Effect of Neonicotinoids on Bacterial Symbionts and Insecticide-Resistant Gene in Whitefly, Bemisia tabaci
Abstract
:Simple Summary
Abstract
1. Introduction
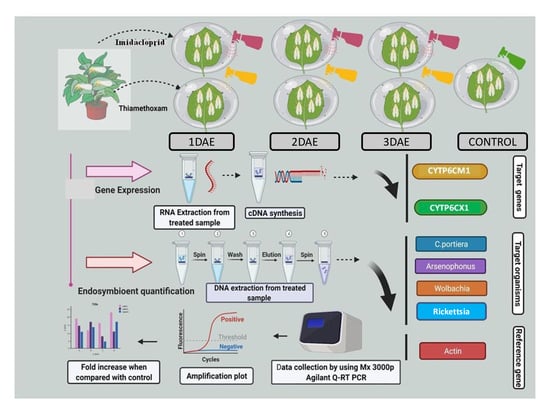
2. Materials and Methods
2.1. Whitefly Rearing
2.2. Genetic Identification of Whitefly and Their Symbiont
2.3. Insecticides
2.4. Bioassays
2.5. Insecticide Treatment on Whiteflies
2.6. DNA Extraction
2.7. RNA Isolation
2.8. cDNA Synthesis
2.9. Quantitative PCR (qPCR) and Quantitative RT-PCR (qRT-PCR) Analysis
2.10. Statistical Analysis
3. Results
3.1. Concentration Mortality Response of Imidacloprid and Thiamethoxam against Whiteflies
3.2. Relative Change in Primary Endosymbiont (Portiera) after Application of Two Neonicotinoids
3.3. Relative Change in Secondary Symbionts after Application of Two Neonicotinoids
3.4. Expression Pattern of Two Cytochrome P450 Genes after Exposure to Chemical Stress
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brown, J.K.; Czosnek, H. Whitefly transmission of plant viruses. In Advanced in Botanical Research; Plant Virus Vector Interactions; Plumb, R.T., Ed.; Academic Press: New York, NY, USA, 2002; Volume 36, pp. 65–76. [Google Scholar]
- Inbar, M.; Gerling, D. Plant-mediated interactions between whiteflies, herbivores, and natural enemies. Annu. Rev. Entomol. 2008, 53, 431–448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, J.K.; Frohlich, D.R.; Rosell, R.C. The sweet potato or silverleaf whiteflies—Biotypes of Bemisia tabaci or a species complex. Annu. Rev. Entomol. 1995, 40, 511–534. [Google Scholar] [CrossRef]
- Perring, T.M. The Bemisia tabaci species complex. Crop Prot. 2001, 20, 725–737. [Google Scholar] [CrossRef]
- Kanakala, S.; Ghanim, M. Global genetic diversity and geographical distribution of Bemisia tabaci and its bacterial endosymbionts. PLoS ONE 2019, 14, e0213946. [Google Scholar] [CrossRef] [Green Version]
- De Barro, P.J.; Liu, S.S.; Boykin, L.M.; Dinsdale, A.B. Bemisia tabaci: A statement of species status. Annu. Rev. Entomol. 2011, 56, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Sethi, A.; Dilawari, V.K. Spectrum of insecticide resistance in whitefly from upland cotton in Indian subcontinent. J. Entomol. 2008, 5, 138–147. [Google Scholar] [CrossRef] [Green Version]
- Peshin, R.; Zhang, W. Integrated pest management and pesticide use. In Integrated Pest Management, 1st ed.; David, P., Rajinder, P., Eds.; Springer: Dordrecht, The Netherlands, 2014; Volume 3, pp. 1–46. [Google Scholar]
- Kranthi, K. Cotton in the climate trap. Cotton Stat. News 2015, 10, 6–9. [Google Scholar]
- Naveen, N.C.; Chaubey, R.; Kumar, D.; Rebijith, K.B.; Rajagopal, R.; Subrahmanyam, B.; Subramanian, S. Insecticide resistance status in the whitefly, Bemisia tabaci genetic groups Asia-I, Asia-II-1 and Asia-II-7 on the Indian subcontinent. Sci. Rep. 2017, 7, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Yan, H.; Yang, Y.; Wu, Y. Biotype and insecticide resistance status of the whitefly Bemisia tabaci from China. Pest Manag. Sci. 2010, 66, 1360–1366. [Google Scholar] [CrossRef] [PubMed]
- Kranthi, K.R.; Jadhav, D.R.; Kranthi, S.; Wanjari, R.R.; Ali, S.S.; Russell, D.A. Insecticide resistance in five major insect pests of cotton in India. Crop Prot. 2002, 21, 449–460. [Google Scholar] [CrossRef]
