Beekeeping Genetic Resources and Retrieval of Honey Bee Apis mellifera L. Stock in the Russian Federation: A Review
Abstract
:Simple Summary
Abstract
1. Introduction
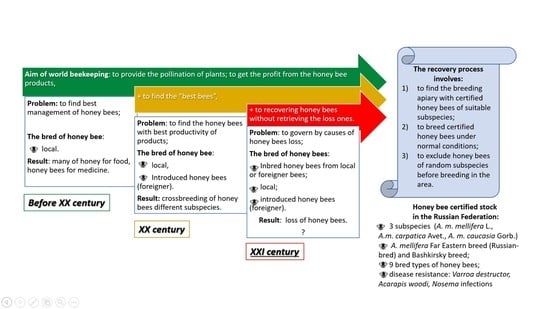
2. Beekeeping Practices in the Russian Federation
2.1. Purposes of Beekeeping: Breeding and Production
2.2. Conservation of A. mellifera L. Honey Bees
2.3. Evaluation of Honey Bees
3. Apis mellifera L. in the Russian Federation
3.1. Apis mellifera mellifera L.
3.2. Breeds and Breed Types of Apis mellifera mellifera L.
3.3. Apis mellifera carpatica Avet.
3.4. Breed Types of Apis mellifera carpatica
3.5. Apis mellifera caucasia Gorb.
3.6. Breed Types of Apis mellifera caucasia Gorb.
4. Honey Bees vs. Varroa and Tracheal Mites
4.1. Apis mellifera Far Eastern Breed (Russian-Bred) Honey Bees
4.2. Apis mellifera Far Eastern (American-Bred, Chinese-Bred) Honey Bees
5. Future Prospects for Beekeeping in the Russian Federation
- The development of modern methodologies for breeding bees, and the evaluation of bee colonies for monitoring the gene pool of honey bees in the Russian Federation.
- The development of tools for the artificial insemination of queen bees and cryopreservation of drone sperm to control the pure breeding and interbreeding of honey bees.
- The establishment of a cryogenic gene bank of local populations of honey bees for breeding in the Russian Federation.
- The conservation of original types and the development of new competitor types of honey bees, considering the needs of commercial beekeeping.
- The breeding of queen bees with highly productive crossbreeding lines for beekeeping commodities.
- The development of nomadic beekeeping regulations to prevent uncontrolled mating on apiaries in the area.
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schlipalius, D.; Ebert, P.R.; Hunt, G.J. Honeybee. In Genome Mapping and Genomics in Arthropods. Genome Mapping Genomics Animals, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 1–16. [Google Scholar] [CrossRef]
- Bommarco, R.; Marini, L.; Vaissière, B.E. Insect pollination enhances seed yield, quality, and market value in oilseed rape. Oecologia 2012, 169, 1025–1032. [Google Scholar] [CrossRef]
- Bradbear, N. Bees and their role in forest livelihoods: A Guide to the services provided by bees and the sustainable harvesting, processing and marketing of their products. Non-Wood For. Prod. 2009, 19, 194. [Google Scholar]
- Neumann, P.; Carreck, N.L. Honey bee colony losses. J. Apic. Res. 2010, 49, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Arundel, J.; Oxley, P.R.; Faiz, A.; Crawford, J.; Winter, S.; Oldroyd, B.P. Remarkable uniformity in the densities of feral honey bee Apis mellifera Linnaeus, 1758 (Hymenoptera: Apidae) colonies in South Eastern Australia. Austral. Entomol. 2014, 53, 328–336. [Google Scholar] [CrossRef]
- Berezin, A.S.; Borodachev, A.V.; Borodachev, V.A.; Mitrofanov, D.V.; Savushkina, L.N. The Loss of Taxonomic Biodiversity of Honey Bees Apis mellifera and Main Breeds in Russia. Phylogenetics Bees 2019, 144–177. [Google Scholar] [CrossRef]
- Bixby, M.; Hoover, S.E.; McCallum, R.; Ibrahim, A.; Ovinge, L.; Olmstead, S. Honey Bee Queen Production: Canadian Costing Case Study and Profitability Analysis. J. Econ. Entomol. 2020, 113, 1618–1627. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, D. Honey bee collapse strikes Japan, up to fifty percent of honey bees gone. Natural News, 28 April 2009.
- Haddad, N. Honey bee viruses, diseases and hive management in the Middle East and their relation to The Colony collapse disorder and bee losses. Uludağ Arıcılık Derg. 2011, 11, 17–24. [Google Scholar]
- Van Engelsdorp, D.; Caron, D.; Hayes, J.; Underwood, R.; Henson, M.; Rennich, K. A national survey of managed honey bee 2010–11 winter colony losses in the USA: Results from the Bee Informed Partnership. J. Apic. Res. 2012, 51, 115–124. [Google Scholar] [CrossRef] [Green Version]
- Borodachev, A.V.; Savushkina, L.N.; Borodachev, V.A. Preservation of biodiversity of Apis mellifera for use in selection. Biomics 2019, 147–157. [Google Scholar] [CrossRef]
- Core, A.; Runckel, C.; Ivers, J.; Quock, C.; Siapno, T.; Denault, S. A new threat to honey bees, the parasitic phorid fly Apocephalus borealis. PLoS ONE 2012, 7, e29639. [Google Scholar] [CrossRef] [Green Version]
- Ricchiuti, L.; Miranda, M.; Venti, R.; Bosi, F.; Marino, L.; Mutinelli, F. Infestation of Apis mellifera colonies by Megaselia scalaris (Loew, 1866) in Abruzzo and Molise regions, central-southern Italy. J. Apic. Res. 2016, 55, 187–192. [Google Scholar] [CrossRef] [Green Version]
- Cham, D.T.; Fombong, A.T.; Ndegwa, P.N.; Irungu, L.W.; Nguku, E.; Raina, S.K. Megaselia scalaris (Diptera: Phoridae), an opportunist parasitoid of honey bees in Cameroon. Afr. Entomol. 2018, 26, 254–258. [Google Scholar] [CrossRef]
- Menail, A.H.; Piot, N.; Meeus, I.; Smagghe, G.; Loucif-Ayad, W. Large pathogen screening reveals first report of Megaselia scalaris (Diptera: Phoridae) parasitizing Apis mellifera intermissa (Hymenoptera: Apidae). J. Invertebr. Pathol. 2016, 137, 33–37. [Google Scholar] [CrossRef]
- Debnath, P.; Roy, D. First Record of Megaselia scalaris (Loew) as a Potential Facultative Parasitoid of Apis mellifera in India. Asian J. Biol. 2019, 1–9. [Google Scholar] [CrossRef]
- Order “About Approval of the List of Quarantine Objects of December 15, 2014” N° 501, Federal Service for Veterinary and Phytosanitary Surveillance of Russian Federation. Available online: https://docs.cntd.ru/document/420245004 (accessed on 15 December 2014).
