Potential of Fermentation and Vacuum Packaging Followed by Chilling to Preserve Black Soldier Fly Larvae (Hermetia illucens)
Abstract
:Simple Summary
Abstract
1. Introduction
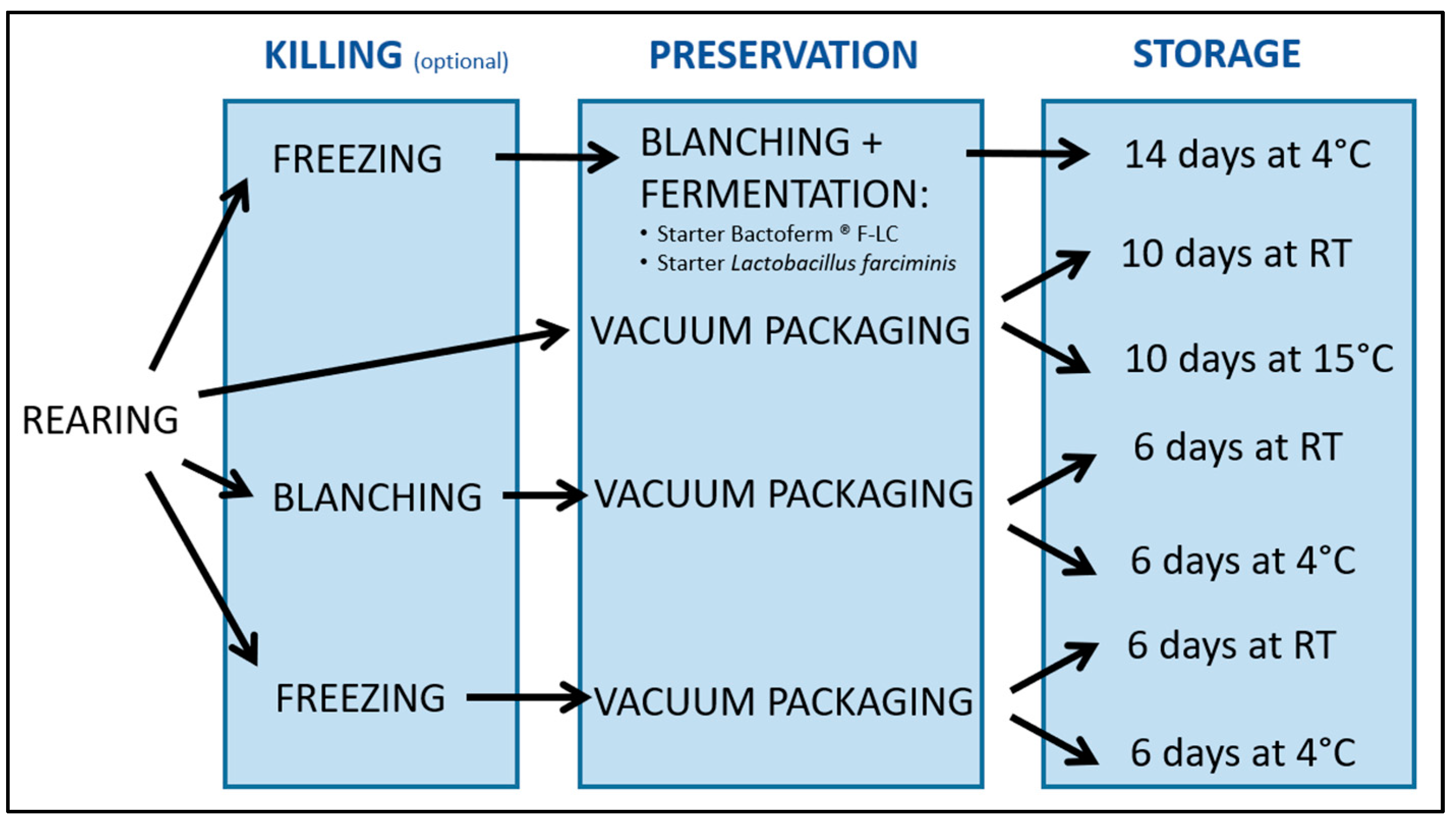
2. Materials and Methods
2.1. Fermentation Followed by Storage and Sampling
2.2. Vacuum Packaging Followed by Storage and Sampling
2.3. Determination of pH during Fermentation and Storage
2.4. Determination of Gas Composition during Vacuum Packaging and Storage