- Basij, M.; Talebi, K.; Ghadamyari, M.; Hosseininaveh, V.; Salami, S.A. Status of resistance of Bemisia tabaci (Hemiptera: Aleyrodidae) to neonicotinoids in Iran and detoxification by cytochrome P450-dependent monooxygenases. Neotrop. Entomol. 2017, 46, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Alon, M.; Alon, F.; Nauen, R.; Morin, S. Organophosphates’ resistance in the B-biotype of Bemisia tabaci (Hemiptera: Aleyrodidae) is associated with a point mutation in an ace1-type acetylcholinesterase and overexpression of carboxylesterase. Insect Biochem. Mol. Biol. 2008, 38, 940–949. [Google Scholar] [CrossRef]
- Nauen, R.; Stumpf, N.; Elbert, A. Toxicological and mechanistic studies on neonicotinoid cross resistance in Q-type Bemisia tabaci (Hemiptera: Aleyrodidae). Pest Manag. Sci. 2002, 58, 868–875. [Google Scholar] [CrossRef]
- Shadmany, M.; Omar, D.; Muhamad, R. Biotype and insecticide resistance status of Bemisia tabaci populations from Peninsular Malaysia. J. Appl. Entomol. 2015, 139, 67–75. [Google Scholar] [CrossRef]
- Ahmad, M.; Arif, M.I.; Naveed, M. Dynamics of resistance to organophosphate and carbamate insecticides in the cotton whitefly Bemisia tabaci (Hemiptera: Aleyrodidae) from Pakistan. J. Pest Sci. 2010, 83, 409–420. [Google Scholar] [CrossRef]
- Prabhaker, N.; Castle, S.; Perring, T.M. Baseline susceptibility of Bemisia tabaci B biotype (Hemiptera: Aleyrodidae) populations from California and Arizona to spirotetramat. J. Econ. Entomol. 2014, 107, 773–780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, N. Insecticide Resistance in Mosquitoes: Impact, Mechanisms, and Research Directions. Annu. Rev. Entomol. 2015, 7, 537–559. [Google Scholar] [CrossRef] [PubMed]
- Feyereisen, R. Insect CYP Genes and P450 Enzymes. In Insect Molecular Biology and Biochemistry, 1st ed.; Gilbert, L., Ed.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 115–128. [Google Scholar]
- Nelson, D.R.; Koymans, L.; Kamataki, T.; Stegeman, J.J.; Feyereisen, R.; Waxman, D.J.; Waterman, M.R.; Gotoh, O.; Coon, M.J.; Estabrook, R.W.; et al. P450 superfamily: Update on new sequences, gene mapping, accession numbers and nomenclature. Pharmacogenetics 1996, 6, 1–42. [Google Scholar] [CrossRef] [PubMed]
- Feyereisen, R. Insect P450 enzymes. Annu. Rev. Entomol. 1999, 44, 507–533. [Google Scholar] [CrossRef]
- Feyereisen, R. Evolution of insect P450. Biochem. Soc. Trans. 2006, 34, 1252–1255. [Google Scholar] [CrossRef] [Green Version]
- Li, X.C.; Schuler, M.A.; Berenbaum, M.R. Molecular mechanisms of metabolic resistance to synthetic and natural xenobiotics. Annu. Rev. Entomol. 2007, 52, 231–253. [Google Scholar] [CrossRef]
- Yang, X.; Xie, W.; Wang, S.L.; Wu, Q.J.; Pan, H.P.; Li, R.M.; Yang, N.N.; Liu, B.M.; Xu, B.Y.; Zhou, X.; et al. Two cytochrome P450 genes are involved in imidacloprid resistance in field populations of the whitefly, Bemisia tabaci, in China. Pestic. Biochem. Physiol. 2013, 107, 343–350. [Google Scholar] [CrossRef]
- Daborn, P.J.; Yen, J.L.; Bogwitz, M.R.; Le Goff, G.; Feil, E.; Jeffers, S.; Tijet, N.; Perry, T.; Heckel, D.; Batterham, P.; et al. A single P450 allele associated with insecticide resistance in Drosophila. Science 2002, 297, 2253–2256. [Google Scholar] [CrossRef]
- Markussen, M.D.; Kristensen, M. Cytochrome P450 monooxygenase-mediated neonicotinoid resistance in the house fly Musca domestica L. Pestic. Biochem. Physiol. 2010, 98, 50–58. [Google Scholar] [CrossRef]
- Nauen, R. Development of a lateral flow test to detect metabolic resistance in Bemisia tabaci mediated by CYP6CM1, a cytochrome P450 with broad-spectrum catalytic efficiency. Pestic. Biochem. Physiol. 2015, 121, 3–11. [Google Scholar] [CrossRef]
