Liquid Baits with Oenococcus oeni Increase Captures of Drosophila suzukii
Abstract
:Simple Summary
Abstract
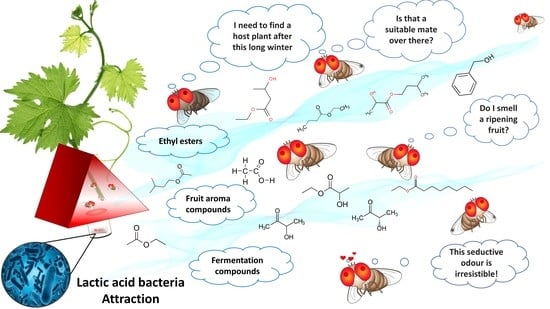
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Composition of Droskidrink for D. suzukii Trapping
2.2. Trapping Experiments with Fermentation in an Open Field
2.2.1. Trial 1: Comparison of New Traps
2.2.2. Trial 2: Fermentation in the Open Field and a Different Trap Design
2.2.3. Trial 3: Evaluation of the New Trap Design
2.3. Statistical Analysis
2.4. Headspace Collection and Chemical Characterization of DD Inoculated with Different Strains by GC-MS
2.5. Droskidrink Preparation with Oenococcus oeni Strain Pn4 (Alpha) and Strain 31 (Beta) for VOC Collection
Volatile Collection
2.6. Chemical Characterization of Volatiles from Droskidrink and Oenococcus oeni Strains
2.6.1. GC-MS Analysis
2.6.2. Electrophysiological Studies and Chemical Identification of Compounds that Elicited a Response in SWD
2.7. Data Analysis from GC-MS and GC-EAD
3. Results
3.1. Trapping Experiments with Fermentation in an Open Field
3.1.1. Trial 1: Comparison of New Traps
3.1.2. Trial 2: Fermentation in the Open Fields and Different Trap Design
3.1.3. Trial 3: Evaluation of the New Trap Design
3.2. Headspace Characterisation of DD Inoculated with Different O. oeni Strains by GC-MS
3.3. Chemical Characterisation of Volatiles from Droskidrink and O. oeni Strains
3.4. Results from Electrophysiological Experiments
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Walsh, D.B.; Bolda, M.P.; Goodhue, R.E.; Dreves, A.J.; Lee, J.; Bruck, D.J.; Walton, V.M.; O’Neal, S.D.; Zalom, F.G. Drosophila suzukii (Diptera: Drosophilidae): Invasive pest of ripening soft fruit expanding its geographic range and damage potential. J. Integr. Pest Manag. 2011, 2, G1–G7. [Google Scholar] [CrossRef]
- Cini, A.; Ioriatti, C.; Anfora, G. A review of the invasion of Drosophila suzukii in Europe and a draft research agenda for integrated pest management. Bull. Insectology 2012, 65, 149–160. [Google Scholar]
- Atallah, J.; Teixeira, L.; Salazar, R.; Zaragoza, G.; Kopp, A. The making of a pest: The evolution of a fruit-penetrating ovipositor in Drosophila suzukii and related species. Proc. R. Soc. B 2014, 281, 20132840. [Google Scholar] [CrossRef] [Green Version]
- Crava, C.M.; Romani, R.; Zanini, D.; Amati, S.; Sollai, G.; Crnjar, R.; Haase, A.; Paoli, M.; Rossi-Stacconi, M.V.; Rota-Stabelli, O. Exploring multiple sensory systems in ovipositors of Drosophila suzukii and related species with different egg-laying behaviour. bioRxiv 2019. [Google Scholar] [CrossRef] [Green Version]
- Rota-Stabelli, O.; Blaxter, M.; Anfora, G. Drosophila suzukii. Curr. Biol. 2013, 23, R8–R9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ioriatti, C.; Walton, V.; Dalton, D.; Anfora, G.; Grassi, A.; Maistri, S.; Mazzoni, V. Drosophila suzukii (Diptera: Drosophilidae) and its potential impact to wine grapes during harvest in two cool climate wine grape production regions. J. Econ. Entomol. 2015, 108, 1148–1155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazzi, D.; Bravin, E.; Meraner, M.; Finger, R.; Kuske, S. Economic impact of the introduction and establishment of Drosophila suzukii on sweet cherry production in Switzerland. Insects 2017, 8, 18. [Google Scholar] [CrossRef] [Green Version]