- Chaika, S. Forensic Entomology; MAX Press: Moscow, Russia, 2003; p. 60. ISBN 5-317-00764-X. (In Russian) [Google Scholar]
- Petukhov, A.V.; (PhD, Perm State Agro-Technological University named after Academician D.N. Pryanishnikov, Perm, Russia). Personal communication, 2021.
- Saltykova, E.S.; (Doctor of Science, Institute of Biochemistry and Genetics of the UNC RAS, Ufa, Russia). Personal communication, 2021.
- Voronin, V.; Tuhtabatov, I. Law enforcement practices of the Federal Service for Veterinary and Phytosanitary Surveillance of the Russia in field of veterinary (Regarding the Russian Federation Subjects). Agrar. Bull. 2019, 12, 49–58. [Google Scholar] [CrossRef]
- Meixner, M.D.; Büchler, R.; Costa, C.; Andonov, S.; Bieńkowska, M.; Bouga, M. Looking for the Best Bee. An Experiment about Interactions Between Origin and Environment of Honey Bee Strains in Europe. Am. Bee J. 2015, 155, 663–666. [Google Scholar]
- Antonova, N.E.; Volkov, L.V.; Sukhomirov, G.I. Bioresource Sector of the Russian Far East. Spat. Econ. Prostranstvennaya Ekon. 2017, 2, 110–137. [Google Scholar] [CrossRef] [Green Version]
- Byatt, M.A.; Chapman, N.C.; Latty, T.; Oldroyd, B.P. The genetic consequences of the anthropogenic movement of social bees. Insectes Soc. 2016, 63, 15–24. [Google Scholar] [CrossRef]
- Cane, J.H.; Schiffhauer, D. Pollinator genetics and pollination: Do honey bee colonies selected for pollen-hoarding field better pollinators of cranberry Varpon? Ecol. Entomol. 2001, 25, 1–7. [Google Scholar] [CrossRef]
- Cao, L.-F.; Zheng, H.-Q.; Pirk, C.W.W.; Hu, F.-L.; Xu, Z.-W. High Royal Jelly-Producing Honeybees (Apis mellifera ligustica) (Hymenoptera: Apidae) in China. J. Econ. Entomol. 2016, 109, 510–514. [Google Scholar] [CrossRef]
- Carreck, N.L.; Dietemann, V.; Neumann, P.; Ellis, J.D. The COLOSS BEEBOOK: Global standards in honey bee research. J. Apic. Res. 2020, 59, 1–4. [Google Scholar] [CrossRef]
- Chapman, N.C.; Harpur, B.A.; Lim, J.; Rinderer, T.E.; Allsopp, M.H.; Zayed, A. Hybrid origins of Australian honeybees (Apis mellifera). Apidologie 2016, 47, 26–34. [Google Scholar] [CrossRef] [Green Version]
- Chmiel, J.A.; Daisley, B.A.; Pitek, A.P.; Thompson, G.J.; Reid, G. Understanding the Effects of Sublethal Pesticide Exposure on Honey Bees: A Role for Probiotics as Mediators of Environmental Stress. Front. Ecol. Evol. 2020, 8, 22. [Google Scholar] [CrossRef] [Green Version]
- Khachaturov, G. Beekeeping in Russia today. Bee World 2010, 87, 30–31. [Google Scholar] [CrossRef]
- Giliba, R.A.; Mpinga, I.H.; Ndimuligo, S.A.; Mpanda, M.M. Changing climate patterns risk the spread of Varroa destructor infestation of African honey bees in Tanzania. Ecol. Process. 2020, 9, 1–11. [Google Scholar] [CrossRef]
- Vercelli, M.; Novelli, S.; Ferrazzi, P.; Lentini, G.; Ferracini, C. A Qualitative Analysis of Beekeepers’ Perceptions and Farm Management Adaptations to the Impact of Climate Change on Honey Bees. Insects 2021, 12, 228. [Google Scholar] [CrossRef]
- Cornuet, J.M.; Garnery, L. Mitochondrial DNA variability in honeybees and its phylogeographic implications. Apidologie 1991, 22, 627–642. [Google Scholar] [CrossRef] [Green Version]
- Larkina, E.O.; Svishchuk, D.V.; Lapynina, E.P. Influence of heavy metal accumulation on the state of honey bee populations on the example of the Ryazan region. In Contemporary Problems of Beekeeping and Apitherapy/Materials of the International Scientific and Practical Conference, 18 December 2021; FNC Beekeeping: Rybnoye, Russia, 2021; Volume 1, pp. 152–158. [Google Scholar] [CrossRef]
- Eskov, E.K.; Eskova, M.D. Reasons for autumn-winter gatherings of bee families. In Contemporary Problems of Beekeeping and Apitherapy/Materials of the International Scientific and Practical Conference, 18 December 2021; FNC Beekeeping: Rybnoye, Russia, 2021; Volume 1, pp. 85–89. [Google Scholar] [CrossRef]
- Berezin, A.S. The relationship of exterior signs, of productivity, of infestation and the hygienic behavior. In Contemporary Problems of Beekeeping and Apitherapy/Materials of the International Scientific and Practical Conference, 18 December 2021; FNC Beekeeping: Rybnoye, Russia, 2021; Volume 1, pp. 25–35. (In Russian) [Google Scholar] [CrossRef]
