Survey for Adventive Populations of the Samurai Wasp, Trissolcus japonicus (Hymenoptera: Scelionidae) in Pennsylvania at Commercial Fruit Orchards and the Surrounding Forest
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
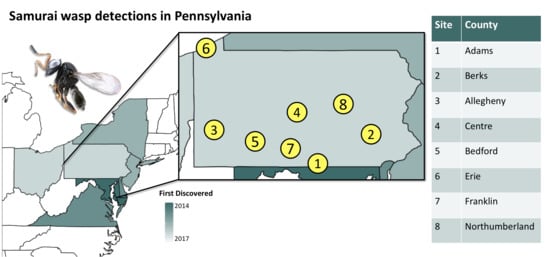
2.1. Survey Locations
2.2. Deployment of Yellow Sticky Cards (YSC)
2.3. Collection and Identification of Parasitoids
2.4. Statistical Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoebeke, E.R.; Carter, M.E. A polyphagous plant pest from Asia newly detected in North America. Proc. Entomol. Soc. Washingt. 2003, 105, 225–237. [Google Scholar]
- Rice, K.B.; Bergh, C.J.; Bergmann, E.J.; Biddinger, D.J.; Dieckhoff, C.; Dively, G.; Fraser, H.; Gariepy, T.; Hamilton, G.; Haye, T.; et al. Biology, ecology, and management of brown marmorated stink bug (Hemiptera: Pentatomidae). J. Integr. Pest Manag. 2014, 5, 1–13. [Google Scholar] [CrossRef]
- Lee, D.; Short, B.D.; Joseph, S.V.; Bergh, J.C.; Leskey, T.C. Review of the biology, ecology, and management of Halyomorpha halys (Hemiptera: Pentatomidae) in China, Japan, and the Republic of Korea. Environ. Entomol. 2013, 42, 627–641. [Google Scholar] [CrossRef]
- Arakawa, R.; Namura, Y. Effects of temperature on development of three Trissolcus spp. (Hymenoptera: Scelionidae), egg parasitoids of the brown marmorated stink bug, Halyomorpha halys (Hemiptera: Pentatomidae). Entomol. Sci. 2002, 5, 215–218. [Google Scholar]
- Talamas, E.J.; Johnson, N.F.; Buffington, M. Key to Nearctic species of Trissolcus Ashmead (Hymenoptera, Scelionidae), natural enemies of native and invasive stink bugs (Hemiptera, Pentatomidae). J. Hymenopt. Res. 2015, 43, 45–110. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.-Q.; Yao, Y.-X.; Qiu, L.-F.; Li, Z.-X. A new species of Trissolcus (Hymenoptera: Scelionidae) parasitizing eggs of Halyomorpha halys (Heteroptera: Pentatomidae) in China with comments on its biology. Ann. Entomol. Soc. Am. 2009, 102, 39–47. [Google Scholar] [CrossRef]
- Scaccini, D.; Falagiarda, M.; Tortorici, F.; Martinez-Sañudo, I.; Tirello, P.; Reyes-Domínguez, Y.; Gallmetzer, A.; Tavella, L.; Zandigiacomo, P.; Duso, C.; et al. An insight into the role of Trissolcus mitsukurii as biological control agent of Halyomorpha halys in Northeastern Italy. Insects 2020, 11, 306. [Google Scholar] [CrossRef] [PubMed]
- Botch, P.S.; Delfosse, E.S. Host-acceptance behavior of Trissolcus japonicus (Hymenoptera: Scelionidae) reared on the invasive Halyomorpha halys (Heteroptera: Pentatomidae) and nontarget species. Environ. Entomol. 2018, 47, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Milnes, J.M.; Beers, E.H. Trissolcus japonicus (Hymenoptera: Scelionidae) causes low levels of parasitism in three North American pentatomids under field conditions. J. Insect Sci. 2019, 19. [Google Scholar] [CrossRef] [Green Version]
- Lara, J.R.; Pickett, C.H.; Kamiyama, M.T.; Figueroa, S.; Romo, M.; Cabanas, C.; Bazurto, V.; Strode, V.; Briseno, K.; Lewis, M.; et al. Physiological host range of Trissolcus japonicus in relation to Halyomorpha halys and other pentatomids from California. BioControl 2019, 64, 513–528. [Google Scholar] [CrossRef]
- Abram, P.K.; Talamas, E.J.; Acheampong, S.; Mason, P.G.; Gariepy, T.D. First detection of the samurai wasp, Trissolcus japonicus (Ashmead) (Hymenoptera, Scelionidae), in Canada. J. Hymenopt. Res. 2019, 68, 29–36. [Google Scholar] [CrossRef] [Green Version]
- Hedstrom, C.; Lowenstein, D.; Andrews, H.; Bai, B.; Wiman, N. Pentatomid host suitability and the discovery of introduced populations of Trissolcus japonicus in Oregon. J. Pest Sci. 2017, 90, 1169–1179. [Google Scholar] [CrossRef]