- Brumin, M.; Kontsedalov, S.; Ghanim, M. Rickettsia influences thermotolerance in the whitefly Bemisia tabaci B biotype. Insect Sci. 2011, 18, 57–66. [Google Scholar] [CrossRef]
- Su, Q.; Xie, W.; Wang, S.L.; Wu, Q.J.; Ghanim, M.; Zhang, Y.J. Location of symbionts in the whitefly Bemisia tabaci affects 498 their densities during host development and environmental stress. PLoS ONE 2014, 9, e91802. [Google Scholar]
- Boush, G.M.; Matsumura, F. Insecticidal degradation by Pseudomonas melophthora, the bacterial symbiont of the apple maggot. J. Econ. Entomol. 1967, 60, 918–920. [Google Scholar] [CrossRef]
- Ghanim, M.; Kontsedalov, S. Susceptibility to insecticides in the Q biotype of Bemisia tabaci is correlated with bacterial symbiont densities. Pest Manag. Sci. 2009, 65, 939–942. [Google Scholar] [CrossRef] [PubMed]
- Zug, R.; Hammerstein, P. Bad guys turned nice? A critical assessment of Wolbachia mutualisms in arthropod hosts. Biol. Rev. 2015, 90, 89–111. [Google Scholar] [CrossRef] [PubMed]
- White, J.A.; Kelly, S.E.; Cockburn, S.N.; Perlman, S.J.; Hunter, M.S. Endosymbiont costs and benefits in a parasitoid infected with both Wolbachia and Cardinium. Heredity 2011, 106, 585–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaw, W.R.; Marcenac, P.; Childs, L.M.; Buckee, C.O.; Baldini, F.; Sawadogo, S.P.; Dabiré, R.K.; Diabaté, A.; Catteruccia, F. Wolbachia infections in natural Anopheles populations affect egg laying and negatively correlate with Plasmodium development. Nat. Commun. 2016, 7, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Pontes, M.H.; Dale, C. Culture and manipulation of insect facultative symbionts. Trends Microbiol. 2006, 14, 406–412. [Google Scholar] [CrossRef]
- Simón, C.; Frati, F.; Beckenbach, A.; Crespi, B.; Liu, H.; Flook, P. Evolution, weighting, and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers. Ann. Entomol. Soc. Am. 1994, 87, 651–701. [Google Scholar] [CrossRef]
- Raina, H.S.; Rawal, V.; Singh, S.; Daimei, G.; Shakarad, M.; Rajagopal, R. Elimination of Arsenophonus and decrease in the bacterial symbionts diversity by antibiotic treatment leads to increase in fitness of whitefly, Bemisia tabaci. Infect. Genet. Evol. 2015, 32, 224–230. [Google Scholar] [CrossRef]
- Pusag, J.C.A.; Jahan, S.H.; Lee, K.S.; Lee, S.; Lee, K.Y. Upregulation of temperature susceptibility in Bemisia tabaci upon acquisition of Tomato yellow leaf curl virus (TYLCV). J. Insect Physiol. 2012, 58, 1343–1348. [Google Scholar] [CrossRef]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Finney, D.J. Probit Analysis, 1st ed.; Cambridge University Press: Cambridge, UK; London, UK, 1971; p. 333. [Google Scholar]
- Dillon, R.J.; Dillon, V.M. The gut bacteria of insects: Nonpathogenic interactions. Annu. Rev. Entomol. 2004, 49, 71–92. [Google Scholar] [CrossRef] [PubMed]
- Shelton, A.M.; Sances, F.V.; Hawley, J.; Tang, J.D.; Boune, M.; Jungers, D.; Collins, H.L.; Farias, J. Assessment of insecticide resistance after the outbreak of diamondback moth (Lepidoptera: Plutellidae) in California in 1997. J. Econ. Entomol. 2000, 93, 931–936. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Li, C.; Su, T.; Jin, J.; Guo, Y.; Ren, D.; Yang, Z.; Liu, Q.; Meng, F. A survey of insecticide resistance in Aedes albopictus (Diptera: Culicidae) during a 2014 dengue fever outbreak in Guangzhou, China. J. Econ. Entomol. 2017, 110, 239–244. [Google Scholar]
- Arthropod Pesticide Resistance Database. 2013. Available online: http://www.pesticideresistance.com/ (accessed on 5 January 2016).