- Brandorf, A.Z.; Shestakova, A.I. Biotechnological methods of prevention and control of honey bee disease. In Contemporary Problems of Beekeeping and Apitherapy/Materials of the International Scientific and Practical Conference, 18 December 2021; FNC Beekeeping: Rybnoye, Russia, 2021; Volume 1, pp. 5–12. [Google Scholar] [CrossRef]
- Abdullabekova, U.B. Structural-typological description of beekeeping terms in the Kumyk, Russian and English languages. Philol. Sci. Res. 2021, 5, 81–88. [Google Scholar] [CrossRef]
- Alpatov, W.W. Porody Medonosnoi Pchely (Honey Bee Breeds); Mosk. O--vo Ispyt Prir: Moscow, Russia, 1948. [Google Scholar]
- Jaffé, R.; Moritz, R. Beekeeping and the Conservation of Native Honeybees in Europe. In Atlas of Biodiversity Risk; Publishing Team, Department of Conservation: Wellington, New Zealand, 2010; pp. 182–183. ISBN 978-954-642-446-4. [Google Scholar]
- Shabarshov, I. Russian Beekeeping; Agropromizdat: Moskow, Russia, 1990; p. 510. ISBN 5-10-001139-4. (In Russian) [Google Scholar]
- Ilyasov, R.A.; Kwon, H.W. Phylogenetics of Bees, 1st ed.; CRC Press Taylor & Francis: New York, NY, USA, 2019; p. 290. ISBN 978-113-850-423-3. [Google Scholar]
- Bourgeois, L.; Sheppard, W.S.; Sylvester, H.A.; Rinderer, T.E. Genetic stock identification of Russian honey bees. J. Econ. Entomol. 2010, 103, 917–924. [Google Scholar] [CrossRef]
- de Grandi-Hoffman, G.; Ahumada, F.; Danka, R.; Chambers, M.; DeJong, E.W.; Hidalgo, G. Population Growth of Varroa destructor (Acari: Varroidae) in Colonies of Russian and Unselected Honey Bee (Hymenoptera: Apidae) Stocks as Related to Numbers of Foragers with Mites. J. Econ. Entomol. 2017, 110, 809–815. [Google Scholar] [CrossRef]
- de Guzman, L.I.; Rinderer, T.E.; Stelzer, J.A.; Beaman, L.; Delatte, G.T.; Harper, C. Hygienic behavior by honey bees from far-eastern Russia. Am. Bee J. 2002, 142, 58–60. [Google Scholar]
- Genersch, E.; Evans, J.D.; Fries, I. Honey bee disease overview. J. Invertebr. Pathol. 2010, 103, S2–S4. [Google Scholar] [CrossRef] [PubMed]
- Goulson, D. Effects of Introduced Bees on Native Ecosystems. Annu. Rev. Ecol. Evol. Syst. 2002, 34, 1–26. [Google Scholar] [CrossRef] [Green Version]
- de Guzman, L.I.; Rinderer, T.E.; Frake, A.M. Comparative reproduction of Varroa destructor in different types of Russian and Italian honey bee combs. Exp. Appl. Acarol. 2008, 44, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Le Conte, Y.; Meixner, M.D.; Brandt, A.; Carreck, N.L.; Costa, C.; Mondet, F. Geographical Distribution and Selection of European Honey Bees Resistant to Varroa destructor. Insects 2020, 11, 873. [Google Scholar] [CrossRef] [PubMed]
- Sharov, M.A. Swarming and swarm ability of honey bee of the Far Eastern breed in the conditions of Primorsky Krai. Vestn. Far East Branch Russ. Acad. Sci. 2021, 3, 81–84. [Google Scholar] [CrossRef]
- Unger, P.; Guzmán-novoa, E. Maternal effects on the hygienic behavior of Russian x Ontario hybrid honeybees (Apis mellifera L.). J. Hered. 2010, 101, 91–96. [Google Scholar] [CrossRef]
- Wang, J.; Xue, Y.; Zhang, F.; Jin, S.; Li, X. Morphometric Analysis of Far East Blank Bee Based on 13 Indexes of Morphometry. Hans J. Agric. Sci. 2014, 4, 87–98. [Google Scholar] [CrossRef]
- de Guzman, L.I.; Rinderer, T.E.; Bigalk, M.; Tubbs, H.; Bernard, S.J. Russian Honey Bee (Hymenoptera: Apidae) Colonies: Acarapis woodi (Acari: Tarsonemidae) Infestations and Overwintering Survival. J. Econ. Entomol. 2005, 98, 1796–1801. [Google Scholar] [CrossRef] [PubMed]
- Rinderer, T.E.; de Guzman, L.I.; Harris, J.; Kuznetsov, V.; Delatte, G.T.; Stelzer, J.A. The release of ARS Russian honey bees. Am. Bee J. 2000, 140, 305–307. [Google Scholar]
- Perminov, A.S. Technological aspects of the Russian beekeeping. In Contemporary Problems of Beekeeping and Apitherapy/Materials of the International Scientific and Practical Conference 18 December 2021; FNC Beekeeping: Rybnoye, Russia, 2021; Volume 1, pp. 5–12. [Google Scholar] [CrossRef]
- Murylev, A.V.; Petukhov, A.V. Habitat of a Prikamskiy Honeybee Population. Achiev. Life Sci. 2014, 5, 75–77. [Google Scholar] [CrossRef] [Green Version]
- Krestov, P.V. Forest Vegetation of Easternmost Russia (Russian Far East). In Forest Vegetation of Northeast Asia; Springer: Dordrecht, The Netherlands, 2003; pp. 93–180. ISBN 978-94-017-0143-3. [Google Scholar] [CrossRef]
