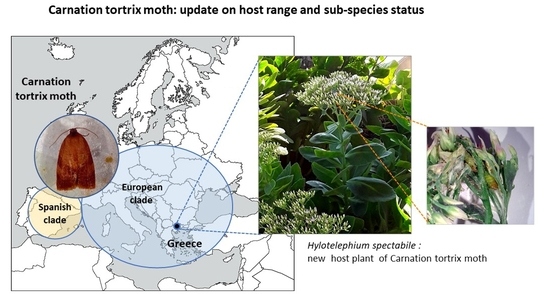
Hylotelephium spectabile, a New Host for Carnation Tortrix Moth (Cacoecimorpha pronubana) and Molecular Characterization in Greece
Abstract
:Simple Summary
Abstract
1. Introduction
2. Identification
3. Biological and Taxonomic Notes of Cacoecimorpha pronubana
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Suffert, M. Re-evaluation of EPPO-listed pests. EPPO Bull. 2012, 42, 181–184. [Google Scholar] [CrossRef]
- Kaçar, G.; Ulusoy, M.R. A new pest of olive trees: Carnation tortrix, Cacoecimorpha pronubana (Hübner), 1796–1799 (Lepidoptera: Tortricidae) in the Eastern Mediterranean Region of Turkey. Turk. J. Entomol. 2008, 32, 211–223, (English Abstract). Available online: https://dergipark.org.tr/en/download/article-file/65044 (accessed on 20 December 2020).
- Alford, D.V. Pests of Fruit Crops: A Colour Handbook, 2nd ed.; CRC Press/Taylor & Francis Group: Boca Raton, FL, USA, 2014; pp. 266–267. [Google Scholar]
- Pencheva, A.; Yovkova, M. New data on alien insect pests of ornamental plants in Bulgaria. For. Ideas 2016, 22, 17–33. Available online: https://forestry-ideas.info/issues/issues_Index.php?pageNum_rsIssue=0&totalRows_rsIssue=9&journalFilter=55 (accessed on 20 December 2020).
- Imre, F. Hedera helix L. a new larval foodplant for Cacoecimorpha pronubana (Hübner, [1796–99]) in Hungary (Lepidoptera: Tortricidae). Microlepidopterahu 2019, 15, 29–34, (English Abstract). [Google Scholar] [CrossRef]
- EPPO Global Database. Available online: https://gd.eppo.int (accessed on 18 December 2020).
- Aarvik, L.E. Fauna Europaea: Tortricidae. In Fauna Europaea: Lepidoptera, Moths. Fauna Europaea Version 2017.06; Karsholt, O., van Nieukerken, E.J., Eds.; Fauna Europaea: Berlin, Germany, 2013; Available online: https://fauna-eu.org (accessed on 18 December 2020).
- Nel, J.; Nel, A. Contribution to the knowledge of the Lepidoptera of the Crete Island (Greece) (Lepidoptera). Le Bull. SEF 2003, 108, 277–282. [Google Scholar]
- Carvalho, P.; Franco, J.C.; Aguiar, A.; Soares, A. Insect pests of citrus in Portugal. International Citrus Congress. In Proceedings of the International Society of Citriculture, Sun City, South Africa, 12–17 May 1996; pp. 613–618. Available online: https://www.researchgate.net/publication/233819663_Insect_pests_of_citrus_in_Portugal (accessed on 20 December 2020).
- Alford, D.V. Pests of Ornamental Trees, Shrubs and Flowers: A Colour Handbook, 2nd ed.; Manson: London, UK, 2012; p. 263. [Google Scholar]
- Hebert, P.D.N.; Cywinska, A.; Ball, S.L.; deWaard, J.R. Biological identifications through DNA barcodes. Proc. R. Soc. Lond. B 2003, 270, 313–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Signorile, L. An unusual, new larval host-plant for Cacoecimorpha pronubana (Hübner, 1799) (Lepidoptera: Tortricidae). Ento-mol. Gaz. 2012, 63, 49–51. [Google Scholar]
- Yang, C.; Qin, Y.; Sun, X.; Yuan, S.; Lin, H. Propagation of Sedum spectabile Boreau in Leaf Culture in Vitro. Not. Bot. Hort. Agrobot. Cluj. 2012, 40, 107. [Google Scholar] [CrossRef] [Green Version]
- Stephenson, R. Sedum. Cultivated Stonecrops; Timber Press: Portland, OR, USA, 1994; p. 272. [Google Scholar]
- Gilligan, T.M.; Baixeras, J.; Brown, J.W. T@RTS: Online World Catalogue of the Tortricidae (Ver. 4.0). Available online: http://www.tortricid.net/catalogue.asp (accessed on 22 December 2020).