2.5. Determination of Survival during Storage of Living Larvae
2.6. Microbial Counts
2.7. Statistical Analyses
3. Results
3.1. Fermentation Followed by Storage
3.1.1. pH Reduction
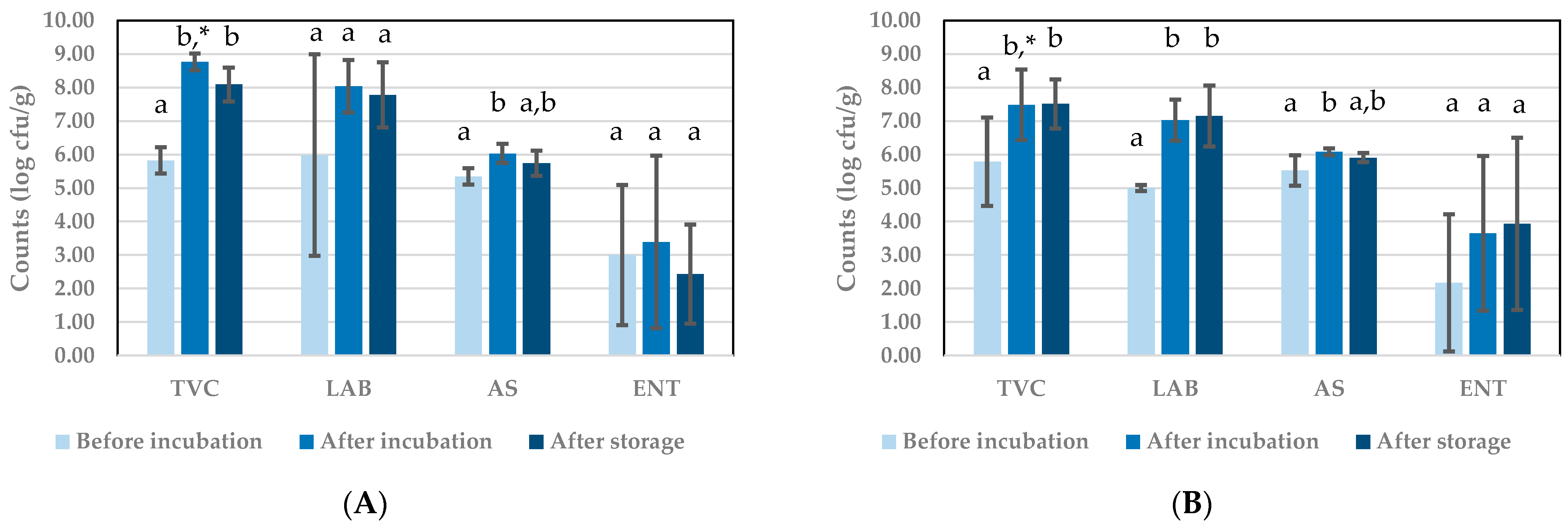
3.1.2. Microbial Counts
3.2. Vacuum Packaging Followed by Storage
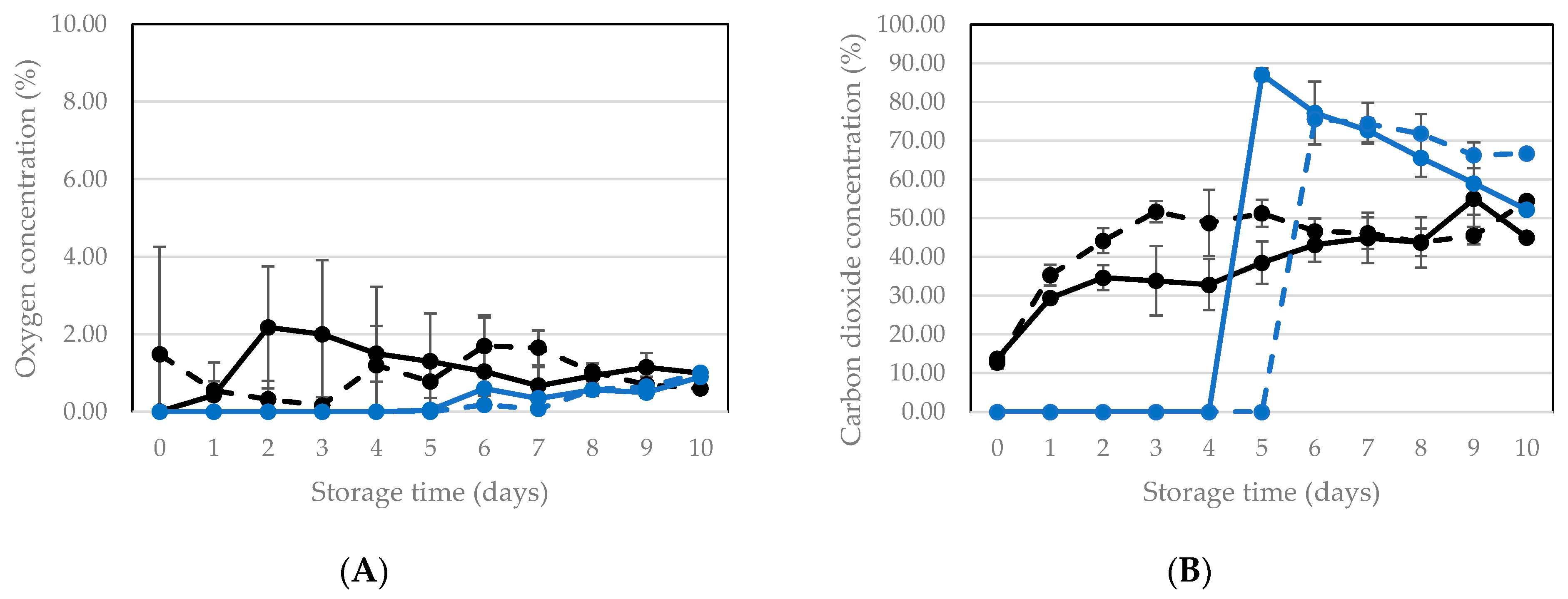
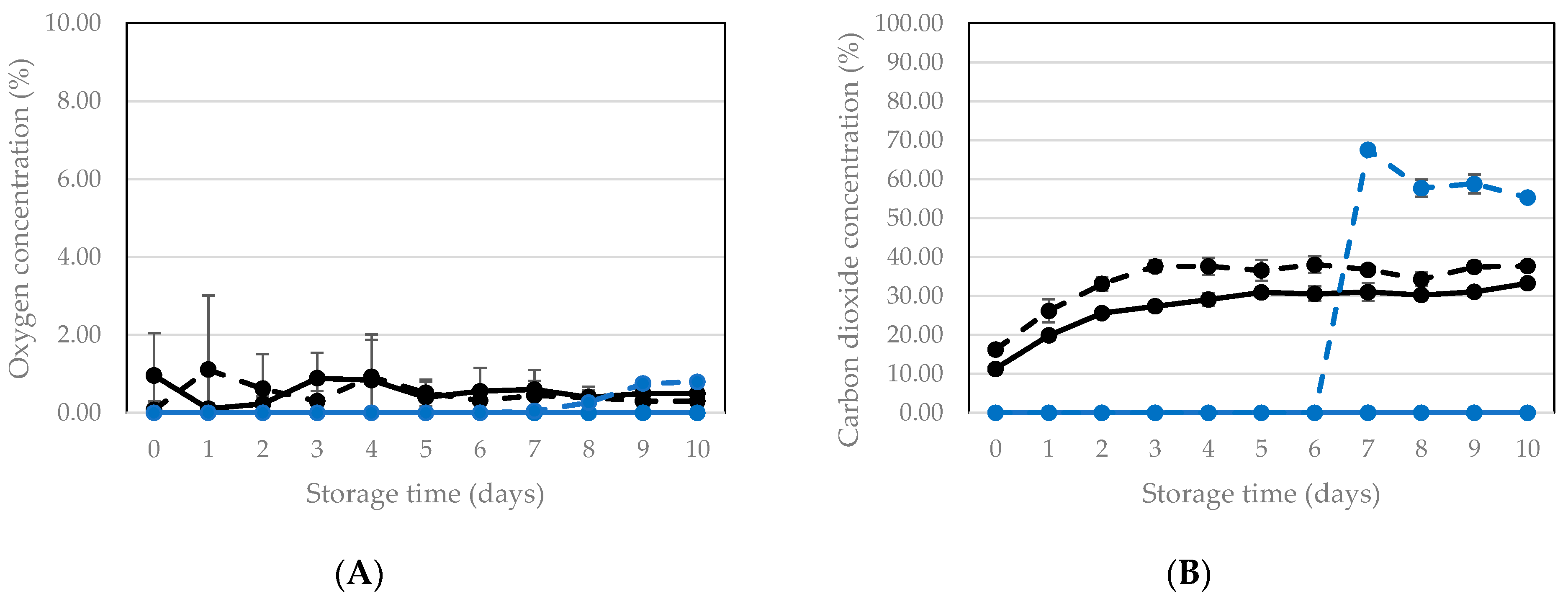
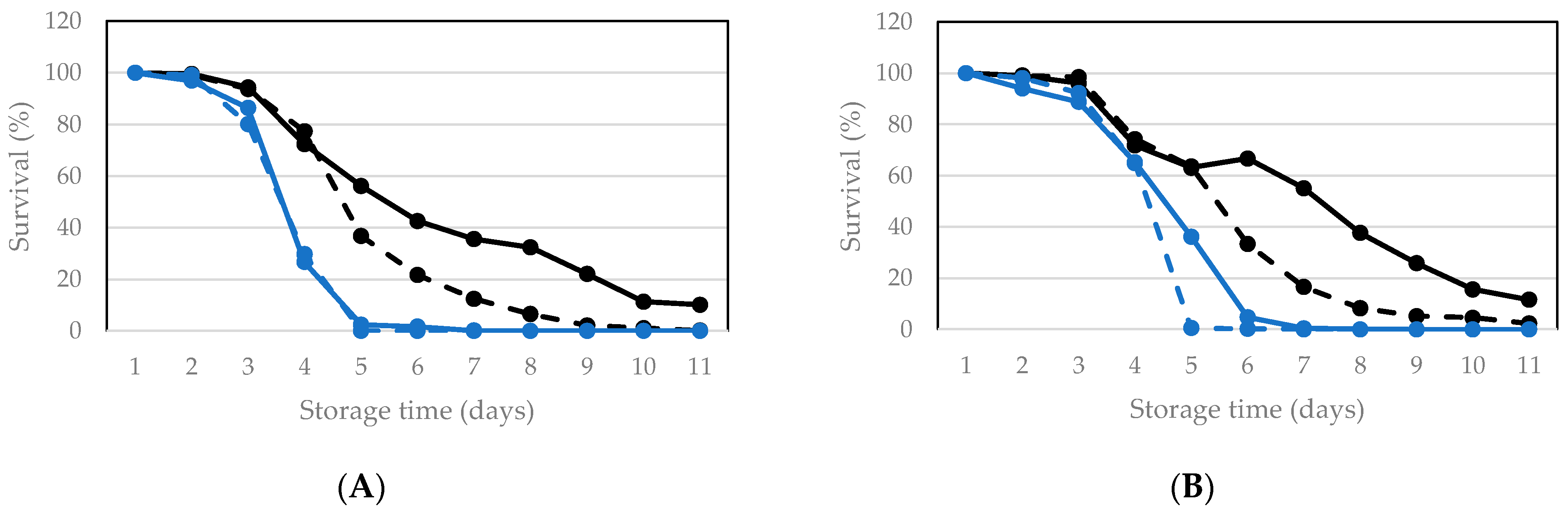
3.2.1. Vacuum Packaging and Storage of Living Larvae
3.2.2. Vacuum Packaging and Storage of Killed Larvae
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scala, A.; Cammack, J.A.; Salvia, R.; Scieuzo, C.; Franco, A.; Bufo, S.A.; Tomberlin, J.K.; Falabella, P. Rearing Substrate Impacts Growth and Macronutrient Composition of Hermetia illucens (L.) (Diptera: Stratiomyidae) Larvae Produced at An Industrial Scale. Sci. Rep. 2020, 10, 19448. [Google Scholar] [CrossRef] [PubMed]
- Wade, M.; Hoelle, J. A Review of Edible Insect Industrialization: Scales of Production and Implications for Sustainability. Environ. Res. Lett. 2020, 15, 123013. [Google Scholar] [CrossRef]
- IPIFF Fact Sheet “Edible Insects on the European Market”. Available online: https://ipiff.org/wp-content/uploads/2020/06/10-06-2020-IPIFF-edible-insects-market-factsheet.pdf (accessed on 28 June 2021).