- Zadvornyi, A.S. Network structures in the development of entrepreneurial practices in beekeeping of Primorsky Krai of the late XIX–early XX centuries. Theor. Appl. Econ. 2020, 1, 80–97. [Google Scholar] [CrossRef]
- Ilyasov, R.A.; Han, G.Y.; Lee, M.L.; Kim, K.W.; Proshchalykin, M.Y.; Lelej, A.S. Genetic Properties and Evolution of Asian Honey Bee Apis cerana ussuriensis from Primorsky Krai, Russia. Russ. J. Genet. 2021, 57, 568–581. [Google Scholar] [CrossRef]
- Krivtsov, N.I. Breed zoning and “best bees” in Russia. Beejournal 2003, 1, 18–20. (In Russian) [Google Scholar]
- Ostroverkhova, N.V.; Konusova, O.L.; Kucher, A.N.; Sharakhov, I.V. A comprehensive characterization of the honeybees in Siberia (Russia). In Beekeeping and Bee Conservation—Advances in Research; InTech: Rijeka, Croatia, 2016; Volume 10, pp. 1–37. ISBN 978-953-51-2411-5. [Google Scholar] [CrossRef] [Green Version]
- Ilyasov, R.A.; Poskryakov, A.V.; Petukhov, A.V.; Nikolenko, A.G. Molecular genetic analysis of five extant reserves of black honeybee Apis melifera melifera in the Urals and the Volga region. Genetika 2016, 52, 931–942. [Google Scholar] [CrossRef]
- Shklyaev, A.S.; Balkov, V.A. Klimat Permskoy Oblasti. Climate of the Perm Region; Izdatel’stvo Permskogo gosudarstvennogo universiteta: Perm, Russia, 1963; p. 189. (In Russian) [Google Scholar]
- Murphy, C.; Robertson, A.W. Preliminary Study of the Effects of Honey Bees (Apis Mellifera) in Tongariro National Park; Department of Conservation: Wellington, New Zealand, 2000; p. 139. ISBN 0-478-21939-3. [Google Scholar]
- Matthews, J.K.; Ridley, A.; Niyigaba, P.; Kaplin, B.A.; Grueter, C.C. Chimpanzee feeding ecology and fallback food use in the montane forest of Nyungwe National Park, Rwanda. Am. J. Primatol. 2019, 81, e22971. [Google Scholar] [CrossRef]
- Beard, C. Honeybees (Apis Mellifera) on Public Conservation Lands; Department of Conservation: Wellington, New Zealand, 2015; p. 25. ISBN 978-0-478-15054-4. [Google Scholar]
- Miguel, I.; Garnery, L.; Iriondo, M.; Baylac, M.; Manzano, C.; Steve Sheppard, W. Origin, evolution and conservation of the honey bees from La Palma Island (Canary Islands): Molecular and morphological data. J. Apic. Res. 2015, 54, 427–440. [Google Scholar] [CrossRef]
- Berezin, A.S. Methods of morphometry in determining the breed of honey bees. Biomics 2019, 11, 167–189. [Google Scholar] [CrossRef]
- del Lama, M.A.; Lobo, J.A.; Soares, A.E.E.; del Lama, S.N. Genetic differentiation estimated by isozymic analysis of Africanized honeybee populations from Brazil and from Central America. Apidologie 1990, 21, 271–280. [Google Scholar] [CrossRef] [Green Version]
- Evans, J.D.; Schwarz, R.S.; Chen, Y.P.; Budge, G.; Cornman, R.S.; De la Rua, P. Standard methods for molecular research in Apis mellifera. J. Apic. Res. 2013, 52, 1–54. [Google Scholar] [CrossRef] [Green Version]
- Hartfelder, K.; Bitondi, M.M.G.; Brent, C.S.; Guidugli-Lazzarini, K.R.; Simões, Z.L.P.; Stabentheiner, A. Standard methods for physiology and biochemistry research in Apis mellifera. J. Apic. Res. 2013, 52, 1–48. [Google Scholar] [CrossRef] [Green Version]
- Ivanova, E.N.; Bienkowska, M.; Petrov, P.P. Allozyme Polymorphism and Phylogenetic Relationships in Apis mellifera Subspecies Selectively Reared in Poland and Bulgaria. Folia Biologica 2011, 59, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Lebedinskiy, I.A.; Lavrskiy, A.Y.; Petukhov, A.V. Changes of rectal pads parameters, as a manifestation of adaptive mechanisms in the life cycle of the bee family Apis mellifera L. Izv. Samar. Univ. 2014, 5, 594–598. (In Russian) [Google Scholar]
- Saltykova, E.S.; Lvov, A.V.; Ben’kovskaya, G.V.; Poskryakov, A.V.; Nikolenko, A.G. Interracial Differences in Expression of Genes of Antibacterial Peptides, Abaecin, Hymenoptaecin, and Defensin, in Bees Apis mellifera mellifera and Apis mellifera caucasia. J. Evol. Biochem. Physiol. 2005, 41, 506–510. [Google Scholar] [CrossRef]
- Nepeivoda, S.N.; Kolbina, L.M. The flight of bees in the small-leaved lime blooming period. In Proceedings of the Materily of Konferenci XLVI Naukowa Konferencja Pszczelarska, 10–11 March 2009; Instytut Sadownictwa i Kwiaciarstwwa: Pullawy, Poland, 2009; Volume 1, pp. 100–101. [Google Scholar]
- Scheiner, R.; Abramson, C.I.; Brodschneider, R.; Crailsheim, K.; Farina, W.M.; Fuchs, S. Standard methods for behavioral studies of Apis mellifera. J. Apic. Res. 2013, 52, 1–58. [Google Scholar] [CrossRef]