- Talamas, E.; Herlihy, M.V.; Dieckhoff, C.; Hoelmer, K.A.; Buffington, M.; Bon, M.-C.; Weber, D.C. Trissolcus japonicus (Ashmead) (Hymenoptera, Scelionidae) emerges in North America. J. Hymenopt. Res. 2015, 43, 119–128. [Google Scholar] [CrossRef] [Green Version]
- Herlihy, M.V.; Talamas, E.J.; Weber, D.C. Attack and success of native and exotic parasitoids on eggs of Halyomorpha halys in three maryland habitats. PLoS ONE 2016, 11, e0150275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milnes, J.M.; Wiman, N.G.; Talamas, E.J.; Brunner, J.F.; Hoelmer, K.A.; Buffington, M.L.; Beers, E.H. Discovery of an exotic egg parasitoid of the brown marmorated stink bug, Halyomorpha halys (Stål) in the Pacific Northwest. Proc. Entomol. Soc. Washingt. 2016, 118, 466–470. [Google Scholar] [CrossRef]
- Stahl, J.; Tortorici, F.; Pontini, M.; Bon, M.-C.; Hoelmer, K.; Marazzi, C.; Tavella, L.; Haye, T. First discovery of adventive populations of Trissolcus japonicus in Europe. J. Pest Sci. 2019, 92, 371–379. [Google Scholar] [CrossRef] [Green Version]
- Jarrett, B.J.M.; Pote, J.; Talamas, E.; Gut, L.; Szucs, M. The Discovery of Trissolcus japonicus (Hymenoptera: Scelionidae) in Michigan. Gt. Lakes Entomol. 2019, 52, 6–11. [Google Scholar]
- Kaser, J.M.; Akotsen-Mensah, C.; Talamas, E.J.; Nielsen, A.L. First report of Trissolcus japonicus parasitizing Halyomorpha halys in North American agriculture. Fla. Entomol. 2018, 101, 680–683. [Google Scholar] [CrossRef]
- Quinn, N.F.; Talamas, E.J.; Acebes-Doria, A.L.; Leskey, T.C.; Bergh, J.C. Vertical sampling in tree canopies for Halyomorpha halys (Hemiptera: Pentatomidae) life stages and its egg parasitoid, Trissolcus japonicus (Hymenoptera: Scelionidae). Environ. Entomol. 2019, 48, 173–180. [Google Scholar] [CrossRef] [Green Version]
- Holthouse, M.; Schumm, Z.; Talamas, E.; Spears, L.; Alston, D. Surveys in northern Utah for egg parasitoids of Halyomorpha halys (Stål) (Hemiptera: Pentatomidae) detect Trissolcus japonicus (Ashmead) (Hymenoptera: Scelionidae). Biodivers. Data J. 2020, 8, e53363. [Google Scholar] [CrossRef]
- Sabbatini Peverieri, G.; Talamas, E.; Bon, M.C.; Marianelli, L.; Bernardinelli, I.; Malossini, G.; Benvenuto, L.; Roversi, P.F.; Hoelmer, K. Two Asian egg parasitoids of Halyomorpha halys (Stål) (Hemiptera, Pentatomidae) emerge in northern Italy: Trissolcus mitsukurii (Ashmead) and Trissolcus japonicus (Ashmead) (Hymenoptera, Scelionidae). J. Hymenopt. Res. 2018, 67, 37–53. [Google Scholar] [CrossRef]
- Quinn, N.F.; Talamas, E.J.; Leskey, T.C.; Bergh, J.C. Sampling methods for adventive Trissolcus japonicus (Hymenoptera: Scelionidae) in a wild tree host of Halyomorpha halys (Hemiptera: Pentatomidae). J. Econ. Entomol. 2019, 112, 1997–2000. [Google Scholar] [CrossRef] [Green Version]
- Lowenstein, D.M.; Andrews, H.; Hilton, R.J.; Kaiser, C.; Wiman, N.G. Establishment in an introduced range: Dispersal capacity and winter survival of Trissolcus japonicus, an adventive egg parasitoid. Insects 2019, 10, 443. [Google Scholar] [CrossRef] [Green Version]
- Tillman, G.; Toews, M.; Blaauw, B.; Sial, A.; Cottrell, T.; Talamas, E.; Buntin, D.; Joseph, S.; Balusu, R.; Fadamiro, H.; et al. Parasitism and predation of sentinel eggs of the invasive brown marmorated stink bug, Halyomorpha halys (Stål) (Hemiptera: Pentatomidae), in the southeastern US. Biol. Control 2020, 145, 104247. [Google Scholar] [CrossRef]
- Johnson, N.F. Systematics of Nearctic Telenomus: Classification and Revisions of the Podisi and Phymatae Species Groups (Hymenoptera: Scelionidae); College of Biological Sciences, Ohio State University: Columbus, OH, USA, 1984; pp. 24–77. [Google Scholar]
- R. Core Team. R: A Language and Environment for Statistical Computing; R Found. Stat. Comput.: Vienna, Austria, 2018; Available online: https//www.R-project.org/ (accessed on 9 February 2021).
