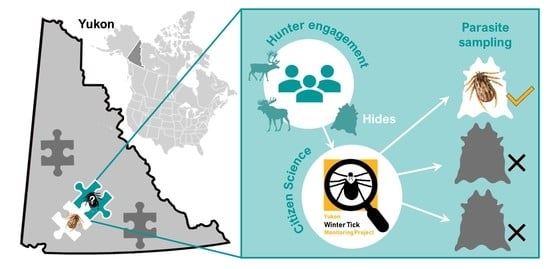
Improving Widescale Monitoring of Ectoparasite Presence in Northern Canadian Wildlife with the Aid of Citizen Science
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Voluntary and Mandated Hunted Hide Submission
2.2. Hide Incentives Program
2.3. Hide Sample Collection Kits
2.4. Sample Processing
2.5. Evaluating YWTMP Success
3. Results
3.1. YWTMP Engagement and Participation
3.2. YWTMP Hide Sample Kit Conformity
3.3. Distribution of Winter Ticks on Hunted Cervids in Yukon
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, X.; Murphy, D.S.; Johnson, D.H.; Paskewitz, S.M. Passive Animal Surveillance to Identify Ticks in Wisconsin, 2011–2017. Insects 2019, 10, 289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyons, L.A.; Brand, M.E.; Gronemeyer, P.; Mateus-Pinilla, N.; Ruiz, M.O.; Stone, C.M.; Tuten, H.C.; Smith, R.L. Comparing Contributions of Passive and Active Tick Collection Methods to Determine Establishment of Ticks of Public Health Concern Within Illinois. J. Med. Entomol. 2021, 58, 1849–1864. [Google Scholar] [CrossRef] [PubMed]
- Nieto, N.C.; Porter, W.T.; Wachara, J.C.; Lowrey, T.J.; Martin, L.; Motyka, P.J.; Salkeld, D.J. Using citizen science to describe the prevalence and distribution of tick bite and exposure to tick-borne diseases in the United States. PLoS ONE 2018, 13, e0199644. [Google Scholar] [CrossRef] [PubMed]
- Ripoche, M.; Gasmi, S.; Adam-Poupart, A.; Koffi, J.K.; Lindsay, L.R.; Ludwig, A.; Milord, F.; Ogden, N.H.; Thivierge, K.; Leighton, P.A. Passive Tick Surveillance Provides an Accurate Early Signal of Emerging Lyme Disease Risk and Human Cases in Southern Canada. J. Med. Entomol. 2018, 55, 1016–1026. [Google Scholar] [CrossRef] [PubMed]
- Eisen, R.J.; Paddock, C.D. Tick and Tickborne Pathogen Surveillance as a Public Health Tool in the United States. J. Med. Entomol. 2021, 58, 1490–1502. [Google Scholar] [CrossRef]
- Lewis, J.; Boudreau, C.R.; Patterson, J.W.; Bradet-Legris, J.; Lloyd, V.K. Citizen Science and Community Engagement in Tick Surveillance—A Canadian Case Study. Healthcare 2018, 6, 22. [Google Scholar] [CrossRef] [Green Version]
- Prunuske, A.; Fisher, C.; Molden, J.; Brar, A.; Ragland, R.; Vanwestrienen, J. Middle-School Student Engagement in a Tick Testing Community Science Project. Insects 2021, 12, 1136. [Google Scholar] [CrossRef]
- Seifert, V.A.; Wilson, S.; Toivonen, S.; Clarke, B.; Prunuske, A. Community Partnership Designed to Promote Lyme Disease Prevention and Engagement in Citizen Science. J. Microbiol. Biol. Educ. 2016, 17, 63–69. [Google Scholar] [CrossRef] [Green Version]