- Demand for Insect Protein Could Hit 500,000 Tons by 2030. Available online: https://www.feednavigator.com/Article/2021/02/24/Demand-for-insect-protein-could-hit-500-000-tons-by-2030 (accessed on 28 June 2021).
- Fellows, P.J. Food Processing Technology: Principles and Practice, 4th ed.; Woodhead Publishing: Amsterdam, The Netherlands, 2017; 1152p. [Google Scholar]
- Larouche, J.; Deschamps, M.-H.; Saucier, L.; Lebeuf, Y.; Doyen, A.; Vandenberg, G.W. Effects of Killing Methods on Lipid Oxidation, Colour and Microbial Load of Black Soldier Fly (Hermetia illucens) Larvae. Animals 2019, 9, 182. [Google Scholar] [CrossRef] [Green Version]
- Kamau, E.; Mutungi, C.; Kinyuru, J.; Imathiu, S.; Tanga, C.; Affognon, H.; Ekesi, S.; Nakimbugwe, D.; Fiaboe, K.K.M. Moisture Adsorption Properties and Shelf-Life Estimation of Dried and Pulverised Edible House Cricket Acheta domesticus (L.) and Black Soldier Fly Larvae Hermetia illucens (L.). Food Res. Int. 2018, 106, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Kamau, E.; Mutungi, C.; Kinyuru, J.; Imathiu, S.; Affognon, H.; Ekesi, S.; Nakimbugwe, D.; Fiaboe, K.K.M. Changes in Chemical and Microbiological Quality of Semi-Processed Black Soldier Fly (Hermetia Illucens L.) Larval Meal during Storage. J. Insects Food Feed 2020, 6, 417. [Google Scholar] [CrossRef]
- Kinyuru, J.N.; Kenji, G.M.; Njoroge, S.M.; Ayieko, M. Effect of Processing Methods on the In Vitro Protein Digestibility and Vitamin Content of Edible Winged Termite (Macrotermes subhylanus) and Grasshopper (Ruspolia differens). Food Bioprocess Technol. 2010, 3, 778. [Google Scholar] [CrossRef]
- Fombong, F.T.; Van Der Borght, M.; Vanden Broeck, J. Influence of Freeze-Drying and Oven-Drying Post Blanching on the Nutrient Composition of the Edible Insect Ruspolia differens. Insects 2017, 8, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azzollini, D.; Derossi, A.; Severini, C. Understanding the Drying Kinetic and Hygroscopic Behaviour of Larvae of Yellow Mealworm (Tenebrio Molitor) and the Effects on Their Quality. J. Insects Food Feed 2016, 2, 233. [Google Scholar] [CrossRef]
- Vandeweyer, D.; Lenaerts, S.; Callens, A.; Van Campenhout, L. Effect of Blanching Followed by Refrigerated Storage or Industrial Microwave Drying on the Microbial Load of Yellow Mealworm Larvae (Tenebrio molitor). Food Control 2017, 71, 311. [Google Scholar] [CrossRef]
- Lenaerts, S.; Van Der Borght, M.; Callens, A.; Van Campenhout, L. Suitability of Microwave Drying for Mealworms (Tenebrio molitor) as Alternative yo Freeze Drying: Impact on Nutritional Quality and Colour. Food Chem. 2018, 254, 129. [Google Scholar] [CrossRef]
- Kröncke, N.; Böschen, V.; Woyzichovski, J.; Demtröder, S.; Benning, R. Comparison of Suitable Drying Processes for Mealworms (Tenebrio molitor). Innov. Food Sci. Emerg. Technol. 2018, 50, 20. [Google Scholar] [CrossRef]
