Development and Evaluation of a New Effective Tool and Method for Assessing Varroadestructor (Acari: Varroidae) Mite Populations in Honey Bee Colonies
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
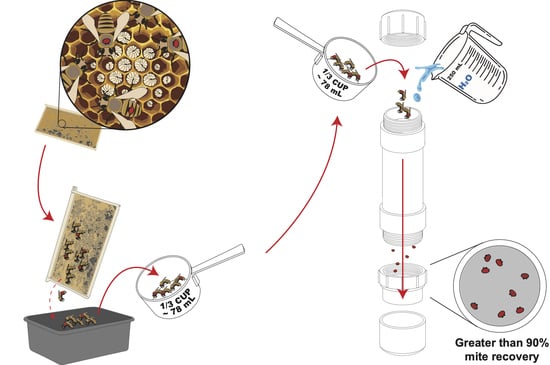
2.1. Construction of the Varroa Shaking Device (VSD)

2.2. Evaluations for Using the VSD
2.2.1. Test of Sample Size (Number of Bees)
2.2.2. Test of Device Shaking Time
2.2.3. Test of Sampling Location within Hives
2.2.4. Evaluation of the VSD Compared with the Sugar Roll Method
3. Results
3.1. Test of Sample Size (Number of Bees)
3.2. Test of Device Shaking Time
3.3. Test of Sampling Location within Hives
3.4. Evaluations of the VSD Compared with the Sugar Roll Method
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rosenkranz, P.; Aumeier, P.; Ziegelmann, B. Biology and control of varroa destructor. J. Invertebr. Pathol. 2010, 103, S96–S119. [Google Scholar] [CrossRef] [PubMed]
- Delaplane, K.S.; Berry, J.A.; Skinner, J.A.; Parkman, J.P.; Hood, W.M. Integrated pest management against varroa destructor reduces colony mite levels and delays treatment threshold. J. Apic. Res. 2005, 44, 157–162. [Google Scholar] [CrossRef]
- Harris, J.; Sheridan, A.B.; MacGown, J. Managing Varroa Mites in Honey Bee Colonies; Mississippi State University Extension Service: Starkville, MS, USA, 2015. [Google Scholar]
- De Jong, D.; Roma, D.D.A.; Goncalves, L. A comparative analysis of shaking solutions for the detection of varroa jacobsoni on adult honeybees. Apidologie 1982, 13, 297–306. [Google Scholar] [CrossRef] [Green Version]
- Rinderer, T.; De Guzman, L.; Sylvester, H.A. Re-examination of the accuracy of a detergent solution for varroa mite detection. Am. Bee J. 2004, 144, 560–562. [Google Scholar]
- Ward, K.; Danka, R.; Ward, R. Comparative performance of two mite-resistant stocks of honey bees (hymenoptera: Apidae) in alabama beekeeping operations. J. Econ. Entomol. 2008, 101, 654–659. [Google Scholar] [CrossRef] [PubMed]
- HBHC. Tools for Varroa Management a Guide to Effective Varroa Sampling & Control; HBHC: London, UK, 2017; pp. 1–25. [Google Scholar]
- Delaplane, K.S.; Hood, W.M. Economic threshold for varroa jacobsoni oud. In the southeastern USA. Apidologie 1999, 30, 383–395. [Google Scholar] [CrossRef] [Green Version]
- Devlin, S.M. Comparative Analyses of Sampling Methods for Varroa; Citeseer: State College, PA, USA, 2001. [Google Scholar]
- Calderone, N.; Turcotte, R. Development of sampling methods for estimating infestation levels of varroa jacobsoni (acari: Varroidae) in colonies of the honey bee, apis mellifera (hymenoptera: Apidae). J. Econ. Entomol. 1998, 91, 851–863. [Google Scholar] [CrossRef]
- Hood, M. Varroa mite control in south carolina. Entomol. Insect Inf. Ser. 2000, 12, 1–7. [Google Scholar]
- Shabanov, M.; Nedjalkov, S.; Toshkov, A. Eine schnelle einfache methode zur varroatose-diagnose. From diagnose und therapie der varroatose; Apimondia Publishing House: Bucharest, Romania, 1980; p. 108. [Google Scholar]