- Almeida-Muradian, L.B.; Barth, O.M.; Dietemann, V.; Eyer, M.; de Freitas, A.S. Standard methods for Apis mellifera honey research. J. Apic. Res. 2020, 59, 1–62. [Google Scholar] [CrossRef]
- Edriss, M.A.; Mostajeran, M.; Ebadi, R. Correlation between honey yield and morphological traits of honey bee in Isfahan. JWSS Isfahan Univ. Technol. 2002, 6, 91–103. [Google Scholar]
- Hu, F.-L.; Bíliková, K.; Casabianca, H.; Daniele, G.; Salmen Espindola, F.; Feng, M. Standard methods for Apis mellifera royal jelly research. J. Apic. Res. 2019, 58, 1–68. [Google Scholar] [CrossRef] [Green Version]
- Szabó, R.T.; Mézes, M.; Szalai, T.; Zajácz, E.; Weber, M. Color identification of honey and methodical development of its instrumental measuring. Columella 2016, 3, 29–36. [Google Scholar] [CrossRef]
- Brandorf, A.Z.; Ivoilova, M.M. Morphogenetic markers of honey bees producing royal jelly with a high content of 10-HDA. Agric. Sci. Euro-North-East 2019, 20, 283–289. [Google Scholar] [CrossRef]
- Brandorf, A.Z.; Ivoilova, M.M. The length of the proboscis by honeybees-pollinators of red clover. In Proceedings of the Materily of Konferenci XLVI Naukowa Konferencja Pszczelarska, 10–11 March 2009; Instytut Sadownictwa i Kwiaciarstwwa: Pullawy, Poland, 2009; Volume 1, pp. 30–31. [Google Scholar]
- Carreck, N.L.; Andree, M.; Brent, C.S.; Cox-Foster, D.; Dade, H.A.; Ellis, J.D. Standard methods for Apis mellifera anatomy and dissection. J. Apic. Res. 2013, 52, 1–40. [Google Scholar] [CrossRef] [Green Version]
- Simankov, M.K.; Likhachev, S.V. Ecological indicator of consequences of southern races Apis mellifera L. introduction to northern regions. Univ. Proc. Volga Reg. Nat. Sci. 2020, 1, 77–85. [Google Scholar] [CrossRef]
- Yeskov, E.K.; Yeskova, M.D. Geographic variability of the number of hooks and length of honey bee (Apis mellifera L.) wings. Russ. Agric. Sci. 2015, 41, 53–54. [Google Scholar] [CrossRef]
- Krivtsov, N.I.; Goryacheva, I.I.; Borodachev, A.V. Investigation of honey bee (Apis mellifera L.) breeds and populations for developing criteria for genetic certification of bees. Russ. Agric. Sci. 2011, 37, 79–82. [Google Scholar] [CrossRef]
- Pozdnyakov, V.N.; Kapakov, V.T.; Abramova, A.B.; Borodachev, A.V.; Krivtsov, N.I. Random Amplified Polymorphic DNA (RAPD) markers of three breed of honey bee Apis mellifera L. Dokl. Biol. Sci. 2000, 372, 309–311. [Google Scholar] [PubMed]
- Suppasat, T. Genetic Relationships between Two Honey Bees (Apis Mellifera Linnaeus, 1758 and Apis Cerana Fabricius, 1753) and Varroa Mites in Thailand; Chulalongkorn University: Chulalongkorn, Thiland, 2007; p. 98. [Google Scholar] [CrossRef]
- Syromyatnikov, M.Y.; Borodachev, A.V.; Kokina, A.V.; Popov, V.N. A Molecular Method for the Identification of Honey Bee Subspecies Used by Beekeepers in Russia. Insects 2018, 9, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirpik, M.A.; Bututaki, O.; Tanrikulu, D. Determining the relative abundance of honey bee (Apis mellifera L.) races in Kars plateau and evaluating some of their characteristics. Kafkas. Univ. Vet. Fak. Derg. 2010, 16, 27–282. [Google Scholar]
- Kirrane, M.J.; de Guzman, L.I.; Holloway, B.; Frake, A.M.; Rinderer, T.E.; Whelan, P.M. Phenotypic and genetic analyses of the Varroa sensitive hygienic trait in Russian honey bee (Hymenoptera: Apidae) colonies. PLoS ONE 2014, 10, e0116672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Page, R.E.; Kerr, W.E. Honey Bee Genetics and Breeding. In The “African” Honey Bee, 1st ed.; CRC Press: Boca Raton, FL, USA, 1981; p. 30. ISBN 9780429308741. [Google Scholar] [CrossRef]
- Sammataro, D.; Yoder, J.A. Honey Bee Colony Health: Challenges and Sustainable Solutions. Fla. Entomol. 2012, 95, 242–243. [Google Scholar] [CrossRef]
- Sperandio, G.; Simonetto, A.; Carnesecchi, E.; Costa, C.; Hatjina, F.; Tosi, S. Beekeeping and honey bee colony health: A review and conceptualization of beekeeping management practices implemented in Europe. Sci. Total Environ. 2019, 696, 1–9. [Google Scholar] [CrossRef]
- Gaga, V.A.; Esaulov, V.N. Innovative Technologies and Modern Facilities in Beekeeping. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Yurga, Russia, 19–21 May 2016; Volume 142, p. 012022. [Google Scholar] [CrossRef]