- Kröncke, N.; Grebenteuch, S.; Keil, C.; Demtröder, S.; Kroh, L.; Thünemann, A.F.; Benning, R.; Haase, H. Effect of Different Drying Methods on Nutrient Quality of the Yellow Mealworm (Tenebrio molitor L.). Insects 2019, 10, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.E.; Bang, W.Y.; Kim, Y.; Bae, S.; Hahn, D.; Jung, Y.H. Storage Characteristics of Two-Spotted Cricket (Gryllus bimaculatus De Geer) Powder According to Drying Method and Storage Temperature. Entomol. Res. 2020, 50, 517–524. [Google Scholar] [CrossRef]
- Lucas-González, R.; Fernández-López, J.; Pérez-Álvarez, J.A.; Viuda-Martos, M. Effect of Drying Processes in the Chemical, Physico-Chemical, Techno-Functional and Antioxidant Properties of Flours Obtained from House Cricket (Acheta domesticus). Eur. Food Res. Technol. 2019, 245, 1451. [Google Scholar] [CrossRef]
- Bawa, M.; Songsermpong, S.; Kaewtapee, C.; Chanput, W. Effects of Microwave and Hot Air Oven Drying on the Nutritional, Microbiological Load, and Color Parameters of the House Crickets (Acheta domesticus). J. Food Process. Preserv. 2020, 44, e14407. [Google Scholar] [CrossRef]
- Vandeweyer, D.; Wynants, E.; Crauwels, S.; Verreth, C.; Viaene, N.; Claes, J.; Lievens, B.; Van Campenhout, L. Microbial Dynamics During Industrial Rearing, Processing, and Storage of Tropical House Crickets (Gryllodes sigillatus) for Human Consumption. Appl. Environ. Microbiol. 2018, 84, e00255-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhen, Y.; Chundang, P.; Zhang, Y.; Wang, M.; Vongsangnak, W.; Pruksakorn, C.; Kovitvadhi, A. Impacts of Killing Process on the Nutrient Content, Product Stability and In Vitro Digestibility of Black Soldier Fly (Hermetia illucens) Larvae Meals. Appl. Sci. 2020, 10, 6099. [Google Scholar] [CrossRef]
- Manniello, M.D.; Moretta, A.; Salvia, R.; Scieuzo, C.; Lucchetti, D.; Vogel, H.; Sgambato, A.; Falabella, P. Insect Antimicrobial Peptides: Potential Weapons to Counteract the Antibiotic Resistance. Cell. Mol. Life Sci. 2021, 78, 4259. [Google Scholar] [CrossRef]
- Van Moll, L.; De Smet, J.; Cos, P.; Van Campenhout, L. Microbial Symbionts of Insects as a Source of New Antimicrobials: A Review. Crit. Rev. Microbiol. 2021. [Google Scholar] [CrossRef]
- Stanbury, P.; Whitaker, A.; Hall, S. Principles of Fermentation Technology, 3rd ed.; Butterworth-Heinemann: Oxford, UK, 2017; 824p. [Google Scholar]
- Bourdichon, F.; Casaregola, S.; Farrokh, C.; Frisvad, J.C.; Gerds, M.L.; Hammes, W.P.; Harnett, J.; Huys, G.; Laulund, S.; Ouwehand, A.; et al. Food fermentations: Microorganisms with technological beneficial use. Int. J. Food Microbiol. 2012, 154, 87. [Google Scholar] [CrossRef]
- Borremans, A.; Lenaerts, S.; Crauwels, S.; Lievens, B.; Van Campenhout, L. Marination and Fermentation of Yellow Mealworm Larvae (Tenebrio molitor). Food Control 2018, 92, 47. [Google Scholar] [CrossRef]
- De Smet, J.; Lenaerts, S.; Borremans, A.; Scholliers, J.; Van Der Borght, M.; Van Campenhout, L. Stability Assessment and Laboratory Scale Fermentation of Pastes Produced on a Pilot Scale from Mealworms (Tenebrio molitor). LWT 2019, 102, 113. [Google Scholar] [CrossRef]