- Sgroi, G.; Iatta, R.; Lia, R.P.; Napoli, E.; Buono, F.; Bezerra-Santos, M.A.; Veneziano, V.; Otranto, D. Tick exposure and risk of tick-borne pathogens infection in hunters and hunting dogs: A citizen science approach. Transbound. Emerg. Dis. 2021, 1–8. [Google Scholar] [CrossRef]
- Porter, W.T.; Motyka, P.J.; Wachara, J.; Barrand, Z.A.; Hmood, Z.; McLaughlin, M.; Pemberton, K.; Nieto, N.C. Citizen science informs human-tick exposure in the Northeastern United States. Int. J. Health Geogr. 2019, 18, 9. [Google Scholar] [CrossRef] [Green Version]
- Brook, R.K.; Kutz, S.J.; Veitch, A.M.; Popko, R.A.; Elkin, B.T.; Guthrie, G. Fostering Community-Based Wildlife Health Monitoring and Research in the Canadian North. EcoHealth 2009, 6, 266–278. [Google Scholar] [CrossRef] [PubMed]
- Tulloch, A.I.; Possingham, H.P.; Joseph, L.N.; Szabo, J.; Martin, T.G. Realising the full potential of citizen science monitoring programs. Biol. Conserv. 2013, 165, 128–138. [Google Scholar] [CrossRef] [Green Version]
- Theobald, E.J.; Ettinger, A.K.; Burgess, H.K.; DeBey, L.B.; Schmidt, N.R.; Froehlich, H.E.; Wagner, C.; HilleRisLambers, J.; Tewksbury, J.; Harsch, M.A.; et al. Global change and local solutions: Tapping the unrealized potential of citizen science for biodiversity research. Biol. Conserv. 2015, 181, 236–244. [Google Scholar] [CrossRef] [Green Version]
- Lieske, D.J.; Lloyd, V.K. Combining public participatory surveillance and occupancy modelling to predict the distributional response of Ixodes scapularis to climate change. Ticks Tick-borne Dis. 2018, 9, 695–706. [Google Scholar] [CrossRef] [PubMed]
- Schvartz, G.; Epp, T.; Burgess, H.J.; Chilton, N.B.; Armstrong, J.S.; Lohmann, K.L. Passive surveillance for ticks on horses in Saskatchewan. Can. Vet. J. 2015, 56, 486–489. [Google Scholar] [PubMed]
- Poh, K.C.; Skvarla, M.; Evans, J.R.; Machtinger, E.T. Collecting Deer Keds (Diptera: Hippoboscidae: Lipoptena Nitzsch, 1818 and Neolipoptena Bequaert, 1942) and Ticks (Acari: Ixodidae) From Hunter-Harvested Deer and Other Cervids. J. Insect Sci. 2020, 20, 19. [Google Scholar] [CrossRef] [PubMed]
- Sine, M.; Morris, K.; Knupp, D. Assessment of a line transect field method to determine winter tick abundance on moose. Alces 2009, 45, 143–146. [Google Scholar]
- Welch, D.A.; Samuel, W.M. Evaluation of random sampling for estimating density of winter ticks (Dermacentor albipictus) on moose (Alces alces) hides. Int. J. Parasitol. 1989, 19, 691–693. [Google Scholar] [CrossRef]
- Apperson, C.S.; Levine, J.F.; Nicholson, W.L.; Carolina, N. Geographic Occurrence of Ixodes scapularis and Amblyomma americanum (Acari: Ixodidae) Infesting White-tailed Deer in North Carolina. J. Wildl. Dis. 1990, 26, 550–553. [Google Scholar] [CrossRef]