- Branco, M.R.; Kidd, N.A.; Pickard, R.S. A comparative evaluation of sampling methods for varroa destructor (acari: Varroidae) population estimation. Apidologie 2006, 37, 452–461. [Google Scholar] [CrossRef] [Green Version]
- Macedo, P.A.; Wu, J.; Ellis, M.D. Using inert dusts to detect and assess varroa infestations in honey bee colonies. J. Apic. Res. 2002, 41, 3–7. [Google Scholar] [CrossRef] [Green Version]
- Kuenen, L.; Calderone, N. Transfers ofvarroa mites from newly emerged bees: Preferences for age-and function-specific adult bees (hymenoptera: Apidae). J. Insect Behav. 1997, 10, 213–228. [Google Scholar] [CrossRef]
- Pietropaoli, M.; Tlak Gajger, I.; Costa, C.; Gerula, D.; Wilde, J.; Adjlane, N.; Aldea-Sánchez, P.; Smodiš Škerl, M.I.; Bubnič, J.; Formato, G. Evaluation of two commonly used field tests to assess varroa destructor infestation on honey bee (Apis mellifera) colonies. Appl. Sci. 2021, 11, 4458. [Google Scholar] [CrossRef]
- Gregorc, A.; Sampson, B. Diagnosis of varroa mite (varroa destructor) and sustainable control in honey bee (apis mellifera) colonies—A review. Diversity 2019, 11, 243. [Google Scholar] [CrossRef] [Green Version]
- Milbrath, M. Varroa mite monitoring using a sugar roll to identify populations of varroa destructor in honey bee colonies. Am. Bee J. 2016, 156, 1119–1122. [Google Scholar]
- Spivak, M.; Reuter, G.S. Honey Bee Diseases and Pests: A Companion to Beekeeping in Northern Climates. University of Minnesota, Department of Entomology and Minnesota Extension: Minneapolis, MN, USA, 2006. [Google Scholar]
- SAS Institute Inc. Version 9.1.3 User Guide; SAS Institute: Cary, NC, USA, 2003. [Google Scholar]
- Caron, D.; Burdick, E.; Danek, K. The Powdered Sugar Sampling to Monitor Varroa Mite Populations in Honey Bee Colonies; MAAREC: University Park, PA, USA, 2009. [Google Scholar]
- Ellis, M.D.; Macedo, P.A. G1430 using the sugar roll technique to detect varroa mites in honey bee colonies. Hist. Mater. Univ. Neb. Linc. Ext. 2001, 116. [Google Scholar]
- Noble, N.I.; Stuhl, C.; Nesbit, M.; Woods, R.; Ellis, J.D. A comparison of varroa destructor (acari: Varroidae) collection methods and survivability in in vitro rearing systems. Fla. Entomol. 2021, 104, 13–17. [Google Scholar] [CrossRef]
- Xie, X.; Huang, Z.; Zeng, Z. Why do varroa mites prefer nurse bees? Sci. Rep. 2016, 6, 28228. [Google Scholar] [CrossRef] [Green Version]
- Berry, J.A.; Afik, O.; Nolan IV, M.P.; Delaplane, K.S. Revisiting powdered sugar for varroa control on honey bees (apis mellifera L.). J. Apic. Res. 2012, 51, 367–368. [Google Scholar] [CrossRef] [Green Version]
- Hoppe, H.; Ritter, W. The influence of the nasonov pheromone on the recognition of house bees and foragers by varroa jacobsoni. Apidologie 1988, 19, 165–172. [Google Scholar] [CrossRef] [Green Version]
- Delaplane, K.S.; Hood, W.M. Effects of delayed acaricide treatment in honey bee colonies parasitized by varroa jacobsoni and a late-season treatment threshold for the south-eastern USA. J. Apic. Res. 1997, 36, 125–132. [Google Scholar] [CrossRef]