- Jandt, J.M.; Gordon, D.M. The behavioral ecology of variation in social insects. Curr. Opin. Insect Sci. 2016, 15, 40–44. [Google Scholar] [CrossRef] [Green Version]
- Alpatov, W.W. Biometrical Studies on Variation and Races of the Honey Bee (Apis mellifera L.). Q. Rev. Biol. 1929, 4. [Google Scholar] [CrossRef]
- de Souza, D.A.; Kaftanoglu, O.; De Jong, D.; Page, R.E.; Amdam, G.V.; Wang, Y. Differences in the morphology, physiology and gene expression of honey bee queens and workers reared in vitro versus in situ. Biol. Open 2018, 7, 1–29. [Google Scholar] [CrossRef] [Green Version]
- Ruttner, F. Morphometric Analysis and Classification. In Biogeography and Taxonomy of Honeybees; Springer: Berlin/Heidelberg, Germany, 1988; pp. 66–78. [Google Scholar] [CrossRef]
- Alpatov, W.W. The races of honey bees and their use in agriculture. Sredi Prir. 1948, 4, 1–65. [Google Scholar]
- Suppasat, T.; Wongsiri, S. Morphometric and Genetic Variation of Tropilaelaps Mites Infesting Apis dorsata and A. mellifera in Thailand. J. Apic. 2018, 33, 227–237. [Google Scholar] [CrossRef]
- Szymula, J.; Skowronek, W.; Bieńkowska, M. Use of various morphological traits measured by microscope or by computer methods in the honeybee taxonomy. J. Apic. Sir. 2010, 54, 91–97. [Google Scholar]
- Krivtsov, N.I. Variability and heritability of economically useful characters of mid-Russian bees. Sel’skokhozyaistvennaya Biol. 1980, 15, 148–151. (In Russian) [Google Scholar]
- Fornara, M.S.; Kramarenko, A.S.; Svistunov, S.V.; Lyubimov, E.M.; Sokolovskii, S.S.; Zinovieva, N.A. Morphometric and molecular genetic differenciation of Apis mellifera caucasia Gorb. honey bee lines reared in Sochi region. Agric. Biol. 2015, 50, 776–784. (In Russian) [Google Scholar] [CrossRef]
- Konusova, O.L.; Ostroverkhova, N.V.; Kucher, A.N.; Kurbatskij, D.V.; Kireeva, T.N. Morphometric variability of honeybees Apis mellifera L., differing in variants of the COI-COII mtDNA locus. Vestn. Tomsk. Gos. Univ. Biol. 2016, 1, 62–81. [Google Scholar] [CrossRef] [PubMed]
- Monachova, M.A.; Goryacheva, I.I.; Krivtsov, N.I. Genetic of honey bee Apis mellifera L. Beejournal 2007, 4, 14–17. (In Russian) [Google Scholar]
- Mostajeran, M.A.; Edriss, M.A.; Basiri, M.R. Analysis of colony and morphological characters in honey bees (Apis mellifera meda). Pak. J. Biol. Sci. 2006, 9, 2685–2688. [Google Scholar] [CrossRef]
- Szabo, T.I. Honeybee Induced Hive Entrance Defrosting. J. Apic.Res. 1987, 27, 115–121. [Google Scholar] [CrossRef]
- Waddington, K.D. Implications of variation in worker body size for the honey bee recruitment system. J. Insect Behav. 1989, 2, 91–103. [Google Scholar] [CrossRef]
- Azikaev, M.G.; Galin, R.R.; Ishkulov, A.A.; Asylguzhin, G.R. To the question of variability of morphometric indicators of Burzyansky bee in the reserve “Shulgan-Tash”. In Modern Problems of Beekeeping and Apiterapy; FNC Beekeeping: Rybnoye, Russia, 2019; pp. 21–26. ISBN 978-5-900205-72-4. [Google Scholar]
- Krivtsov, N.I. Apicultural in Russia: State and place in the world. Achiev. Sci. Technol. AIC 2011, 9, 15–16. (In Russian) [Google Scholar]
- Mannapov, A.; Moskovskaya, N. Influence of adaptogens on some physiological indicators of bee colonies. Feeding of agricultural Anim. Feed. Prod. 2020, 9, 67–75. [Google Scholar] [CrossRef]
- Kolmes, S.A.; Sam, Y. Relationships between Sizes of Morphological Features in Worker Honey Bees (Apis mellifera). J. N. Y. Entomol. Soc. 1991, 99, 684–690. [Google Scholar]
- Milne, C.P.; Pries, K.J. Honeybee Corbicular Size and Honey Production. J. Apic. Res. 1984, 23, 11–14. [Google Scholar] [CrossRef]
- Il’yasov, R.A.; Petukhov, A.V.; Poskryakov, A.V.; Nikolenko, A.G. Local honeybee (Apis mellifera mellifera L.) populations in the Urals. Russ. J. Genet. 2007, 43, 709–711. [Google Scholar] [CrossRef]
- Ilyasov, R.A.; Poskryakov, A.V.; Nikolenko, A.G. Modern methods of assessing the taxonomic affiliation of honeybee colonies. Ecol. Genet. 2017, 4, 41–51. [Google Scholar] [CrossRef] [Green Version]
- Kaskinova, M.D.; Gaifullina, L.R.; Saltykova, E.S.; Poskryakov, A.V.; Nikolenko, A.G. Genetic markers for the resistance of honey bee to Varroa destructor. Vavilov J. Genet. Breed. 2020, 24, 853–860. [Google Scholar] [CrossRef]
- Kalashnikov, A.E.; Udina, I.G. Distribution of rna-containing bee viruses in honey bee (Apis mellifera) in several regions of Russia. Mol. Genet. Microbiol. Virol. 2017, 35, 31–35. (In Russian) [Google Scholar] [CrossRef]
