of extracellular matrix proteins. Matrix Biol. 1999, 18, 391–399. [Google Scholar] [CrossRef]
- Mauck, R.L.; Soltz, M.A.; Wang, C.C.B.; Wong, D.D.; Chao, P.-H.G.; Valhmu, W.B.; Hung, C.T.; Ateshian, G.A. Functional Tissue Engineering of Articular Cartilage Through Dynamic Loading of Chondrocyte-Seeded Agarose Gels. J. Biomech. Eng. 2000, 122, 252–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, K.W.; Mauck, R.L.; Wang, C.C.-B.; Kelly, T.-A.N.; Ho, M.M.-Y.; Chen, F.H.; Ateshian, G.A.; Hung, C.T. Duty Cycle of Deformational Loading Influences the Growth of Engineered Articular Cartilage. Cell. Mol. Bioeng. 2009, 2, 386–394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, R.L.; Lin, J.; Trindade, M.C.D.; Shida, J.; Kajiyama, G.; Vu, T.; Hoffman, A.R.; Van Der Meulen, M.C.H.; Goodman, S.B.; Schurman, D.J.; et al. Time-dependent effects of intermittent hydrostatic pressure on articular chondrocyte type II collagen and aggrecan mRNA expression. J. Rehabil. Res. Dev. 2000, 37, 153–161. [Google Scholar] [PubMed]
- Smith, R.L.; Rusk, S.F.; Ellison, B.E.; Wessells, P.; Tsuchiya, K.; Carter, D.R.; Caler, W.E.; Sandell, L.J.; Schurman, D.J. In vitro stimulation of articular chondrocyte mRNA and extracellular matrix synthesis by hydrostatic pressure. J. Orthop. Res. 1996, 14, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Guilak, F.; Meyer, B.C.; Ratcliffe, A.; Mow, V.C. The effects of matrix compression on proteoglycan metabolism in articular cartilage explants. Osteoarthr. Cartil. 1994, 2, 91–101. [Google Scholar] [CrossRef]
- Nugent, G.E.; Aneloski, N.M.; Schmidt, T.A.; Schumacher, B.L.; Voegtline, M.S.; Sah, R.L. Dynamic shear stimulation of bovine cartilage biosynthesis of proteoglycan 4. Arthritis Rheum. 2006, 54, 1888–1896. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yao, S.-J.; Alini, M.; Stoddart, M.J. Chondrogenesis of Human Bone Marrow Mesenchymal Stem Cells in Fibrin–Polyurethane Composites Is Modulated by Frequency and Amplitude of Dynamic Compression and Shear Stress. Tissue Eng. Part A 2010, 16, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Schätti, O.; Grad, S.; Goldhahn, J.; Salzmann, G.; Li, Z.; Alini, M.; Stoddart, M.J. A combination of shear and dynamic compression leads to mechanically induced chondrogenesis of human mesenchymal stem cells. Eur. Cells Mater. 2011, 22, 214–225. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.H.; Baker, B.M.; Ateshian, G.A.; Mauck, R.L. Sliding contact loading enhances the tensile properties of mesenchymal stem cell-seeded hydrogels. Eur. Cell Mater. 2012, 24, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Carter, D.R.; Blenman, E.R.; Beaupré, G.S. Correlations between mechanical stress history and tissue differentiation in initial fracture healing. J. Orthop. Res. 1988, 6, 736–748. [Google Scholar] [CrossRef] [PubMed]
- Biot, M.A. General Theory of Three-Dimensional Consolidation. J. Appl. Phys. 1941, 12, 155–164. [Google Scholar] [CrossRef]
- Biot, M.A. Theory of Elasticity and Consolidation for a Porous Anisotropic Solid. J. Appl. Phys. 1955, 26, 182–185. [Google Scholar] [CrossRef]
- Biot, M.A. Theory of propagation of elastic waves in a fluid-saturated porous solid. I. Low frequency range. J. Acoust. Soc. Am. 1956, 28, 168–178. [Google Scholar] [CrossRef]
- Biot, M.A. Theory of propagation of elastic waves in a fluid-saturated porous solid. II. Higher frequency range. J. Acoust. Soc. Am. 1956, 28, 179–191. [Google Scholar] [CrossRef]
- Biot, M.A.; Willis, D.G. The elastic coefficients of the theory of consolidation. J. Appl. Mech. 1957, 24, 594–601. [Google Scholar]
- Biot, M.A. Generalized Theory of Acoustic Propagation in Porous Dissipative Media. J. Acoust. Soc. Am. 1962, 34, 1254–1264. [Google Scholar] [CrossRef]
- Biot, M.A. Theory of finite deformation of porous solid. Indiana Univ. Math. J. 1972, 21, 597–620. [Google Scholar] [CrossRef]
- Biot, M.A. Generalized Lagrangian equations of non-linear reaction-diffusion. Chem. Phys. 1982, 66, 11–26. [Google Scholar] [CrossRef]
- Cowin, S.C. Bone poroelasticity. J. Biomech. 1999, 32, 217–238. [Google Scholar] [CrossRef]