- Cortinas, M.R.; Kitron, U. County-Level Surveillance of White-Tailed Deer Infestation by Ixodes scapularis and Dermacentor albipictus (Acari: Ixodidae) Along the Illinois River. J. Med. Entomol. 2006, 43, 810–819. [Google Scholar] [CrossRef]
- Addison, E.M.; Smith, L.M. Productivity of winter ticks (Dermacentor albipictus) collected from moose killed on Ontario roads. Alces 1981, 17, 136–145. [Google Scholar]
- Bergeron, D.H.; Pekins, P.J. Evaluating the usefulness of three indices for assessing winter tick abundance in northern New Hampshire. Alces 2014, 50, 1–15. [Google Scholar]
- Curry, P. Caribou Herds and Arctic Communities: Exploring a New Tool for Caribou Health Monitoring. InfoNorth 2009, 62, 495–499. [Google Scholar] [CrossRef] [Green Version]
- Kashivakura, C. Detecting Dermacentor albipictus, the Winter Tick, at the Northern Extent of Its Range: Hunter-Based Monitoring and Serological Assay Development. Master’s Thesis, University of Calgary, Calgary, AB, Canada, 2013. [Google Scholar]
- Kutz, S.J.; Jenkins, E.J.; Veitch, A.M.; Ducrocq, J.; Polley, L.; Elkin, B.; Lair, S. The Arctic as a model for anticipating, preventing, and mitigating climate change impacts on host–parasite interactions. Vet. Parasitol. 2009, 163, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Seton, E.T. Life-Histories of Northern Animals: An Account of the Mammals of Manitoba; Charles Schriebers Sons: New York, NY, USA, 1909; Volume 1. [Google Scholar]
- Cameron, A.E.; Fulton, J.S. A local Outbreak of the Winter or Moose Tick, Dermacentor albipictus, Pack. (Ixodoidea) in Saskatchewan. Bull. Entomol. Res. 1927, 17, 249–257. [Google Scholar] [CrossRef]
- Pybus, M.J. Moose and Ticks in Alberta: A Dieoff in 1998/99; Fisheries and Wildlife Management Division; Alberta Environment: Edmonton, AB, Canada, 1999.
- Samuel, W.M. Factors affecting epizootics of winter ticks and mortality of moose. Alces 2007, 43, 39–48. [Google Scholar]
- Jones, H.; Pekins, P.J.; Kantar, L.E.; O’neil, M.; Ellingwood, D. Fecundity and summer calf survival of moose during 3 successive years of winter tick epizootics. Alces 2017, 53, 85–98. [Google Scholar]
- Pekins, P.J. Metabolic and Population Effects of Winter Tick Infestations on Moose: Unique Evolutionary Circumstances? Front. Ecol. Evol. 2020, 8, 176. [Google Scholar] [CrossRef]
- Mooring, M.S.; Samuel, W. The biological basis of grooming in moose: Programmed versus stimulus-driven grooming. Anim. Behav. 1998, 56, 1561–1570. [Google Scholar] [CrossRef] [Green Version]
- Samuel, W.M.; Welch, D.A. Winter ticks on moose and other ungulates: Factors influencing their population size. Alces 1991, 27, 169–182. [Google Scholar]
- Samuel, B. White as a Ghost: Winter Ticks and Moose; Natural History Series; Federation of Alberta Naturalists: Edmonton, AB, Canada, 2004.