- Karageorgi, M.; Bracker, L.B.; Lebreton, S.; Minervino, C.; Cavey, M.; Siju, K.P.; Grunwald Kadow, I.C.; Gompel, N.; Prud’homme, B. Evolution of multiple sensory systems drives novel egg-laying behavior in the fruit pest Drosophila suzukii. Curr. Biol. 2017, 27, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Tait, G.; Park, K.; Nieri, R.; Crava, M.C.; Mermer, S.; Clappa, E.; Boyer, G.; Dalton, D.T.; Carlin, S.; Brewer, L.; et al. Reproductive Site Selection: Evidence of an Oviposition Cue in a Highly Adaptive Dipteran, Drosophila suzukii (Diptera: Drosophilidae). Environ. Entomol. 2020, 49, 355–363. [Google Scholar] [CrossRef]
- Tait, G.; Grassi, A.; Pfab, F.; Crava, C.M.; Dalton, D.T.; Magarey, R.; Ometto, L.; Vezzulli, S.; Rossi-Stacconi, M.V.; Gottardello, A. Large-scale spatial dynamics of Drosophila suzukii in Trentino, Italy. J. Pest Sci. 2018, 91, 1213–1224. [Google Scholar] [CrossRef]
- Loreto, F.; Dicke, M.; Schnitzler, J.P.; Turlings, T.C. Plant volatiles and the environment. Plant Cell Environ. 2014, 37, 1905–1908. [Google Scholar] [CrossRef]
- Bruce, T.J.; Wadhams, L.J.; Woodcock, C.M. Insect host location: A volatile situation. Trends Plant Sci. 2005, 10, 269–274. [Google Scholar] [CrossRef] [PubMed]
- De Bruyne, M.; Baker, T.C. Odor detection in insects: Volatile codes. J. Chem. Ecol. 2008, 34, 882–897. [Google Scholar] [CrossRef] [PubMed]
- Keesey, I.W.; Knaden, M.; Hansson, B.S. Olfactory specialization in Drosophila suzukii supports an ecological shift in host preference from rotten to fresh fruit. J. Chem. Ecol. 2015, 41, 121–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheidler, N.H.; Liu, C.; Hamby, K.A.; Zalom, F.G.; Syed, Z. Volatile codes: Correlation of olfactory signals and reception in Drosophila-yeast chemical communication. Sci. Rep. 2015, 5, 14059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becher, P.G.; Flick, G.; Rozpędowska, E.; Schmidt, A.; Hagman, A.; Lebreton, S.; Larsson, M.C.; Hansson, B.S.; Piškur, J.; Witzgall, P. Yeast, not fruit volatiles mediate Drosophila melanogaster attraction, oviposition and development. Funct. Ecol. 2012, 26, 822–828. [Google Scholar] [CrossRef]
- Bruce, T.J.; Pickett, J.A. Perception of plant volatile blends by herbivorous insects–finding the right mix. Phytochemistry 2011, 72, 1605–1611. [Google Scholar] [CrossRef]
- Hamby, K.A.; Hernández, A.; Boundy-Mills, K.; Zalom, F.G. Associations of yeasts with spotted-wing Drosophila (Drosophila suzukii; Diptera: Drosophilidae) in cherries and raspberries. Appl. Environ. Microbiol. 2012, 78, 4869–4873. [Google Scholar] [CrossRef] [Green Version]
