Effects of Temperature and Relative Humidity on the Embryonic Development of Hypera postica Gyllenhal (Col.: Curculionidae)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
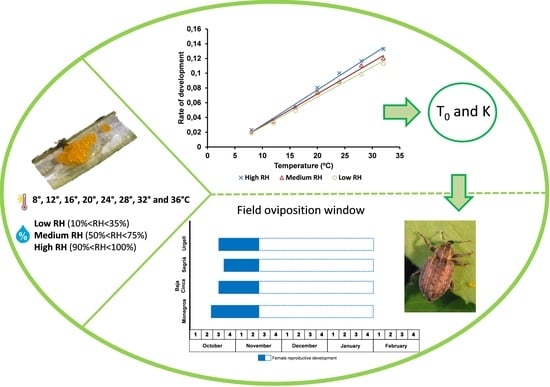
2.1. Laboratory Tests
2.2. Field Sampling
2.3. Data Analysis
3. Results
3.1. Survival
3.2. Developmental Time
3.3. External Egg Colour
3.4. Developmental Rate
3.5. Oviposition Window in the Ebro-Valley
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoffmann, A. Sous-famille des Curculionidae, Tribu des Hyperini, Les Hypera (syn: Phytonomus). In Entomologie Appliquée a L’agriculture. Tome I. Coléoptères. Second Volume; Balachowsky, A.S., Ed.; Masson et Cíe: Paris, France, 1963; pp. 984–989. [Google Scholar]
- Goosey, H.B. A degree-day model of sheep grazing influence on alfalfa weevil and crop characteristics. J. Econ. Entomol. 2012, 105, 102–112. [Google Scholar] [CrossRef]
- Saeidi, M.; Moharramipour, S. Physiology of Cold Hardiness, Seasonal Fluctuations, and Cryoprotectant Contents in Overwintering Adults of Hypera postica (Coleoptera: Curculionidae). Environ. Entomol. 2017, 46, 960–966. [Google Scholar] [CrossRef]
- Soroka, J.; Grenkow, L.; Cárcamo, H.; Meers, S.; Barkley, S.; Gavloski, J. An assessment of degree-day models to predict the phenology of alfalfa weevil (Coleoptera: Curculionidae) on the Canadian Prairies. Can. Entomol. 2019, 152, 110–129. [Google Scholar] [CrossRef]
- Pons, X.; Nuñez, E. Plagas da la alfalfa: Importancia, daños y estrategias de control. In La alfalfa, Agronomía y Utilización; Lloveras, J., Delgado, I., Chocarro, C., Eds.; Edicions de la Universitat de Lleida: Lleida, Spain, 2020; pp. 167–202. [Google Scholar]
- Pons, X.; Lumbierres, B.; Comas, J.; Madeira, F.; Starý, P. Effects of surrounding landscape on parasitism of alfalfa aphids in an IPM crop system in northern Catalonia. BioControl 2013, 58, 733–744. [Google Scholar] [CrossRef]
- Pons, X. Control Químico de Plagas de Alfalfa, Situación Actual y Perspectivas. Vida Rural 2019, 462, 48–52. [Google Scholar]
- Price, P.W. Insect Ecology, 3rd ed.; John Wiley and Sons: New York, NY, USA, 1997. [Google Scholar]
- Huffaker, C.B.; Berryman, P.; Turchin, A. Dynamics and regulation of insect populations. In Ecological Entomology; Huffaker, C.B., Gutierrez, A.P., Eds.; Wiley: New York, NY, USA, 1999; p. 776. [Google Scholar]
- Gillott, C. Entomology, 3rd ed.; Springer: Dordrecht, The Netherlands, 2005. [Google Scholar]
- García-Ruiz, E.; Marco, V.; Pérez-Moreno, I. Effects of Variable and Constant Temperatures on the Embryonic Development and Survival of a New Grape Pest, Xylotrechus arvicola (Coleoptera: Cerambycidae). Environ. Entomol. 2011, 40, 939–947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arbab, A.; Mcneill, M.R. Determining suitability of thermal development models to estimate temperature parameters for embryonic development of Sitona lepidus Gyll. (Coleoptera: Curculionidae). J. Pest Sci. 2011, 84, 303–311. [Google Scholar] [CrossRef]
- Lumbierres, B.; Madeira, F.; Roca, M.; Pons, X. Effects of temperature and diet on the development and reproduction of the ladybird Oenopia conglobata. Entomol. Gen. 2020. [Google Scholar] [CrossRef]