- Jeschke, P.; Nauen, R.; Schindler, M.; Elbert, A. Overview of the status and global strategy for neonicotinoids. J. Agric. Food Chem. 2010, 59, 2897–2908. [Google Scholar] [CrossRef]
- Naggar, J.B.; Zidan, N.E.A. Field evaluation of imidacloprid and thiamethoxam against sucking insects and their side effects on soil fauna. J. Plant Prot. Res. 2013, 53, 375–387. [Google Scholar] [CrossRef]
- Liu, X.D.; Guo, H.F. Importance of endosymbionts Wolbachia and Rickettsia in insect resistance development. Curr. Opin. Insect Sci. 2019, 33, 84–90. [Google Scholar] [CrossRef]
- Feldhaar, H. Bacterial symbionts as mediators of eco-logically important traits of insect hosts. Ecol. Entomol. 2011, 36, 533–543. [Google Scholar] [CrossRef]
- Bosch, T.J.; Welte, C.U. Detoxifying symbionts in agriculturally important pest insects. Microbiol. Biotechnol. 2017, 10, 531–540. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, C.; He, Z.; Wang, Z.G.; Wang, J.L.; Wang, Y.F. Wolbachia infection decreased the resistance of Drosophila to lead. PLoS ONE 2012, 7, e32643. [Google Scholar] [CrossRef] [Green Version]
- Brownlie, J.C.; Cass, B.N.; Riegler, M.; Witsenburg, J.J.; Iturbe-Ormaetxe, I.; McGraw, E.A.; O’Neill, S.L. Evidence for metabolic provisioning by a common invertebrate endosymbiont, Wolbachia pipientis, during periods of nutritional stress. PLoS Pathog. 2009, 5, e1000368. [Google Scholar] [CrossRef] [Green Version]
- Walker, T.J.P.H.; Johnson, P.H.; Moreira, L.A.; Iturbe-Ormaetxe, I.; Frentiu, F.D.; McMeniman, C.J.; Leong, Y.S.; Dong, Y.; Axford, J.; Kriesner, P.; et al. The w Mel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature 2011, 476, 450–453. [Google Scholar] [CrossRef] [PubMed]
- Panteleev, D.Y.; Goryacheva, I.I.; Andrianov, B.V.; Reznik, N.L.; Lazebny, O.E.; Kulikov, A.M. The endosymbiotic bacterium Wolbachia enhances the nonspecific resistance to insect pathogens and alters behavior of Drosophila melanogaster. Russ. J. Genet. 2007, 43, 1066–1069. [Google Scholar] [CrossRef]
- Ceja-Navarro, J.A.; Vega, F.E.; Karaoz, U.; Hao, Z.; Jenkins, S.; Lim, H.C.; Kosina, P.; Infante, F.; Northen, T.R.; Brodie, E.L. Gut microbiota mediate caffeine detoxification in the primary insect pest of coffee. Nat. Commun. 2015, 6, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Kontsedalov, S.; Zchori-Fein, E.; Chiel, E.; Gottlieb, Y.; Inbar, M.; Ghanim, M. The presence of Rickettsia is associated with increased susceptibility of Bemisia tabaci (Homoptera: Aleyrodidae) to insecticides. Pest Manag. Sci. 2008, 64, 789–792. [Google Scholar] [CrossRef]
- Pang, R.; Chen, M.; Yue, L.; Xing, K.; Li, T.; Kang, K.; Liang, Z.; Yuan, L.; Zhang, W. A distinct strain of Arsenophonus symbiont decreases insecticide resistance in its insect host. PLoS Genet. 2018, 14, e1007725. [Google Scholar] [CrossRef]