- Zinovieva, N.A.; Krivtsov, N.I.; Fornara, M.S.; Gladyr’, E.A.; Borodachev, A.V.; Berezin, S.A. Microsatellites as a tool for evaluation of allele pool dynamics when creation of Prioksky type of Middle Russian honey bee. Agric. Biol. 2011, 6, 75–79. [Google Scholar]
- Zinovieva, N.A.; Soloshenko, V.A.; Fornara, M.S.; Shatokhin, K.S.; Kharchenko, G.I.; Borodachev, A.V. Genetic differentiation of the Novosibirsk population of Primorsky honey bee. Russ. Agric. Sci. 2013, 39, 346–349. [Google Scholar] [CrossRef]
- Papachristoforou, A.; Rortais, A.; Bouga, M.; Arnold, G.; Garnery, L. Genetic characterization of the cyprian honey bee (Apis mellifera cypria) based on microsatellites and mitochondrial DNA polymorphisms. J. Apic. Sci. 2013, 57, 127–134. [Google Scholar] [CrossRef] [Green Version]
- Kandemir, I.; Meixner, M.D.; Ozkan, A.; Sheppard, W.S. Genetic characterization of honey bee (Apis mellifera cypria) populations in Northern Cyprus. Apidologie 2006, 37, 547–555. [Google Scholar] [CrossRef] [Green Version]
- Kandemir, İ.; Özkan, A.; Fuchs, S. Re-evaluation of honeybee (Apis mellifera) microtaxonomy: A geometric morphometric approach. Apidologie 2011, 42, 618–627. [Google Scholar] [CrossRef] [Green Version]
- Kekeçoğlu, M. Morphometric Divergence of Anatolian Honeybees through Loss of Original Traits: A Dangerous Outcome of Turkish Apiculture. Sociobiology 2018, 65, 232. [Google Scholar] [CrossRef]
- Sheppard, W.S.; Berlocher, S.H. Allozyme variation and differentiation among four Apis species. Apidologie 1989, 20, 419–431. [Google Scholar] [CrossRef] [Green Version]
- Gemeda, M.; Legesse, G.; Damto, T.; Kebaba, D. Harvesting Royal Jelly Using Splitting and Grafting Queen Rearing Methods in Ethiopia. Bee World 2020, 97, 114–116. [Google Scholar] [CrossRef]
- Hussein, M.H. A review of beekeeping in Arab countries. Bee World 2015, 81, 56–71. [Google Scholar] [CrossRef]
- Nikonorov, I.M.; Ben’kovskaia, G.V.; Poskriakov, A.V.; Nikolenko, A.G.; Vakhitov, V.A. Use of a PCR method for controlling pure-breeding of honeybees Apis mellifera mellifera L. in the southern Urals. Genetika 1998, 34, 1574–1577. [Google Scholar] [PubMed]
- Ilyasov, R.A.; Kosarev, M.N.; Neal, A.; Yumaguzhin, F.G. Burzyan Wild-Hive Honeybee A. m. mellifera in South Ural. Bee World 2015, 47, 7–11. [Google Scholar] [CrossRef]
- Peng, Y.-S.; Fang, Y.; Xu, S.; Ge, L. The resistance mechanism of the Asian honey bee, Apis cerana Fabr., to an ectoparasitic mite, Varroa jacobsoni Oudemans. J. Invertebr. Pathol. 1987, 49, 54–60. [Google Scholar] [CrossRef]
- Sumpter, D.J.T.; Martin, S.J. The dynamics of virus epidemics in Varroa-infested honey bee colonies. J. Anim. Ecol. 2004, 73, 51–63. [Google Scholar] [CrossRef]
- Guichard, M.; Dietemann, V.; Neuditschko, M.; Dainat, B. Three decades of selecting honey bees that survive infestations by the parasitic mite Varroa destructor: Outcomes, limitations and strategy. Preprints 2020, 1, 1–84. [Google Scholar] [CrossRef]
- Francis, R.M.; Nielsen, S.L.; Kryger, P. Varroa-virus interaction in collapsing honey bee colonies. PLoS ONE 2013, 8, e57540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kraus, B.; Page, R.E. Effect of Varroa jacobsoni (Mesostigmata: Varroidae) on Feral Apis mellifera (Hymenoptera: Apidae) in California. Environ. Entomol. 1995, 24, 1473–1480. [Google Scholar] [CrossRef]
- Rinderer, T.; de Guzman, L.; Sylvester, H.A. Re-examination of the accuracy of a detergent solution for Varroa mite detection. Am. Bee J. 2004, 144, 560–562. [Google Scholar]
- Van der Zee, R.; Pisa, L.; Andonov, S.; Brodschneider, R.; Charrière, J.-D.; Chlebo, R. Managed honey bee colony losses in Canada, China, Europe, Israel and Turkey, for the winters of 2008–2009 and 2009–2010. J. Apic. Res. 2012, 51, 100–114. [Google Scholar] [CrossRef]
- Van Engelsdorp, D.; Hayes, J.J.; Underwood, R.M.; Pettis, J. A survey of honey bee colony losses in the U.S., fall 2007 to spring 2008. PLoS ONE 2008, 3, e4071. [Google Scholar] [CrossRef]
- Maggi, M.; Antúnez, K.; Invernizzi, C.; Aldea, P.; Vargas, M.; Negri, P. Honeybee health in South America. Apidologie 2016, 47, 835–854. [Google Scholar] [CrossRef] [Green Version]