- Zahiri, B.; Fathipour, Y.; Khanjani, M.; Moharramipour, S.; Zalucki, M.P. Preimaginal development response to constant temperatures in Hypera postica (Coleoptera: Curculionidae): Picking the best model. Environ. Entomol. 2010, 39, 177–189. [Google Scholar] [CrossRef]
- Wiggleswort, V.B. The Principles of Insect Physiology, 7th ed.; Chapman and Hall: London, UK, 1972. [Google Scholar]
- Ofuya, T.I.; Reichmuth, C. Effect of relative humidity on the susceptibility of Callosobruchus maculatus (Fabricius) (Coleoptera: Bruchidae) to two modified atmospheres. J. Stored Prod. Res. 2001, 38, 139–146. [Google Scholar] [CrossRef]
- Aslam, A.; Jafir, M.; Wajid Javed, M.; Shehzad, M.; Zubair Chaudhary, M.; Aftab, M.; Muhammad Jafir, C. Effect of temperature and relative humidity on development of Sitophilus oryzae L. (coleoptera: Curculionidae). J. Entomol. Zool. Stud. 2017, 5, 85–90. [Google Scholar]
- Sweetman, H.L.; Wedemeyer, J. Further studies of the physical ecology of the alfafa weevil, Hypera Postica (Gyllenhal). Ecology 1933, 14, 46–60. [Google Scholar] [CrossRef]
- Sanaei, E.; Seiedy, M. Developmental differences of local populations of alfalfa weevil (Hypera Postica) (Coleoptera: Curculionidae). Turkish J. Zool. 2016, 40, 471–479. [Google Scholar] [CrossRef]
- Sanaei, E.; Husemann, M.; Seiedy, M.; Rethwisch, M.; Tuda, M.; Toshova, T.B.; Kim, M.J.; Atanasova, D.; Kim, I. Global genetic diversity, lineage distribution, and Wolbachia infection of the alfalfa weevil Hypera postica (Coleoptera: Curculionidae). Ecol. Evol. 2019, 9, 9546–9563. [Google Scholar] [CrossRef] [Green Version]
- Soroka, J.; Bennett, A.M.R.; Kora, C.; Schwarzfeld, M.D. Distribution of alfalfa weevil (Coleoptera: Curculionidae) and its parasitoids on the Canadian Prairies, with a key to described species of Nearctic Bathyplectes (Hymenoptera: Ichneumonidae). Can. Entomol. 2020, 152, 663–701. [Google Scholar] [CrossRef]
- Stark, J.A.; Berberet, R.C.; Cuperus, G.W. Mortality of overwintering eggs and larvae of the alfalfa weevil in Oklahoma. Environ. Entomol. 1994, 23, 35–40. [Google Scholar] [CrossRef]
- Roberts, S.J.; De Witt, J.R.; Armbrust, E.J. Predicting Spring hatch of the alfalfa weevil. J. Econ. Entomol. 1970, 63, 921–923. [Google Scholar] [CrossRef]
- Therneau, T.M. A Package for Survival Analysis in R. Available online: https://cran.r-project.org/web/packages/survival/index.html (accessed on 14 August 2020).
- Kassambara, A.; Kosinski, M.; Biecek, P. Survminer: Drawing Survival Curves Using “ggplot2”. Available online: https://cran.rproject.org/web/packages/survminer/survminer.pdf (accessed on 14 August 2020).
- Mendiburu, F.D. Agricolae: Statistical Procedures for Agricultural Research. Available online: http://cran.r-project.org/package=agricolae (accessed on 14 August 2020).
- Campbell, A.; Frazer, B.D.; Gilbert, N.; Gutierrez, A.P.; Mackauer, M. Temperature requirements of some aphids and their parasites. J. Appl. Ecol. 1974, 11, 431. [Google Scholar] [CrossRef]
- State Meteorological Agency AEMET. Available online: http://www.aemet.es/es (accessed on 5 October 2020).
- Levi-Mourao, A.; Meseguer, R.; Pons, X. Effect of temperature on the post-embryonic development and reproduction of the alfalfa weevil, Hypera postica Gyllenhal (Col.: Curculionidae). Unpublished, Manuscript in Preparation.