- Skaljac, M.; Kirfel, P.; Grotmann, J.; Vilcinskas, A. Fitness costs of infection with Serratia symbiotica are associated with greater susceptibility to insecticides in the pea aphid Acyrthosiphon pisum. Pest Manag. Sci. 2018, 74, 1829–1836. [Google Scholar] [CrossRef]
- Hosokawa, T.; Kikuchi, Y.; Nikoh, N.; Shimada, M.; Fukatsu, T. Strict host-symbiont cospeciation and reductive genome evolution in insect gut bacteria. PLoS Biol. 2006, 4, 1841–1851. [Google Scholar] [CrossRef] [PubMed]
- Nakabachi, A.; Ueoka, R.; Oshima, K.; Teta, R.; Mangoni, A.; Gurgui, M. Defensive bacteriome symbiont with a drastically reduced genome. Curr. Biol. 2013, 23, 1478–1484. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Liu, X.; Guo, H. Variations in endosymbiont infection between buprofezin-resistant and susceptible strains of Laodelphax striatellus (Falle’ n). Curr. Microbiol. 2018, 75, 709–715. [Google Scholar] [CrossRef]
- Pan, H.P.; Chu, D.; Liu, B.M.; Xie, W.; Wang, S.L.; Wu, Q.J.; Zhang, Y.J. Relative amount of symbionts in insect hosts changes with host-plant adaptation and insecticide resistance. Environ. Entomol. 2013, 42, 74–78. [Google Scholar] [CrossRef] [Green Version]
- Marubayashi, J.M.; Kliot, A.; Yuki, V.A.; Rezende, J.A.M.; Krause-Sakate, R.; Pavan, M.A.; Ghanim, M. Diversity and localization of bacterial endosymbionts from whitefly species collected in Brazil. PLoS ONE 2014, 9, e108363. [Google Scholar] [CrossRef]
- Rana, V.S.; Singh, S.T.; Priya, N.G.; Kumar, J.; Rajagopal, R. Arsenophonus GroEL interacts with CLCuV and is localized in midgut and salivary gland of whitefly B. tabaci. PLoS ONE 2012, 7, e42168. [Google Scholar] [CrossRef]
- Duron, O.; Labbe, P.; Berticat, C.; Rousset, F.; Guillot, S.; Raymond, M.; Weill, M. High Wolbachia density correlates with cost of infection for insecticide resistant Culex pipiens mosquitoes. Evolution 2006, 60, 303–314. [Google Scholar] [CrossRef]
- Liu, N.; Scott, J.G. Genetic analysis of factors controlling elevated cytochrome P450, CYP6D1, cytochrome b5, P450 reductase and monooxygenase activities in LPR house flies, Musca domestica. Biochem. Genet. 1996, 34, 133–148. [Google Scholar] [CrossRef]
- Zhuang, H.M.; Wang, K.F.; Zheng, L.; Wu, Z.J.; Miyata, T.; Wu, G. Identification and characterization of a cytochrome P450 CYP6CX1 putatively associated with insecticide resistance in Bemisia tabaci. Insect Sci. 2011, 18, 484–494. [Google Scholar] [CrossRef]








| Organism | Accession Number | Primer Name | Primer Sequences (5′→3′) | Annealing Temperature (°C) |
| Diagnostic PCR | ||||
| “Ca. Portiera aleyrodidarum” | MT585785 | Por-F Por-R | CGTACGGAAACGTACGCTAA TAAGCATAGGGCTTTCACATAAA | 60 |