- Welch, D.A.; Samuel, W.M.; Wilke, C.J. Dermacentor albipictus (Acari, Ixodidae) on Captive Reindeer and Free-ranging Woodland Caribou. J. Wildl. Dis. 1990, 26, 410–411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bondo, K.J.; Macbeth, B.; Schwantje, H.; Orsel, K.; Culling, D.; Culling, B.; Tryland, M.; Nymo, I.H.; Kutz, S. Health Survey of Boreal Caribou (Rangifer Tarandus Caribou) in Northeastern British Columbia, Canada. J. Wildl. Dis. 2019, 55, 544–562. [Google Scholar] [CrossRef] [PubMed]
- Calvente, E.; Chinnici, N.; Brown, J.; Banfield, J.E.; Brooks, J.W.; Yabsley, M.J. Winter Tick (Dermacentor albipictus)–Associated Dermatitis in a Wild Elk (Cervus canadensis) in Pennsylvania, USA. J. Wildl. Dis. 2020, 56, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Calvente, E.; Pelletier, S.; Banfield, J.; Brown, J.; Chinnici, N. Prevalence of Winter Ticks (Dermacentor albipictus) in Hunter-Harvested Wild Elk (Cervus canadensis) from Pennsylvania, USA (2017–2018). Vet. Sci. 2020, 7, 177. [Google Scholar] [CrossRef]
- Machtinger, E.T.; Springer, H.R.; Brown, J.E.; Olafson, P.U. Sudden Mortality in Captive White-Tailed Deer with Atypical Infestation of Winter Tick. J. Med. Entomol. 2021, 58, 1962–1965. [Google Scholar] [CrossRef] [PubMed]
- Chenery, E.S.; Harms, N.J.; Mandrak, N.E.; Molnár, P.K. First records of Dermacentor albipictus larvae collected by flagging in Yukon, Canada. Parasites Vectors 2020, 13, 565. [Google Scholar] [CrossRef]
- Government of Yukon. Management Plan for Elk in Yukon; Environment Yukon: Whitehorse, YT, Canada, 2016; p. 22.
- Government of Yukon. Hair Transect Sampling Trials for Winter Tick Monitoring on Elk Hides: Draft Report; Environment Yukon: Whitehorse, YT, Canada, 2012.
- Lakomý, M.; Hlavová, R.; Machackova, H.; Bohlin, G.; Lindholm, M.; Bertero, M.G.; Dettenhofer, M. The motivation for citizens’ involvement in life sciences research is predicted by age and gender. PLoS ONE 2020, 15, e0237140. [Google Scholar] [CrossRef]
- Lotfian, M.; Ingensand, J.; Brovelli, M.A. A Framework for Classifying Participant Motivation that Considers the Typology of Citizen Science Projects. ISPRS Int. J. Geo-Inf. 2020, 9, 704. [Google Scholar] [CrossRef]
- Milligan, H. Licensed Harvest Trends in Yukon: 1980 To 2014; Yukon Fish and Wildlife Branch Report MR-18-05; Department of Environment, Government of Yukon: Whitehorse, YT, Canada, 2018.
- Commissioner of Yukon. Wildlife Act: Wildlife Regulation; O.I.C. 2012/084. Available online: https://laws.yukon.ca/cms/images/LEGISLATION/SUBORDINATE/2012/2012-0084/2012-0084_1.pdf (accessed on 5 February 2022).
- Addison, E.M.; McLaughlin, R.F. Growth and Development of Winter Tick, Dermacentor albipictus, on Moose, Alces alces. J. Parasitol. 1988, 74, 670. [Google Scholar] [CrossRef]
- Lindquist, E.E.; Galloway, T.D.; Artsob, H.; Lindsay, L.R.; Drebot, M.; Wood, H.; Robbins, R. A Handbook to the Ticks of Canada (Ixodida: Ixodidae, Argasidae); Biological Survey of Canada Monograph Series No. 7; Biological Survey of Canada: Sackville, NB, Canada, 2016. [Google Scholar]
- Cox, J.; Oh, E.Y.; Simmons, B.; Lintott, C.; Masters, K.; Greenhill, A.; Graham, G.; Holmes, K. Defining and Measuring Success in Online Citizen Science: A Case Study of Zooniverse Projects. Comput. Sci. Eng. 2015, 17, 28–41. [Google Scholar] [CrossRef]
- Freitag, A.; Pfeffer, M.J. Process, Not Product: Investigating Recommendations for Improving Citizen Science “Success”. PLoS ONE 2013, 8, e64079. [Google Scholar] [CrossRef] [PubMed]
- Government of Yukon. Yukon Hunting Regulations Summary 2020–2021. 2020. Available online: https://emrlibrary.gov.yk.ca/environment/yukon-hunting-regulations-summary/2020-21.pdf (accessed on 24 February 2022).