- Mazzetto, F.; Gonella, E.; Crotti, E.; Vacchini, V.; Syrpas, M.; Pontini, M.; Mangelinckx, S.; Daffonchio, D.; Alma, A. Olfactory attraction of Drosophila suzukii by symbiotic acetic acid bacteria. J. Pest Sci. 2016, 89, 783–792. [Google Scholar] [CrossRef]
- Davis, T.S.; Crippen, T.L.; Hofstetter, R.W.; Tomberlin, J.K. Microbial volatile emissions as insect semiochemicals. J. Chem. Ecol. 2013, 39, 840–859. [Google Scholar] [CrossRef]
- Bueno, E.; Martin, K.R.; Raguso, R.A.; McMullen, J.G.; Hesler, S.P.; Loeb, G.M.; Douglas, A.E. Response of wild spotted wing drosophila (Drosophila suzukii) to microbial volatiles. J. Chem. Ecol. 2019, 46, 688–698. [Google Scholar] [CrossRef]
- Vet, L.E.; Dicke, M. Ecology of infochemical use by natural enemies in a tritrophic context. Annu. Rev. Entomol. 1992, 37, 141–172. [Google Scholar] [CrossRef]
- Hamby, K.A.; Becher, P.G. Current knowledge of interactions between Drosophila suzukii and microbes, and their potential utility for pest management. J. Pest Sci. 2016, 89, 621–630. [Google Scholar] [CrossRef]
- Grassi, A.; Anfora, G.; Maistri, S.; Maddalena, G.; De Cristofaro, A.; Savini, G.; Ioriatti, C. Development and efficacy of Droskidrink, a food bait for trapping Drosophila suzukii. In Proceedings of the IOBC VIII Workshop on Integrated soft fruit production, Vigalzano di Pergine, Trento, Italy, 26–28 May 2014; pp. 197–204. [Google Scholar]
- Cloonan, K.R.; Abraham, J.; Angeli, S.; Syed, Z.; Rodriguez-Saona, C. Advances in the chemical ecology of the spotted wing drosophila (Drosophila suzukii) and its applications. J. Chem. Ecol. 2018, 44, 922–939. [Google Scholar] [CrossRef] [PubMed]
- Clymans, R.; Van Kerckvoorde, V.; Bangels, E.; Akkermans, W.; Alhmedi, A.; De Clercq, P.; Belien, T.; Bylemans, D. Olfactory preference of Drosophila suzukii shifts between fruit and fermentation cues over the season: Effects of physiological status. Insects 2019, 10, 200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asplen, M.K.; Anfora, G.; Biondi, A.; Choi, D.-S.; Chu, D.; Daane, K.M.; Gibert, P.; Gutierrez, A.P.; Hoelmer, K.A.; Hutchison, W.D. Invasion biology of spotted wing drosophila (Drosophila suzukii): A global perspective and future priorities. J. Pest Sci. 2015, 88, 469–494. [Google Scholar] [CrossRef]
- Feng, Y.; Bruton, R.; Park, A.; Zhang, A. Identification of attractive blend for spotted wing drosophila, Drosophila suzukii, from apple juice. J. Pest Sci. 2018, 91, 1251–1267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cha, D.H.; Hesler, S.P.; Wallingford, A.K.; Zaman, F.; Jentsch, P.; Nyrop, J.; Loeb, G.M. Comparison of commercial lures and food baits for early detection of fruit infestation risk by Drosophila suzukii (Diptera: Drosophilidae). J. Econ. Entomol. 2018, 111, 645–652. [Google Scholar] [CrossRef] [Green Version]
- Cha, D.H.; Adams, T.; Werle, C.T.; Sampson, B.J.; Adamczyk, J.J., Jr.; Rogg, H.; Landolt, P.J. A four-component synthetic attractant for Drosophila suzukii (diptera: Drosophilidae) isolated from fermented bait headspace. Pest Manag. Sci. 2014, 70, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, D.; McGhee, P.; Gut, L.; Miller, J. Improving monitoring tools for spotted wing drosophila, Drosophila suzukii. Entomol. Exp. Appl. 2017, 164, 87–93. [Google Scholar] [CrossRef] [Green Version]