- Koehler, C.S.; Gyrisco, G.G. Responses of the alfalfa weevil, Hypera postica, to controlled environments. J. Econ. Entomol. 1961, 54, 625–627. [Google Scholar] [CrossRef]
- Guppy, J.C.; Mukerji, M.K. Effects of temperature on developmental rate of the immature stages of the alfalfa weevil, Hypera postica (Coleoptera: Curculionidae). Can. Entomol. 1974, 106, 93–100. [Google Scholar] [CrossRef]
- Guarneri, A.A.; Lazzari, C.; Xavier, A.A.P.; Diotaiuti, L.; Lorenzo, M.G. The effect of temperature on the behaviour and development of Triatoma brasiliensis. Physiol. Entomol. 2003, 28, 185–191. [Google Scholar] [CrossRef]
- Norhisham, A.R.; Abood, F.; Rita, M.; Hakeem, K.R. Effect of humidity on egg hatchability and reproductive biology of the bamboo borer (Dinoderus minutus Fabricius). Springerplus 2013, 2, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Lincoln, D.C.R. The oxygen and water requirements of the egg of Ocypus olens Müller (Staphylinidae, Coleoptera). J. Insect Physiol. 1961, 7, 265–272. [Google Scholar] [CrossRef]
- Prakash, M. Encyclopaedia of Entomology, 2nd ed.; Discovery Pub: Delhi, India, 2008. [Google Scholar]
- Guarneri, A.A.; Lazzari, C.; Diotaiuti, L.; Lorenzo, M.G. The effect of relative humidity on the behaviour and development of Triatoma brasiliensis. Physiol. Entomol. 2002, 27, 142–147. [Google Scholar] [CrossRef]
- Hirose, E.; Panizzi, A.R.; Cattelan, A.J. Effect of relative humidity on emergence and on dispersal and regrouping of first instar Nezara viridula (L.) (Hemiptera: Pentatomidae). Neotrop. Entomol. 2006, 35, 757–761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ewer, R.F. The biology and behaviour of Ptinus tectus bole (Coleoptera, Ptinidae), a pest of stored products: The effect of temperature and humidity on oviposition, feeding and duration of life cycle. J. Exp. Biol. 1942, 18, 290–305. [Google Scholar]
- Zrubek, B.; Woods, H.A. Insect eggs exert rapid control over an oxygen-water tradeoff. Proc. R. Soc. B Biol. Sci. 2006, 273, 831–834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morrison, W.P.; Pass, B.C. The Effect of Subthreshold Temperatures on Eggs of the Alfalfa Weevil. Environ. Entomol. 1974, 3, 353–355. [Google Scholar] [CrossRef]
- Shade, R.E.; Hintz, T.R. Factors Influencing Alfalfa Weevil (Coleoptera: Curculionidae) Egg Hatch and Larval Establishment1. Environ. Entomol. 1983, 12, 1129–1132. [Google Scholar] [CrossRef]
- Kishaba, A.N.; Henneberry, T.J. Effects of cold storage on egg viability of the cabbage looper and some aspects of the biology of the progeny survivors. J. Econ. Entomol. 1966, 59, 1169–1171. [Google Scholar] [CrossRef] [Green Version]
- Armbrust, E.J.; White, C.E.; Dewitt, J.R. Lethal Limits of Low Temperature for the Alfalfa Weevil in Illinois. J. Econ. Entomol. 1969, 62, 464–467. [Google Scholar] [CrossRef]
- Casagrande, R.A.; Stehr, F.W. Evaluating the effects of harvesting alfalfa on alfalfa weevil (coleoptera: Curculionidae) and parasite populations in michigan. Can. Entomol. 1973, 105, 1119–1128. [Google Scholar] [CrossRef]
- Dowdy, A.K.; Berberet, R.C.; Stritzke, J.F.; Caddel, J.L.; McNew, R.W. Late Fall Harvest, Winter Grazing, and Weed Control for Reduction of Alfalfa Weevil (Coleoptera: Curculionidae) Populations. J. Econ. Entomol. 1992, 85, 1946–1953. [Google Scholar] [CrossRef]
- GENCAT Servei Metereològic de Catalunya. Available online: https://www.meteo.cat (accessed on 15 October 2020).