- Government of Yukon. Yukon Hunting Regulations Summary 2021–2022. 2021. Available online: https://emrlibrary.gov.yk.ca/environment/yukon-hunting-regulations-summary/2021-22.pdf (accessed on 24 February 2022).
- Zutlevics, T. Could providing financial incentives to research participants be ultimately self-defeating? Res. Ethics 2016, 12, 137–148. [Google Scholar] [CrossRef]
- Martin, V.Y. Citizen Science as a Means for Increasing Public Engagement in Science: Presumption or possibility? Sci. Commun. 2017, 39, 142–168. [Google Scholar] [CrossRef]
- Khadjesari, Z.; Murray, E.; Kalaitzaki, E.; White, I.; McCambridge, J.; Thompson, S.G.; Wallace, P.; Godfrey, C. Impact and Costs of Incentives to Reduce Attrition in Online Trials: Two Randomized Controlled Trials. J. Med. Internet Res. 2011, 13, e26. [Google Scholar] [CrossRef]
- Eisen, L.; Eisen, R.J. Benefits and Drawbacks of Citizen Science to Complement Traditional Data Gathering Approaches for Medically Important Hard Ticks (Acari: Ixodidae) in the United States. J. Med. Entomol. 2021, 58, 1–9. [Google Scholar] [CrossRef]
- Kullenberg, C.; Kasperowski, D. What Is Citizen Science?–A Scientometric Meta-Analysis. PLoS ONE 2016, 11, e0147152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ginsberg, H.S.; Rulison, E.L.; Miller, J.L.; Pang, G.; Arsnoe, I.M.; Hickling, G.J.; Ogden, N.H.; LeBrun, R.A.; Tsao, J.I. Local abundance of Ixodes scapularis in forests: Effects of environmental moisture, vegetation characteristics, and host abundance. Ticks Tick-Borne Dis. 2020, 11, 101271. [Google Scholar] [CrossRef]
- Eogden, N.; Mechai, S.; Margos, G. Changing geographic ranges of ticks and tick-borne pathogens: Drivers, mechanisms and consequences for pathogen diversity. Front. Cell. Infect. Microbiol. 2013, 3, 46. [Google Scholar] [CrossRef] [Green Version]
- Martıínez, M.; Rodrıíguez-Vigal, C.; Jones, O.R.; Coulson, T.; Miguel, A.S. Different hunting strategies select for different weights in red deer. Biol. Lett. 2005, 1, 353–356. [Google Scholar] [CrossRef] [Green Version]
- Bunnefeld, N.; Baines, D.; Newborn, D.; Milner-Gulland, E.J. Factors affecting unintentional harvesting selectivity in a monomorphic species. J. Anim. Ecol. 2009, 78, 485–492. [Google Scholar] [CrossRef]
- Yoder, J.A.; Pekins, P.J.; Dobrotka, C.J.; Fisher, K.A.; Kantar, L.; McLellan, S.; O’Neal, M.; Klompen, H. Tick development on sexually-active bull moose is more advanced compared to that of cow moose in the winter tick, Dermacentor albipictus. Int. J. Parasitol. 2019, 9, 56–59. [Google Scholar] [CrossRef] [PubMed]
- Swei, A.; O’Connor, K.E.; Couper, L.I.; Thekkiniath, J.; Conrad, P.A.; Padgett, K.A.; Burns, J.; Yoshimizu, M.H.; Gonzales, B.; Munk, B.; et al. Evidence for transmission of the zoonotic apicomplexan parasite Babesia duncani by the tick Dermacentor albipictus. Int. J. Parasitol. 2019, 49, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Baldridge, G.D.; Scoles, G.A.; Burkhardt, N.Y.; Schloeder, B.; Kurtti, T.J.; Munderloh, U.G. Transovarial transmission of Francisella-like endosymbionts and Anaplasma phagocytophilum variants in Dermacentor albipictus (Acari: Ixodidae). J. Med. Entomol. 2009, 46, 625–632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schotthoefer, A.; Stinebaugh, K.; Martin, M.; Munoz-Zanzi, C. Tickborne disease awareness and protective practices among U.S. Forest Service employees from the upper Midwest, USA. BMC Public Health 2020, 20, 1575. [Google Scholar] [CrossRef] [PubMed]
- Samuel, W.M. Locations of Moose in Northwestern Canada with Hair Loss Probably Caused by the Winter Tick, Dermacentor albipictus (Acari: Ixodidae). J. Wildl. Dis. 1989, 25, 436–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watt, K. British Columbia Provincial Moose Winter Tick Surveillance Program. 2021. Available online: https://www2.gov.bc.ca/assets/gov/environment/plants-animals-and-ecosystems/wildlife-wildlife-habitat/wildlife-health/wildlife-health-documents/provincial_moose_winter_tick_program_report.pdf (accessed on 24 February 2022).