| Rickettsia sp. | MT027499 | Ric-F Ric-R | GCTCAGAACGAACGCTGG GAAGGAAAGCATCTCTGC | 56 |
| Wolbachia | MT 032316 | Wol-F Wol-R | CGGGGGAAAATTTATTGCT AGCTGTAATACAGAAAGGAAA | 56 |
| Arsenophonus | MT026928 | Arse-F Arse_R | CGTTTGATGAATTCATAGTCAAA GGTCCTCCAGTTAGTGTTACCCAAC | 54 |
| B. tabaci | PRJEB41468 | C1-J-2195 L2-N-3014 | TTGATTTTTTGGTCATCCAGAAGT TCCAATGCACTAATCTGCCATATTA | 53 |
| qPCR | Target Gene | Primer Name | Primer Sequences (5′→3′) | Annealing Temperature (°C) |
| “Ca. Portiera aleyrodidarum” | 16 S rDNA | Port73-F Port266-R | TAGTCCACGCTGTAAACG AGGCACCCTTCCATCT | 60 |
| Rickettsia sp. | gltA | glt375-F glt574-R | AAAGGTTGCTCATCATGCGTT GCCATAGGATGCGAAGAGCT | 60 |
| Arsenophonus | 23 S rDNA | 23 S-F 23 S-R | CGTTTGATGAATTCATAGTCAAA GGTCCTCCAGTTAGTGTTACCCAAC | 60 |
| Wolbachia | Wsp | Wsp-F Wsp-R | TGGTCCAATAAGTGATGAAGAAAC AAAAATTAAACGCTACTCCA | 60 |
| B. tabaci | CYTP6CM1 | CM1-F CM1-R | CACTCTTTTGGATTTACTGC GTGAAGCTGCCTCTTTAATG | 60 |
| B. tabaci | CYTP6CX1 | CX1-F CX1-R | GTGCCCTACATCTCGCCTATC CATTTCTTTCGTCGTCTCCAAC | 60 |
| B. tabaci | β-actin | Actin-F Actin-R | ACCGCAAGATTCCATACCC CGCTGCCTCCACCTCATT | 60 |
| Insecticide | HAE | LC Values (mg/L) | χ2 | Slope ± SE | 95% CL at LC50 (mg/L) | ||
|---|---|---|---|---|---|---|---|
| LC30 | LC50 | LC90 | |||||
| Imidacloprid | 24 | 20.3 | 89.64 | 3379.55 | 0.528 | 0.813 ± 0.178 | 48.09–250.28 |
| 48 | 7.11 | 26.16 | 630.61 | 0.843 | 0.927 ± 0.179 | 14.39–45.61 | |
| 72 | 4.19 | 12.73 | 192.04 | 0.801 | 1.087 ± 0.194 | 6.73–20.46 | |
| 96 | 2.382 | 7.18 | 106.46 | 0.215 | 1.094 ± 0.206 | 3.14–12.06 | |
| Thiamethoxam | 24 | 2.23 | 12.28 | 794.76 | 3.012 | 0.708 ± 0.156 | 5.72–26.03 |
| 48 | 0.872 | 5.28 | 430.35 | 1.239 | 0.671 ± 0.155 | 1.76–10.89 | |
| 72 | 0.631 | 2.34 | 57.53 | 0.622 | 0.921 ± 0.175 | 0.86–4.33 | |
| 96 | 0.492 | 1.73 | 37.21 | 2.616 | 0.961 ± 0.183 | 0.58–3.27 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barman, M.; Samanta, S.; Thakur, H.; Chakraborty, S.; Samanta, A.; Ghosh, A.; Tarafdar, J. Effect of Neonicotinoids on Bacterial Symbionts and Insecticide-Resistant Gene in Whitefly, Bemisia tabaci. Insects 2021, 12, 742. https://0-doi-org.brum.beds.ac.uk/10.3390/insects12080742
Barman M, Samanta S, Thakur H, Chakraborty S, Samanta A, Ghosh A, Tarafdar J. Effect of Neonicotinoids on Bacterial Symbionts and Insecticide-Resistant Gene in Whitefly, Bemisia tabaci. Insects. 2021; 12(8):742. https://0-doi-org.brum.beds.ac.uk/10.3390/insects12080742
Chicago/Turabian StyleBarman, Mritunjoy, Snigdha Samanta, Himanshu Thakur, Swati Chakraborty, Arunava Samanta, Amalendu Ghosh, and Jayanta Tarafdar. 2021. "Effect of Neonicotinoids on Bacterial Symbionts and Insecticide-Resistant Gene in Whitefly, Bemisia tabaci" Insects 12, no. 8: 742. https://0-doi-org.brum.beds.ac.uk/10.3390/insects12080742