- Lee, K.; Moon, R.; Burkness, E.; Hutchison, W.; Spivak, M. Practical sampling plans for varroa destructor (acari: Varroidae) in apis mellifera (hymenoptera: Apidae) colonies and apiaries. J. Econ. Entomol. 2010, 103, 1039–1050. [Google Scholar] [CrossRef] [PubMed]



| Item No. | Material | Description | QTY |
|---|---|---|---|
| 1 | PVC * Pipe | Main Body, 5.8 cm W, 15 cm L | 1 |
| 2 | PVC Adaptor | Threaded Male–Female, 5.8 cm–6.0 cm W, ~4.8 cm L | 2 |
| 3 | Metal Mesh | #8 Wire Mesh 6.0 cm W | 1 |
| 4 | PVC Spigot | Threaded Female–Male 6.0 cm–5.8 cm W, ~4.8 cm L | 1 |
| 5 | Metal Screen | #80 Wire Mesh 5.8 cm W | 1 |
| 6 | PVC Top Cap | Threaded, 5.8 cm W, ~3.5 cm L | 1 |
| 7 | PVC Bottom Cap | Non-threaded, 6.0 cm W | 1 |
| Sample Size Group | No. Hives | No. Varroa | No. Bees | No. Varroa/100 Bees |
|---|---|---|---|---|
| <250 bees | 31 | 18.5 ± 2.7 | 218.0 ± 4.2 | 8.4 ± 1.2 |
| >250 bees | 16 | 47.7 ± 25.9 | 278.2 ± 11.6 | 17.9 ± 9.8 |
| Sample Shaking Time (Seconds) | No. Hives | No. Varroa | No. Bees | No. Varroa/100 Bees |
|---|---|---|---|---|
| 40 | 4 | 19.0 ± 9.8 | 243.8 ± 11.43 | 8.5 ± 4.6 |
| 50 | 3 | 16.7 ± 11.6 | 241.0 ± 19.7 | 6.3 ± 4.5 |
| 60 | 4 | 22.8 ± 8.5 | 229.8 ± 15.4 | 9.5 ± 3.2 |
| 90 | 4 | 38.3 ± 17.5 | 233.8 ± 11.8 | 15.5 ± 6.4 |
| Location Bees Collected | No. Hives | No. Varroa | No. Bees | No. Varroa/100 Bees |
|---|---|---|---|---|
| Brood Frame (BF) | 6 | 21.5 ± 4.4 | 227.0 ± 10.5 | 9.9 ± 2.4 |
| Forager Frame (FF) | 6 | 9.8 ±2.6 | 218.8 ± 11.6 | 4.5 ± 1.1 |
| Blend (BF + FF) | 6 | 13.5 ± 2.6 | 241.8 ± 5.5 | 5.7 ± 1.2 |
| Year and Method | No. Hives | No. Varroa in Sample | No. Bees in Sample | No. Varroa per 100 Bees | Removal Efficiency (%) |
|---|---|---|---|---|---|
| 2017 | Mean ± se | Mean ± se | Mean ± se | Mean ± se | |
| VSD | 21 | 20.2 ± 4.6 | 245.5 ± 12.9 | 8.3 ± 1.8 | 90.3 ± 4.2 |
| Sugar Roll | 21 | 22.5 ± 5.2 | 255.6 ± 12.6 | 9.1 ± 2.0 | 97.4 ± 1.3 |
| 2018 | |||||
| VSD | 10 | 38.4 ± 9.7 | 226.0 ± 3.70 | 17.0 ± 4.2 | 95.8 ± 1.3 |
| Sugar Roll | 10 | 34.5 ± 8.18 | 227.0 ± 5.27 | 15.0 ± 3.7 | 98.4 ± 0.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Posada-Flórez, F.J.; Abban, S.K.; Smith, I.B., Jr.; Cook, S.C. Development and Evaluation of a New Effective Tool and Method for Assessing Varroadestructor (Acari: Varroidae) Mite Populations in Honey Bee Colonies. Insects 2022, 13, 457. https://0-doi-org.brum.beds.ac.uk/10.3390/insects13050457
Posada-Flórez FJ, Abban SK, Smith IB Jr., Cook SC. Development and Evaluation of a New Effective Tool and Method for Assessing Varroadestructor (Acari: Varroidae) Mite Populations in Honey Bee Colonies. Insects. 2022; 13(5):457. https://0-doi-org.brum.beds.ac.uk/10.3390/insects13050457
Chicago/Turabian StylePosada-Flórez, Francisco J., Samuel K. Abban, I. Barton Smith, Jr., and Steven C. Cook. 2022. "Development and Evaluation of a New Effective Tool and Method for Assessing Varroadestructor (Acari: Varroidae) Mite Populations in Honey Bee Colonies" Insects 13, no. 5: 457. https://0-doi-org.brum.beds.ac.uk/10.3390/insects13050457