- Carlson, C.J.; Burgio, K.R.; Dougherty, E.R.; Phillips, A.J.; Bueno, V.M.; Clements, C.F.; Castaldo, G.; Dallas, T.A.; Cizauskas, C.A.; Cumming, G.S.; et al. Parasite biodiversity faces extinction and redistribution in a changing climate. Sci. Adv. 2017, 3, 1602422. [Google Scholar] [CrossRef] [Green Version]
- Kuba, K.; Larivee, M.; Kloppers, E.; Ward, R.; Florkiewicz, R.; VanderKop, M.; Harms, N.J. Hair Transect Sampling Method Trials for Monitoring Winter Tick (Dermacentor albipictus) Burdens on Elk (Cervus elaphus) Hides in Yukon; Yukon Fish and Wildlife Branch Report TR-16; Government of Yukon: Whitehorse, YT, Canada, 2016.





| Harvested Species | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 |
|---|---|---|---|---|---|---|---|---|---|---|
| Moose | 0.2 | 0 | 0 | 0 | 0 | 0.3 | 0.1 | 0.3 | 7.5 | 9.0 |
| Caribou | 0 | 0 | 0.3 | 0.4 | 0 | 0 | 0 | 0.8 | 5.5 | 8.5 |
| Number of: | 2019 | 2020 |
|---|---|---|
| Hunting licenses | 5135 | 5125 |
| Hunting seals issued—Moose | 3957 | 3950 |
| Hunting seals issued—Caribou | 3467 | 3668 |
| Harvested animals (Moose + Caribou) | 1167 | 860 |
| Kits distributed | 435 | 617 |
| Kits returned | 44 | 56 |
| Engagement | ||
| All hunters | 8.5% | 12.0% |
| With seals (average) | 11.7% | 16.2% |
| Successful harvest | 37.3% | 71.7% |
| Participation | 10.1% | 9.1% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chenery, E.S.; Henaff, M.; Magnusson, K.; Harms, N.J.; Mandrak, N.E.; Molnár, P.K. Improving Widescale Monitoring of Ectoparasite Presence in Northern Canadian Wildlife with the Aid of Citizen Science. Insects 2022, 13, 380. https://0-doi-org.brum.beds.ac.uk/10.3390/insects13040380
Chenery ES, Henaff M, Magnusson K, Harms NJ, Mandrak NE, Molnár PK. Improving Widescale Monitoring of Ectoparasite Presence in Northern Canadian Wildlife with the Aid of Citizen Science. Insects. 2022; 13(4):380. https://0-doi-org.brum.beds.ac.uk/10.3390/insects13040380
Chicago/Turabian StyleChenery, Emily S., Maud Henaff, Kristenn Magnusson, N. Jane Harms, Nicholas E. Mandrak, and Péter K. Molnár. 2022. "Improving Widescale Monitoring of Ectoparasite Presence in Northern Canadian Wildlife with the Aid of Citizen Science" Insects 13, no. 4: 380. https://0-doi-org.brum.beds.ac.uk/10.3390/insects13040380