- Fisher, R.C. The life-history and habits of Tortrix pronubana, HB, with special reference to the larval and pupal stages. Ann. Appl. Biol. 1924, 11, 395–447. [Google Scholar] [CrossRef]
- Alford, D.V. A Textbook of Agricultural Entomology, 1st ed.; Blackwell Science: Oxford, MA, USA, 1999; p. 214. [Google Scholar]
- Gilligan, T.M.; Brown, J.W.; Baixeras, J. Immigrant Tortricidae: Holarctic versus Introduced Species in North America. Insects 2020, 11, 594. [Google Scholar] [CrossRef] [PubMed]
- Avtzis, D.N.; Markoudi, V.; Mizerakis, V.; Devalez, J.; Naka, G.; Poulakakis, N.; Petanidou, T. The Aegean Archipelago as cradle: Divergence of the glaphyrid genus Pygopleurus and phylogeography of P. foina. Syst. Biodivers 2021. under publication. [Google Scholar]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotech. 1994, 3, 294–299. Available online: https://www.researchgate.net/publication/15316743_DNA_primers_for_amplification_of_mitochondrial_Cytochrome_C_oxidase_subunit_I_from_diverse_metazoan_invertebrates (accessed on 20 December 2020).
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Damos, P.; Bonsignore, C.P.; Gardi, F.; Avtzis, D.N. Phenological Responses and a Comparative Phylogenetic Insight of Anarsia Lineatella and Grapholita Molesta between Distinct Geographical Regions within the Mediterranean Basin. J. Appl. Entomol. 2014, 138, 528–538. [Google Scholar] [CrossRef]
- Huemer, P.; Hebert, P.D.N.; Mutanen, M.; Wieser, C.; Wiesmair, B.; Hausmann, A.; Yakovlev, R.; Möst, M.; Gottsberger, B.; Strutzenberger, P.; et al. Large Geographic Distance versus Small DNA Barcode Divergence: Insights from a Comparison of European to South Siberian Lepidoptera. PLoS ONE 2018, 13, e0206668. [Google Scholar] [CrossRef]
- Arthofer, W.; Avtzis, D.; Riegler, M.; Stauffer, C. Mitochondrial Phylogenies in the Light of Pseudogenes and Wolbachia: Re-Assessment of a Bark Beetle Dataset. ZooKeys 2010, 56, 269–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Razowski, J. Tortricidae of Europe. Tortricinae and Chlidanotinae; Slamka: Bratislava, Slovakia, 2002; Volume 1, p. 247. [Google Scholar]
- Chambon, J.P. Atlas des genitalia Males des lépidoptères Tortricidae France et Belgique; INRA Editions: Paris, France, 1999; p. 400. [Google Scholar]


| Host Category | Common Name | Scientific Name | Botanic Family | References |
|---|---|---|---|---|
| Crop plants | strawberry | Fragaria × ananassa Duchesne | Rosaceae | [3,6,9] |
| almond | Prunus dulcis (Mill.) D.A. Webb | Rosaceae | ||
| cherry | Prunus avium L. | Rosaceae | ||
| apple | Malus domestica Borkh. | Rosaceae | ||
| raspberry | Rubus spp. | Rosaceae | ||
| grapevine | Vitis vinifera L. | Vitaceae | ||
| citrus | Citrus spp. | Rutaceae | ||
| olive | Olea europaea L. | Oleaceae | ||
| Ornamental plants | carnation | Dianthus spp. | Caryophyllaceae | [10,11] |
| bay laurel | Laurus nobilis L. | Lauraceae | ||
| cypress | Cupressus spp. | Cupressaceae | ||
| broom | Cytisus spp. | Fabaceae | ||
| daphne | Daphne spp. | Thymeleaceae | ||
| false acacia | Robinia pseudoacacia L. | Fabaceae | ||
| fuchsia | Fuchsia spp. | Onagraceae | ||
| spider flower | Grevillea spp. | Proteaceae | ||
| honeysuckle | Lonicera spp. | Caprifoliaceae | ||
| St. John’s wort | Hypericum spp. | Hypericaceae | ||
| ivy | Hedera spp. | Araliaceae | ||
| Japanese spindle | Euonymus japonicus Thunb. | Celastraceae | ||
| privet | Ligustrum vulgare L. | Oleaceae | ||
| laurustinus | Viburnum tinus L. | Adoxaceae | ||
| Japanese laurel | Aucuba japonica Thunb. | Garryaceae | ||
| Cape sundew | Drosera capensis L. | Droseraceae | [12] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simoglou, K.B.; Avtzis, D.N.; Baixeras, J.; Sarigkoli, I.; Roditakis, E. Hylotelephium spectabile, a New Host for Carnation Tortrix Moth (Cacoecimorpha pronubana) and Molecular Characterization in Greece. Insects 2021, 12, 245. https://0-doi-org.brum.beds.ac.uk/10.3390/insects12030245
Simoglou KB, Avtzis DN, Baixeras J, Sarigkoli I, Roditakis E. Hylotelephium spectabile, a New Host for Carnation Tortrix Moth (Cacoecimorpha pronubana) and Molecular Characterization in Greece. Insects. 2021; 12(3):245. https://0-doi-org.brum.beds.ac.uk/10.3390/insects12030245
Chicago/Turabian StyleSimoglou, Konstantinos B., Dimitrios N. Avtzis, Joaquín Baixeras, Ioanna Sarigkoli, and Emmanouil Roditakis. 2021. "Hylotelephium spectabile, a New Host for Carnation Tortrix Moth (Cacoecimorpha pronubana) and Molecular Characterization in Greece" Insects 12, no. 3: 245. https://0-doi-org.brum.beds.ac.uk/10.3390/insects12030245