- Landolt, P.J.; Adams, T.; Davis, T.S.; Rogg, H. Spotted wing drosophila, Drosophila suzukii (Diptera: Drosophilidae), trapped with combinations of wines and vinegars. Fla. Entomol. 2012, 95, 326–333. [Google Scholar] [CrossRef]
- Tonina, L.; Grassi, A.; Caruso, S.; Mori, N.; Gottardello, A.; Anfora, G.; Giomi, F.; Vaccari, G.; Ioriatti, C. Comparison of attractants for monitoring Drosophila suzukii in sweet cherry orchards in Italy. J. Appl. Entomol. 2018, 142, 18–25. [Google Scholar] [CrossRef]
- Burrack, H.J.; Asplen, M.; Bahder, L.; Collins, J.; Drummond, F.A.; Guedot, C.; Isaacs, R.; Johnson, D.; Blanton, A.; Lee, J.C.; et al. Multistate comparison of attractants for monitoring Drosophila suzukii (diptera: Drosophilidae) in blueberries and caneberries. Environ. Entomol. 2015, 44, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Bolda, M.P.; Goodhue, R.E.; Zalom, F.G. Spotted wing drosophila: Potential economic impact of a newly established pest. Agric. Resour. Econ. Rev. 2010, 13, 5–8. [Google Scholar]
- Schetelig, M.; Lee, K.Z.; Otto, S.; Talmann, L.; Stökl, J.; Degenkolb, T.; Vilcinskas, A.; Halitschke, R. Environmentally sustainable pest control options for Drosophila suzukii. J. Appl. Entomol. 2018, 142, 3–17. [Google Scholar] [CrossRef]
- Chandler, J.A.; James, P.M.; Jospin, G.; Lang, J.M. The bacterial communities of Drosophila suzukii collected from undamaged cherries. PeerJ 2014, 2, e474. [Google Scholar] [CrossRef] [Green Version]
- Barata, A.; Malfeito-Ferreira, M.; Loureiro, V. The microbial ecology of wine grape berries. Int. J. Food Microbiol. 2012, 153, 243–259. [Google Scholar] [CrossRef]
- Hamby, K.A.; Bellamy, D.E.; Chiu, J.C.; Lee, J.C.; Walton, V.M.; Wiman, N.G.; York, R.M.; Biondi, A. Biotic and abiotic factors impacting development, behavior, phenology, and reproductive biology of Drosophila suzukii. J. Pest Sci. 2016, 89, 605–619. [Google Scholar] [CrossRef]
- Cha, D.H.; Adams, T.; Rogg, H.; Landolt, P.J. Identification and field evaluation of fermentation volatiles from wine and vinegar that mediate attraction of spotted wing drosophila, Drosophila suzukii. J. Chem. Ecol. 2012, 38, 1419–1431. [Google Scholar] [CrossRef]
- Kleiber, J.R.; Unelius, C.R.; Lee, J.C.; Suckling, D.M.; Qian, M.C.; Bruck, D.J. Attractiveness of fermentation and related products to spotted wing Drosophila (Diptera: Drosophilidae). Environ. Entomol. 2014, 43, 439–447. [Google Scholar] [CrossRef]
- Cordente, A.G.; Curtin, C.D.; Varela, C.; Pretorius, I.S. Flavour-active wine yeasts. Appl. Microbiol. Biotechnol. 2012, 96, 601–618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nielsen, J.C.; Richelieu, M. Control of flavor development in wine during and after malolactic fermentation by Oenococcus oeni. Appl. Environ. Microbiol. 1999, 65, 740–745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zapparoli, G.; Fracchetti, F.; Stefanelli, E.; Torriani, S. Genetic and phenotypic strain heterogeneity within a natural population of Oenococcus oeni from A marone wine. J. Appl. Microbiol. 2012, 113, 1087–1096. [Google Scholar] [CrossRef] [PubMed]