- Borremans, A.; Crauwels, S.; Vandeweyer, D.; Smets, R.; Verreth, C.; Van der Borght, M.; Lievens, B.; Van Campenhout, L. Comparison of Six Commercial Meat Starter Cultures for the Fermentation of Yellow Mealworm (Tenebrio molitor) Paste. Microorganisms 2019, 7, 540. [Google Scholar] [CrossRef] [Green Version]
- Borremans, A.; Smets, R.; Van Campenhout, L. Fermentation Versus Meat Preservatives to Extend the Shelf Life of Mealworm (Tenebrio molitor) Paste for Feed and Food Applications. Front. Microbiol. 2020, 11, 1510. [Google Scholar] [CrossRef]
- Cutter, C.N. Microbial Control by Packaging: A Review. Crit. Rev. Food Sci. Nutr. 2002, 42, 151. [Google Scholar] [CrossRef] [PubMed]
- Farmer, N. Trends in Packaging of Food, Beverages and Other Fast-Moving Consumer Goods (FMCG), 1st ed.; Woodhead Publishing: Amsterdam, The Netherlands, 2013; 344p. [Google Scholar]
- Dijk, R.; van den Berg, D.; Beumer, R.; de Boer, E.; Dijkstra, A.; Mout, L.; Stegeman, H.; Uyttendaele, M.; In’t Veld, S. Microbiologie van Voedingsmiddelen: Methoden, Principes en Criteria, 5th ed.; MYbusinessmedia: Capelle aan den Ijssel, The Netherlands, 2015; pp. 403–516. [Google Scholar]
- Janda, J.M.; Abbott, S.L. The Enterobacteriaceae, 2nd ed.; ASM Press: Washington, DC, USA, 2006; 411p. [Google Scholar]
- Zhang, M.; Meng, X.; Bhandari, B.; Fang, Z. Recent Developments in Film and Gas Research in Modified Atmosphere Packaging of Fresh Foods. Crit. Rev. Food Sci. Nutr. 2016, 56, 2174. [Google Scholar] [CrossRef]
- Mogodiniyai Kasmaei, K. Ensiling as a Method for Storage and Processing of Black Soldier Fly Larvae for Use as Animal Feed. Research Report from Swedish Energy Agency. 2018. Available online: http://www.energimyndigheten.se/ (accessed on 28 May 2021).
- Kube, K.; Resch, R.; Gierus, M. Ensiling Hermentia illucens Larvae for Storage Purposes. In Proceedings of the INSECTA Congress, Potsdam, Germany, 5–6 September 2019; p. 102. [Google Scholar]
- Mennah-Govela, Y.A.; Cai, H.; Chu, J.; Kim, K.; Maborang, M.K.; Sun, W.; Bornhorst, G.M. Buffering Capacity of Commercially Available Foods Is Influenced by Composition and Initial Properties in the Context of Gastric Digestion. Food Funct. 2020, 11, 2255. [Google Scholar] [CrossRef]
- Wynants, E.; Frooninckx, L.; Crauwels, S.; Verreth, C.; De Smet, J.; Sandrock, C.; Wohlfahrt, J.; Van Schelt, J.; Depraetere, S.; Lievens, B.; et al. Assessing the Microbiota of Black Soldier Fly Larvae (Hermetia illucens) Reared on Organic Waste Streams on Four Different Locations at Laboratory and Large Scale. Microb. Ecol. 2019, 77, 913. [Google Scholar] [CrossRef]
- Foerster, H.F. Activation and Germination Characteristics Observed in Endospores of Thermophilic Strains of Bacillus. Arch. Microbiol. 1983, 134, 175. [Google Scholar] [CrossRef]