- Cramer, W.; Guiot, J.; Fader, M.; Garrabou, J.; Gattuso, J.-P.; Iglesias, A.; Lange, M.A.; Lionello, P.; Lla-sat, M.C.; Paz, S.; et al. Climate change and interconnected risks to sustainable development in the Mediterranean. Nat. Clim. Chang. 2018, 8, 972–980. [Google Scholar] [CrossRef] [Green Version]
- Pellissier, M.E.; Nelson, Z.; Jabbour, R. Ecology and Management of the Alfalfa Weevil (Coleoptera: Curculionidae) in Western United States Alfalfa. J. Integr. Pest Manag. 2017, 8, 5. [Google Scholar] [CrossRef] [Green Version]





| RH Regimes | 8 °C | 12 °C | 16 °C | 20 °C | 24 °C | 28 °C | 32 °C | 36 °C | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| χ2 | df | p | χ2 | df | p | χ2 | df | p | χ2 | df | p | χ2 | df | p | χ2 | df | p | χ2 | df | p | χ2 | df | p | |
| 3 RH | 174 | 2 | <2 × 10−16 | 29.7 | 2 | 4 × 10−7 | 26.3 | 2 | 2.2 × 10−6 | 14.7 | 2 | 7 × 10−4 | 63.6 | 2 | 2 × 10−14 | 31.1 | 2 | 2 × 10−7 | 134 | <2 × 10−16 | 62.2 | 2 | 3 × 10−14 | |
| High vs. medium | 20.4 | 1 | 6 × 10−6 | 13.4 | 1 | 3 × 10−4 | 19.6 | 1 | 1 × 10−5 | 7.3 | 1 | 0.007 | 1.5 | 1 | 0.2 | 0.7 | 1 | 0.4 | 2 | 1 | 0.2 | 11.4 | 1 | 7 × 10−4 |
| High vs. low | 74.2 | 1 | <2 × 10−16 | 29.8 | 1 | 5 × 10−8 | 26.7 | 1 | 2 × 10−7 | 14.8 | 1 | 1 × 10−4 | 49.6 | 1 | 2 × 10−12 | 15.6 | 1 | 8 × 10−5 | 88.4 | 1 | <2 × 10−16 | 24.3 | 1 | 8 × 10−7 |
| Medium vs. low | 129 | 1 | <2 × 10−16 | 4.0 | 1 | 0.06 | 0.2 | 1 | 0.7 | 1.1 | 1 | 0.3 | 28.7 | 1 | 8 × 10−8 | 25.6 | 1 | 4 × 10−7 | 83.1 | 1 | <2 × 10−16 | 47.7 | 1 | 5 × 10−12 |
| RH | Parameters of the Linear Regression | T0 (°C) | K (DD) | ||||
|---|---|---|---|---|---|---|---|
| Coefficient (b) | Intercept (a) | F | p | R2 | |||
| High | 0.0048 | −0.0183 | 757.4 | <0.0001 | 0.9934 | 3.82 | 209.05 |
| Medium | 0.0043 | −0.0147 | 1019.3 | <0.0001 | 0.9907 | 3.40 | 230.83 |
| Low | 0.0041 | −0.0141 | 373.3 | <0.0001 | 0.9868 | 3.30 | 246.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Levi-Mourao, A.; Madeira, F.; Meseguer, R.; García, A.; Pons, X. Effects of Temperature and Relative Humidity on the Embryonic Development of Hypera postica Gyllenhal (Col.: Curculionidae). Insects 2021, 12, 250. https://0-doi-org.brum.beds.ac.uk/10.3390/insects12030250
Levi-Mourao A, Madeira F, Meseguer R, García A, Pons X. Effects of Temperature and Relative Humidity on the Embryonic Development of Hypera postica Gyllenhal (Col.: Curculionidae). Insects. 2021; 12(3):250. https://0-doi-org.brum.beds.ac.uk/10.3390/insects12030250
Chicago/Turabian StyleLevi-Mourao, Alexandre, Filipe Madeira, Roberto Meseguer, Addy García, and Xavier Pons. 2021. "Effects of Temperature and Relative Humidity on the Embryonic Development of Hypera postica Gyllenhal (Col.: Curculionidae)" Insects 12, no. 3: 250. https://0-doi-org.brum.beds.ac.uk/10.3390/insects12030250