- McCullagh, P. Generalized Linear Models; Routledge: Abingdon-on-Thames, UK, 2018; ISBN 10:10-412-31760-31765. [Google Scholar]
- Payne, R.W. GenStat. Wiley Interdiscip. Rev. Comput. Stat. 2009, 1, 255–258. [Google Scholar] [CrossRef]
- Anfora, G.; Tasin, M.; De Cristofaro, A.; Ioriatti, C.; Lucchi, A. Synthetic grape volatiles attract mated Lobesia botrana females in laboratory and field bioassays. J. Chem. Ecol. 2009, 35, 1054. [Google Scholar] [CrossRef] [PubMed]
- Dalton, D.T.; Walton, V.M.; Shearer, P.W.; Walsh, D.B.; Caprile, J.; Isaacs, R. Laboratory survival of Drosophila suzukii under simulated winter conditions of the Pacific Northwest and seasonal field trapping in five primary regions of small and stone fruit production in the United States. Pest Manag. Sci. 2011, 67, 1368–1374. [Google Scholar] [CrossRef] [PubMed]
- Revadi, S.; Vitagliano, S.; Rossi Stacconi, M.V.; Ramasamy, S.; Mansourian, S.; Carlin, S.; Vrhovsek, U.; Becher, P.G.; Mazzoni, V.; Rota-Stabelli, O. Olfactory responses of Drosophila suzukii females to host plant volatiles. Physiol. Entomol. 2015, 40, 54–64. [Google Scholar] [CrossRef]
- De Cristofaro, A.; Rotundo, G.; Germinara, G.S. Electrophysiological and olfactory responses of Ephestia kuehniella Zeller adults to cereals’ semiochemicals. IOBC WPRS Bull. 2000, 23, 189–194. [Google Scholar]
- Van Den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Riu-Aumatell, M.; Castellari, M.; López-Tamames, E.; Galassi, S.; Buxaderas, S. Characterisation of volatile compounds of fruit juices and nectars by HS/SPME and GC/MS. Food Chem. 2004, 87, 627–637. [Google Scholar] [CrossRef]
- Landolt, P.J.; Adams, T.; Zack, R.S.; Crabo, L. A diversity of moths (Lepidoptera) trapped with two feeding attractants. Ann. Entomol. Soc. Am. 2011, 104, 498–506. [Google Scholar] [CrossRef] [Green Version]
- Knight, A.L.; Basoalto, E.; Yee, W.; Hilton, R.; Kurtzman, C.P. Adding yeasts with sugar to increase the number of effective insecticide classes to manage Drosophila suzukii (Matsumura) (Diptera: Drosophilidae) in cherry. Pest Manag. Sci. 2016, 72, 1482–1490. [Google Scholar] [CrossRef]
- Knight, S.; Goddard, M.R. Quantifying separation and similarity in a Saccharomyces cerevisiae metapopulation. ISME J. 2015, 9, 361–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piñero, J.C.; Barrett, B.A.; Bolton, L.G.; Follett, P.A. β-cyclocitral synergizes the response of adult Drosophila suzukii (Diptera: Drosophilidae) to fruit juices and isoamyl acetate in a sex-dependent manner. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, A. The Pherobase: Database of Insect Pheromones and Semiochemicals. Available online: http://www.pherobase.com (accessed on 12 August 2020).