- Martin, S.J.; Kryger, P. Imparct of Varroa destructor on the honey bees of South Africa. In Proceedings of the Conference Proceedings Apimondia, Durban, South Africa, 28 October–1 November 2001; Volume 1, p. 174. [Google Scholar]
- Mendoza, Y.; Tomasco, I.H.; Antúnez, K.; Castelli, L.; Branchiccela, B.; Santos, E. Unraveling Honey Bee-Varroa destructor Interaction: Multiple Factors Involved in Differential Resistance between Two Uruguayan Populations. Vet. Sci. 2020, 7, 116. [Google Scholar] [CrossRef]
- Danka, R.G.; Villa, J.D. Evidence of auto grooming as a mechanism of honey bee resistance to tracheal mite infestation. J. Apic. Res. 1998, 37, 39–46. [Google Scholar] [CrossRef]
- Hawkins, G.P.; Martin, S.J. Elevated recapping behavior and reduced Varroa destructor reproduction in natural Varroa resistant Apis mellifera honey bees from the UK. Apidologie 2021, 52, 647–657. [Google Scholar] [CrossRef]
- Simonov, E.; Egidarev, E. Intergovernmental cooperation on the Amur River basin management in the twenty-first century. Int. J. Water Resour. Dev. 2018, 34, 771–791. [Google Scholar] [CrossRef]
- Rinderer, T.E.; Kuznetsov, V.N.; Danka, R.G.; Delatte, G.T. An importation of potentially Varroa-resistant honey bees from far-eastern Russia. Am. Bee J. 1997, 137, 787–790. [Google Scholar]
- Villa, J.D.; Rinderer, T.E.; Bigalk, M. Overwintering of Russian honey bees in northeastern Iowa. Sci. Bee Cult. 2009, 1, 19–21. [Google Scholar]
- Codes, L.G.; Popova, B.D. Breeds of Far Eastern Bees. Beejournal 2016, 7, 12–13. (In Russian) [Google Scholar]
- Domènech, R.; Tracy, E.; Rovira, M.; Lepeshkin, E. Beekeeping in Primorsky Province: Challenges and Opportunities: A Needs Assessment; Forest Science and Technology Centre of Catalonia (CTFC) and WWF Russia: Solsona, Spain; Moscow, Russia, 2019; p. 52. [Google Scholar] [CrossRef]
- Tracy, E.F. Virtuous Bees: How Beekeeping Helps Protect Primary Forests in the Russian Far East; Rachel Carson Center for Environment and Society: Arcadia, CA, USA, 2020; Volume 11, pp. 1–7. ISSN 2199-3408. [Google Scholar]
- Tubbs, H.; Harper, C.; Bigalk, M.; Bernard, S.J. Commercial management of ARS Russian honey bees. Am. Bee J. 2003, 143, 819–820. [Google Scholar]






| Breed, Type | Length of Proboscis, mm M ± SE | Width of Third Tergite, mm M ± SE | Cubital Index, % M ± SE | Tarsal Index, % M ± SE | Author |
|---|---|---|---|---|---|
| 1. A. m. mellifera | 6.20 ± 0.02 | 5.0 ± 0.04 | 62.3 ± 1.5 | 55.6 ± 0.2 | [11] |
| 1.1 Prioksky | 6.70 ± 0.03 | 4.8 ± 0.01 | 56.4 ± 1.0 | 59.4 ± 0.3 | [11] |
| 1.2 Orlovsky | 6.30 ± 0.04 | 4.9 ± 0.06 | 60.2 ± 1.7 | 55.8 ± 0.6 | [11] |
| 1.3 Tatarsky | 6.30 ± 0.04 | 5.0 ± 0.01 | 60.6 ± 0.4 | 55.2 ± 0.2 | [11] |
| 1.4 Burzyansky | 6.20 ± 0.03 5.85 ± 0.01 | 4.9 ± 0.01 4.9 ± 0.04 | 59.2 ± 0.5 58.4 ± 0.7 | 57.0 ± 0.2 55.6 ± 0.3 | [11,110] |
| 1.5 Bashkirsky | 6.15 ± 0.01 | 5.0 ± 0.01 | 63.0 ± 0.2 | 55.1 ± 0.1 | [11] |
| 2. A.m. Far Eastern breed (Russian-bred) | 6.70 ± 0.03 6.40 ± 0.08 | 5.1 ± 0.03 4.9 ± 0.01 | 45.4 ± 0.5 43.9 ± 0.39 | 57.7 ± 2.3 56.8 ± 0.2 | [11,111] |
| 3. A.m. carpatica | 6.60 ± 0.02 6.57 ± 0.02 | 4.7 ± 0.01 4.6 ± 0.02 | 43.1 ± 0.4 39.7 ± 1.9 | 52.0 ± 0.6 52.6 ± 0.3 | [11,112] |
| 3.1 Maikopsky | 6.70 ± 0.02 | 4.9 ± 0.01 | 47.9 ± 0.02 | 52.0 ± 0.1 | [11] |
| 3.2 Moscovsky | 6.70 ± 0.01 6.84 ± 0.01 | 4.6 ± 0.01 4.6 ± 0.01 | 40.3 ± 1.4 38.5 ± 1.2 | 54.9 ± 0.2 52.5 ± 1.0 | [11,112] |
| 4. A.m. caucasia | 6.90 ± 0.01 | 4.7 ± 0.01 | 51.2 ± 0.2 | 55.0 ± 0.2 | [11] |
| 4.1 Krasnopolyansky | 7.00 ± 0.01 | 4.8 ± 0.01 | 52.4 ± 0.2 | 55.4 ± 0.3 | [11] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frunze, O.; Brandorf, A.; Kang, E.-J.; Choi, Y.-S. Beekeeping Genetic Resources and Retrieval of Honey Bee Apis mellifera L. Stock in the Russian Federation: A Review. Insects 2021, 12, 684. https://0-doi-org.brum.beds.ac.uk/10.3390/insects12080684
Frunze O, Brandorf A, Kang E-J, Choi Y-S. Beekeeping Genetic Resources and Retrieval of Honey Bee Apis mellifera L. Stock in the Russian Federation: A Review. Insects. 2021; 12(8):684. https://0-doi-org.brum.beds.ac.uk/10.3390/insects12080684
Chicago/Turabian StyleFrunze, Olga, Anna Brandorf, Eun-Jin Kang, and Yong-Soo Choi. 2021. "Beekeeping Genetic Resources and Retrieval of Honey Bee Apis mellifera L. Stock in the Russian Federation: A Review" Insects 12, no. 8: 684. https://0-doi-org.brum.beds.ac.uk/10.3390/insects12080684