- Cowin, S.C. Anisotropic poroelasticity: Fabric tensor formulation. Mech. Mater. 2004, 36, 665–677. [Google Scholar] [CrossRef]
- Maslov, L.B. Algorithm for the numerical analysis of biological tissues based on a two-phase medium model. Bull. Ivanovo State Power Univ. 2005, 3, 1–9. (In Russian) [Google Scholar]
- Maslov, L.B. Poroelastic models of vibrations of biological tissues. Bull. Nizhny Novgorod Univ. 2011, 4, 499–501. (In Russian) [Google Scholar]
- Maslov, L.B. Mathematical model of bone tissue structural rearrangement. Russ. J. Biomech. 2013, 17, 39–63. (In Russian) [Google Scholar]
- Maslov, L.B. Mathematical Model of Bone Regeneration. Mech. Compos. Mater. 2017, 53, 399–414. [Google Scholar] [CrossRef]
- Carter, D.R.; Rapperport, D.J.; Fyhrie, D.P.; Schurman, D.J. Relation of coxarthrosis to stresses and morphogenesis: A finite element analysis. Acta Orthop. Scand. 1987, 58, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Carter, D.R.; Wong, M. The role of mechanical loading histories in the development of diarthrodial joints. J. Orthop. Res. 1988, 6, 804–816. [Google Scholar] [CrossRef] [PubMed]
- Carter, D.R.; Beaupre, C.S. Linear elastic and poroelastic models of cartilage can produce comparable stress results: A comment on Tanck et al. (J. Biomech. 32, 153–161, 1999). J. Biomech. 1999, 32, 1255–1257. [Google Scholar]
- Carter, D.R.; Wong, M. Modelling cartilage mechanobiology. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2003, 358, 1461–1471. [Google Scholar] [CrossRef] [Green Version]
- Carter, D.R.; Beaupré, G.S.; Wong, M.; Smith, R.L.; Andriacchi, T.P.; Schurman, D.J. The Mechanobiology of Articular Cartilage Development and Degeneration. Clin. Orthop. Relat. Res. 2004, 427S, S69–S77. [Google Scholar] [CrossRef]
- Soltz, M.A.; Ateshian, G.A. Experimental verification and theoretical prediction of cartilage interstitial fluid pressurization at an impermeable contact interface in confined compression. J. Biomech. 1998, 31, 927–934. [Google Scholar] [CrossRef]
- Soltz, M.A.; Ateshian, G.A. Interstitial fluid pressurization during confined compression cyclical loading of articular cartilage. Ann. Biomed. Eng. 2000, 28, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Rose, L.F.; Wolf, E.J.; Brindle, T.; Cernich, A.; Dean, W.K.; Dearth, C.L.; Grimm, M.; Kusiak, A.; Nitkin, R.; Potter, K.; et al. The convergence of regenerative medicine and rehabilitation: Federal perspectives. npj Regen. Med. 2018, 10, 3–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poliakov, A.; Pakhaliuk, V.; Popov, V.L. Current Trends in Improving of Artificial Joints Design and Technologies for Their Arthroplasty. Front. Mech. Eng. 2020, 6, 4. [Google Scholar] [CrossRef]
- Poliakov, O.; Olinichenko, G.; Pashkov, Y.; Kalinin, M.; Kramar, V.; Burkov, D. A new design of a unipolar hip endoprosthesis focused on the best quality of life of the patients during the postoperative period. In Proceedings of the 2012 International Conference on Biomedical Engineering, Penang, Malaysia, 27–28 February 2012; pp. 22–27. [Google Scholar] [CrossRef]
- Polyakov, A.; Pakhaliuk, V.; Kalinin, M.; Kramar, V.; Kolesova, M.; Kovalenko, O. System Analysis and Synthesis of Total Hip Joint Endoprosthesis. Procedia Eng. 2015, 100, 530–538. [Google Scholar] [CrossRef] [Green Version]













Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popov, V.L.; Poliakov, A.M.; Pakhaliuk, V.I. Synovial Joints. Tribology, Regeneration, Regenerative Rehabilitation and Arthroplasty. Lubricants 2021, 9, 15. https://0-doi-org.brum.beds.ac.uk/10.3390/lubricants9020015
Popov VL, Poliakov AM, Pakhaliuk VI. Synovial Joints. Tribology, Regeneration, Regenerative Rehabilitation and Arthroplasty. Lubricants. 2021; 9(2):15. https://0-doi-org.brum.beds.ac.uk/10.3390/lubricants9020015
Chicago/Turabian StylePopov, Valentin L., Aleksandr M. Poliakov, and Vladimir I. Pakhaliuk. 2021. "Synovial Joints. Tribology, Regeneration, Regenerative Rehabilitation and Arthroplasty" Lubricants 9, no. 2: 15. https://0-doi-org.brum.beds.ac.uk/10.3390/lubricants9020015