- Rochat, D.; Morin, J.P.; Kakul, T.; Beaudoin-Ollivier, L.; Prior, R.; Renou, M.; Malosse, I.; Stathers, T.; Embupa, S.; Laup, S. Activity of male pheromone of Melanesian rhinoceros beetle Scapanes australis. J. Chem. Ecol. 2002, 28, 479–500. [Google Scholar] [CrossRef]
- Arguello, J.R.; Sellanes, C.; Lou, Y.R.; Raguso, R.A. Can yeast (S. cerevisiae) metabolic volatiles provide polymorphic signaling? PLoS ONE 2013, 8, e70219. [Google Scholar] [CrossRef] [Green Version]
- Keesey, I.W.; Koerte, S.; Retzke, T.; Haverkamp, A.; Hansson, B.S.; Knaden, M. Adult frass provides a pheromone signature for drosophila feeding and aggregation. J. Chem. Ecol. 2016, 42, 739–747. [Google Scholar] [CrossRef] [Green Version]
- Guzzon, R.; Anfora, G.; Grassi, A.; Ioriatti, C. A new tool against Drosophila suzukii improving the efficacy of the D. suzukii food baits by the addition of Oenococcus oeni strains. In Proceedings of the 9th Symposium In Vino Analytica Scientia 2015: Analytical Chemistry for Wine, Brandy and Spirits, Mezzocorona, Italy, 14–17 July 2015; pp. 123–124. [Google Scholar]
- Romano, P.; Suzzi, G. Origin and production of acetoin during wine yeast fermentation. Appl. Environ. Microbiol. 1996, 62, 309–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abraham, J.; Zhang, A.; Angeli, S.; Abubeker, S.; Michel, C.; Feng, Y.; Rodriguez-Saona, C. Behavioral and antennal responses of Drosophila suzukii (Diptera: Drosophilidae) to volatiles from fruit extracts. Environ. Entomol. 2015, 44, 356–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Sayed, A.; Suckling, D.; Wearing, C.; Byers, J. Potential of mass trapping for long-term pest management and eradication of invasive species. J. Econ. Entomol. 2006, 99, 1550–1564. [Google Scholar] [CrossRef]
- Suckling, D.M.; Stringer, L.D.; Kean, J.M.; Lo, P.L.; Bell, V.; Walker, J.T.; Twidle, A.M.; Jiménez-Pérez, A.; El-Sayed, A.M. Spatial analysis of mass trapping: How close is close enough? Pest Manag. Sci. 2015, 71, 1452–1461. [Google Scholar] [CrossRef] [PubMed]
- Lasa, R.; Velazquez, O.E.; Ortega, R.; Acosta, E. Efficacy of commercial traps and food odor attractants for mass trapping of Anastrepha ludens (Diptera: Tephritidae). J. Econ. Entomol. 2014, 107, 198–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossi-Stacconi, M.V.; Kaur, R.; Mazzoni, V.; Ometto, L.; Grassi, A.; Gottardello, A.; Rota-Stabelli, O.; Anfora, G. Multiple lines of evidence for reproductive winter diapause in the invasive pest Drosophila suzukii: Useful clues for control strategies. J. Pest Sci. 2016, 89, 689–700. [Google Scholar] [CrossRef]
- Tait, G.; Cabianca, A.; Grassi, A.; Pfab, F.; Oppedisano, T.; Puppato, S.; Mazzoni, V.; Anfora, G.; Walton, V.M. Drosophila suzukii daily dispersal between distinctly different habitats. Entomol. Gen. 2019, 40, 25–37. [Google Scholar] [CrossRef]