- Lenth, R. Emmeans: Estimated Marginal Means, Aka Least-Squares Means. Available online: https://rdrr.io/cran/emmeans/man/emmeans-package.html (accessed on 9 February 2021).
- Todd, J.H.; Pearce, B.M.; Barratt, B.I.P. Using qualitative food webs to predict species at risk of indirect effects from a proposed biological control agent. BioControl 2021, 66, 45–58. [Google Scholar] [CrossRef]
- Lowenstein, D.M.; Andrews, H.; Mugica, A.; Wiman, N.G.; Nielsen, A. sensitivity of the egg parasitoid Trissolcus japonicus (Hymenoptera: Scelionidae) to field and laboratory-applied insecticide residue. J. Econ. Entomol. 2019, 112, 2077–2084. [Google Scholar] [CrossRef] [PubMed]
- Haye, T.; Moraglio, S.T.; Stahl, J.; Visentin, S.; Gregorio, T.; Tavella, L. Fundamental host range of Trissolcus japonicus in Europe. J. Pest Sci. 2020, 93, 171–182. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Zhang, F.; Gariepy, T.; Mason, P.; Gillespie, D.; Talamas, E.; Haye, T. Seasonal parasitism and host specificity of Trissolcus japonicus in northern China. J. Pest Sci. 2017, 90, 1127–1141. [Google Scholar] [CrossRef] [Green Version]



| Year | County (Location) | N (n) per Habitat | Tree Fruit with T. japonicus | ||
|---|---|---|---|---|---|
| Orchard | Forest Border | Forest | |||
| 2018 | Berks (1) | 13 (28) | 2 (29) | 0 (30) | Apple, Peach |
| Adams | — | 1 (18) | — | — | |
| Allegheny | — | 6 (12) | 0 (6) | — | |
| Centre | 19 (18) | — | — | Apple | |
| 2019 | Bedford | 0 (9) | 2 (12) | 0 (6) | — |
| Berks (1) | 5 (6) | 1 (4) | 0 (8) | Apple, Peach | |
| Northumberland | 2 (9) | 0 (9) | 0 (9) | Apple | |
| Erie | — | 2 (24) | — | — | |
| Franklin | 1 (9) | 0 (9) | 0 (9) | Apple | |
| Berks (2) | 5 (9) | 0 (8) | 0 (8) | Apple, Pear | |
| Lycoming | — | 0 (27) | — | — | |
| Allegheny | — | 9 (18) | 0 (9) | — | |
| Centre | 1 (8) | 0 (8) | 0 (9) | Apple | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peterson, H.M.; Talamas, E.; Krawczyk, G. Survey for Adventive Populations of the Samurai Wasp, Trissolcus japonicus (Hymenoptera: Scelionidae) in Pennsylvania at Commercial Fruit Orchards and the Surrounding Forest. Insects 2021, 12, 258. https://0-doi-org.brum.beds.ac.uk/10.3390/insects12030258
Peterson HM, Talamas E, Krawczyk G. Survey for Adventive Populations of the Samurai Wasp, Trissolcus japonicus (Hymenoptera: Scelionidae) in Pennsylvania at Commercial Fruit Orchards and the Surrounding Forest. Insects. 2021; 12(3):258. https://0-doi-org.brum.beds.ac.uk/10.3390/insects12030258
Chicago/Turabian StylePeterson, Hillary M., Elijah Talamas, and Grzegorz Krawczyk. 2021. "Survey for Adventive Populations of the Samurai Wasp, Trissolcus japonicus (Hymenoptera: Scelionidae) in Pennsylvania at Commercial Fruit Orchards and the Surrounding Forest" Insects 12, no. 3: 258. https://0-doi-org.brum.beds.ac.uk/10.3390/insects12030258