- Ishida, Y.; Ishido, T.; Kadota, H. Temperature-pH Effect upon Germination of Bacterial Spores. Can. J. Microbiol. 1976, 22, 322. [Google Scholar] [CrossRef]
- Valero, A.; Olague, E.; Medina-Pradas, E.; Garrido-Fernández, A.; Romero-Gil, V.; Cantalejo, M.J.; García-Gimeno, R.M.; Pérez-Rodríguez, F.; Posada-Izquierdo, G.D.; Arroyo-López, F.N. Influence of Acid Adaptation on the Probability of Germination of Clostridium sporogenes Spores Against pH, NaCl and Time. Foods 2020, 9, 127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, D.M.; Young-Perkins, K.E.; Merson, R.L. Factors influencing Clostridium botulinum Spore Germination, Outgrowth, and Toxin Formation in Acidified Media. Appl. Environ. Microbiol. 1988, 54, 1446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hutkins, R.W. Microbiology and Technology of Fermented Foods, 1st ed.; Blackwell Publishing: Ames, IA, USA, 2007; 473p. [Google Scholar]


| Starter Tested | Treatment | pH | ||
|---|---|---|---|---|
| Before Incubation | After Incubation | After Storage | ||
| Bactoferm® F-LC | No starter (control) | 7.06 ± 0.37 A,a | 6.18 ± 0.83 B,a | 5.96 ± 0.16 B,a |
| Starter | 7.12 ± 0.38 A,a | 6.09 ± 0.92 B,a | 6.43 ± 0.83 A,B,a | |
| L. farciminis | No starter (control) | 7.00 ± 0.24 A,a | 6.99 ± 1.34 A,a | 6.93 ± 1.13 A,a |
| Starter | 7.19 ± 0.09 A,a | 6.40 ± 1.07 A,B,a | 6.23 ± 0.60 B,a | |
| Killing Method | Storage Temperature | Packaging Condition | Microbial Counts (log cfu/g) | |||
|---|---|---|---|---|---|---|
| Total Aerobic Viable Counts | Entero- Bacteriaceae | Lactic Acid Bacteria | Aerobic Bacterial Endospores | |||
| Blanching | Before packaging | 4.75 | <1.00 † | 2.33 | 3.97 | |
| RT | Control | 9.05 | 8.40 | 7.63 | 6.00 | |
| Vacuum | 8.85 | 8.45 | 6.89 | 5.26 | ||
| 4 °C | Control | <4.97 † | <4.97 † | <3.97 † | 3.25 | |
| Vacuum | <4.95 † | <4.95 † | <3.95 † | 3.09 | ||
| Freezing | Before packaging | 8.28 | 6.37 | 7.42 | 4.83 | |
| RT | Control | 9.56 | 8.28 | 8.13 | 3.66 | |
| Vacuum | 9.16 | 8.24 | 7.74 | 4.73 | ||
| 4 °C | Control | 7.23 | 6.35 | 5.23 | 4.38 | |
| Vacuum | 7.18 | 7.05 | <5.00 † | 4.46 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van Campenhout, L.; Lachi, D.; Vandeweyer, D. Potential of Fermentation and Vacuum Packaging Followed by Chilling to Preserve Black Soldier Fly Larvae (Hermetia illucens). Insects 2021, 12, 714. https://0-doi-org.brum.beds.ac.uk/10.3390/insects12080714
Van Campenhout L, Lachi D, Vandeweyer D. Potential of Fermentation and Vacuum Packaging Followed by Chilling to Preserve Black Soldier Fly Larvae (Hermetia illucens). Insects. 2021; 12(8):714. https://0-doi-org.brum.beds.ac.uk/10.3390/insects12080714
Chicago/Turabian StyleVan Campenhout, Leen, Dario Lachi, and Dries Vandeweyer. 2021. "Potential of Fermentation and Vacuum Packaging Followed by Chilling to Preserve Black Soldier Fly Larvae (Hermetia illucens)" Insects 12, no. 8: 714. https://0-doi-org.brum.beds.ac.uk/10.3390/insects12080714