| Code | Trial | Base Solution | pH | CA | Alpha/Beta | Alpha/Beta Rate g/L−1 | Trap | Amount of Liquid (mL) | Liquid Replaced Weekly? |
|---|---|---|---|---|---|---|---|---|---|
| A: Cw | 1 | DD | 2.6 | Cup | 200 | y | |||
| A: Cw | 2 | DD | 2.6 | Cup | 200 | y | |||
| A: Cn | 3 | DD | 2.6 | Cup | 200 | n | |||
| B: Kw | 1 | DD | 3.8 | Cup | 200 | y | |||
| B: Kw | 2 | DD | 3.8 | Cup | 200 | y | |||
| B: Kn | 3 | DD | 3.8 | Cup | 200 | n | |||
| C: ChLn | 1 | CL | 6.5 | Cup | 200 | n | |||
| C: ChLw | 2 | CL | 6.5 | Cup | 200 | y | |||
| C: ChLn | 3 | CL | 6.5 | Cup | 200 | n | |||
| D: α5Kn | 1 | DD | 3.8 | Alpha | 0.5 | Cup | 200 | n | |
| D: α2Kw | 2 | DD | 3.8 | Alpha | 0.2 | Cup | 200 | y | |
| D: α2KΔn | 3 | DD | 3.8 | Alpha | 0.2 | DC | 15 | n | |
| E: β5Kn | 1 | DD | 3.8 | Beta | 0.5 | Cup | 200 | n | |
| E: β2Kw | 2 | DD | 3.8 | Beta | 0.2 | Cup | 200 | y | |
| E: β2KΔn | 3 | DD | 3.8 | Beta | 0.2 | DC | 15 | n | |
| F: β5KnCA | 1 | DD | 3.8 | Y | Beta | 0.5 | Cup | 200 | n |
| F: β2KwCA | 2 | DD | 3.8 | Y | Beta | 0.2 | Cup | 200 | y |
| F: β2KΔnCA | 3 | DD | 3.8 | Y | Beta | 0.2 | DC | 15 | n |
| G: β5KΔIBn | 1 | DD | 3.8 | Beta | 0.5 | DIB | 200 | n | |
| G: β5KΔCw | 2 | DD | 3.8 | Beta | 0.2 | DC | 20 | y | |
| H: β2KΔUBn | 1 | DD | 3.8 | Beta | 0.5 | DUB | 200 | n | |
| H: β2KΔCw | 2 | DD | 3.8 | Beta | 0.2 | DC | 15 | y |
| Volatile Compound | Retention Time | Extracted Ions | Droskidrink | MRI1000 | α | β | T1 | S18 | P1 |
|---|---|---|---|---|---|---|---|---|---|
| Min | m/z | % | |||||||
| Acid | |||||||||
| Acetic acid | 2.2 | 60 | 100 | 47.78 | 55.75 | 55.9 | 874.39 | 64.88 | 669.25 |
| Alcohols | |||||||||
| Ethanol | 1.55 | 45 | 100 | 126.07 | 129.25 | 138.499 | 165.14 | 119.49 | 154.68 |
| Isoamyl alcohol | 4.7 | 41 | 100 | 123.66 | 136.69 | 188.1 | 115.98 | 99.73 | 109.61 |
| Aldehyde | |||||||||
| Acetoin | 3.78 | 88 | 100 | 149.45 | 169.16 | 184.76 | 78.7 | 126.11 | 44.61 |
| Esters | |||||||||
| Ethyl acetate | 2.27 | 88 | 100 | 5.04 | 5.53 | 6.98 | 3.65 | 4.33 | 3.89 |
| 2-Butyl acetate | 5.55 | 87 | 100 | 114.63 | 105.54 | 112.49 | 70.92 | 81.63 | 68.71 |
| Isobutyl acetate | 6.17 | 56 | 100 | 70.72 | 69.19 | 106.79 | 48.35 | 51.06 | 43.88 |
| Ethyl butyrate | 7.36 | 71 | 100 | 64.95 | 62.2 | 90.78 | 52.81 | 46.44 | 51.73 |
| Isoamyl acetate | 10.84 | 43 | 100 | 40.16 | 37.22 | 42.18 | 31.41 | 33.42 | 29.5 |
| Ethyl caproate | 16.73 | 88 | 100 | 95.83 | 92.47 | 147.36 | 72.36 | 57.51 | 50.62 |
| Ethyl octanoate | 25.35 | 88 | 100 | 143.35 | 135.62 | 241.42 | 97.7 | 91.79 | 72.75 |
| Ketone | |||||||||
| 2-Butanone | 2.13 | 72 | 100 | 139.39 | 149.26 | 164.71 | 75.32 | 110.97 | 84.98 |
| Compound Chemical Name | CAS | RT | Retention Index | ||
|---|---|---|---|---|---|
| ZB-WAX | ZB-WAX | HP-5 MS | |||
| 1 | Acetic acid | 64-19-7 | 8.32 | 1480 | 600 |
| 2 | 2-Butanone, 3-hydroxy | 513-86-0 | 6.02 | 1288 | 662 |
| 3 | 3-Methyl-1-butanol | 123-51-3 | 4.9 | 1117 | 720 |
| 4 | Ethyl butyrate | 105-54-4 | 10.58 | 1591 | 788 |
| 5 | Ethyl lactate | 97-64-3 | 6.76 | 1338 | 781 |
| 6 | Butanoic acid | 107-92-6 | 11.15 | 1630 | 843.7 |
| 7 | 1-Butanol, 3-methyl-, acetate | 123-92-2 | 4.05 | 1148 | 879.8 |
| 8 | 3-(Acetyloxy)-2-butanone | 4906-24-5 | 7.36 | 1376 | 899.1 |
| 9 | 1-Hexanol | 111-27-3 | 6.86 | 1344 | 862 |
| 10 | Grape butyrate | 5405-41-4 | 10.11 | 1518 | 907 |
| 11 | Benzaldehyde | 100-52-7 | 9.58 | 1534 | 947 |
| 12 | Ethyl hexanoate | 123-66-0 | 5.26 | 1234 | 980 |
| 13 | Butanoic acid, 3-methyl- | 503-74-2 | 11.57 | 1668 | 913.1 |
| 14 | 3-Hexenoic acid, ethyl ester | 2396-83-0 | 6.08 | 1293 | 956.8 |
| 15 | Nonanal | 124-19-6 | 7.6 | 1392 | 1074 |
| 16 | 1-Hexanol, 2-ethyl | 104-76-7 | 8.9 | 1486 | 1034.9 |
| 17 | Hexanoic acid | 142-62-1 | 13.98 | 1833 | 908.0 |
| 18 | 1- Octanol | 111-87-5 | 10.02 | 1553 | 1050 |
| 19 | Acetic acid, hexyl ester | 142-92-7 | 5.78 | 1271 | 1018.1 |
| 20 | Sorbic Acid | 110-44-1 | 17.74 | 2133 | 1089.8 |
| 21 | Acetic acid, phenylmethyl ester | 140-11-4 | 12.43 | 1719 | 1167.3 |
| 22 | Phenylethyl Alcohol | 60-12-8 | 14.87 | 1900 | 1120.1 |
| 23 | Benzenemethanol, benzyl alcohol | 100-51-6 | 14.4 | 1865 | 1039.0 |
| 24 | Acetic acid, 2-phenylethyl ester | 103-45-7 | 13.63 | 1793 | 1262.5 |
| 25 | Benzeneacetic acid, ethyl ester | 101-97-3 | 13.21 | 1777 | 1250.3 |
| 26 | Eugenol | 97-53-0 | 18.07 | 2162 | 1361.5 |
| 27 | Triacetin | 102-76-1 | 16.85 | 2059 | 1354.4 |
| 28 | Diethyl succinate | 123-25-1 | 11.66 | 1674 | 1182.8 |
| 29 | Benzothiazole | 95-16-9 | 15.62 | 1960 | 1225.5 |
| 30 | Octanoic acid, ethyl ester | 106-32-1 | 8.14 | 1428 | 1198.0 |
| 31 | Benzoic acid, 2-hydroxy-, methyl ester | 119-36-8 | 13.12 | 1769 | 1197.8 |
| 32 | Phenol, 2,4-bis(1,1-dimethylethyl) | 96-76-4 | 19.57 | 2293 | 1511.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ðurović, G.; Alawamleh, A.; Carlin, S.; Maddalena, G.; Guzzon, R.; Mazzoni, V.; Dalton, D.T.; Walton, V.M.; Suckling, D.M.; Butler, R.C.; et al. Liquid Baits with Oenococcus oeni Increase Captures of Drosophila suzukii. Insects 2021, 12, 66. https://0-doi-org.brum.beds.ac.uk/10.3390/insects12010066
Ðurović G, Alawamleh A, Carlin S, Maddalena G, Guzzon R, Mazzoni V, Dalton DT, Walton VM, Suckling DM, Butler RC, et al. Liquid Baits with Oenococcus oeni Increase Captures of Drosophila suzukii. Insects. 2021; 12(1):66. https://0-doi-org.brum.beds.ac.uk/10.3390/insects12010066
Chicago/Turabian StyleÐurović, Gordana, Amani Alawamleh, Silvia Carlin, Giuseppe Maddalena, Raffaele Guzzon, Valerio Mazzoni, Daniel T. Dalton, Vaughn M. Walton, David M. Suckling, Ruth C. Butler, and et al. 2021. "Liquid Baits with Oenococcus oeni Increase Captures of Drosophila suzukii" Insects 12, no. 1: 66. https://0-doi-org.brum.beds.ac.uk/10.3390/insects12010066