1. Introduction
As in many tropical countries around the world, dengue continues to be a major public health concern in Indonesia. Data from the Indonesian Ministry of Health recorded that the number of dengue haemorrhagic fever cases in 2010 reached a peak of 156,086 with 1358 deaths [
1]. Fewer cases have been reported since 2010, but numbers continue to be high (e.g., 101,218 cases with 736 deaths in 2013 [
2]) and the disease burden on the population remains substantial. While dengue vaccine development has been an area of active research, the main way to prevent and control dengue virus transmission is to target its primary vector, the mosquito,
Aedes aegypti (L.). Current dengue control in Indonesia focuses on community-based elimination of mosquito breeding sites [
3,
4,
5,
6], however chemical insecticides have been an important tool for more than 40 years of dengue vector control [
6,
7,
8,
9]. The two classes of insecticide that have been utilized intensively to prevent or reduce dengue transmission are organophosphates (temephos and malathion) and pyrethroids. Malathion fogging to control adult mosquitoes has been in use since 1973 [
10] and, starting in 1980, was supplemented with use of temephos larvicides [
7].
In the late 1980s, synthetic pyrethroids were introduced to control the dengue vector [
11,
12,
13] and have been used widely since as a control measure to eliminate adult mosquitoes particularly during dengue outbreaks. Community-wide applications of pyrethroids have been used for ongoing seasonal vector control, and they have also been applied extensively at the household level. Specific pyrethroids advised by Indonesian vector borne diseases control programs include the adulticides cyfluthrin 50% EC; cypermethrin 25% ULV; lamdacyhalothrin 25% EC and permethrin and
S-bioallethrin 10/1.5 OS; and alpha-
cypermethrin [
8].
Despite many ongoing efforts to combat dengue in Indonesia, control of the disease is facing many challenges. Perhaps the most important issue involves the development of insecticide resistance in
Ae. aegypti, which probably has evolved due to a range of practices including frequent and/or indiscriminate application of insecticides. There are several ways that insect populations can become resistant to insecticides; resistance mechanisms include increased metabolic detoxification of the insecticide before it reaches its target site by a series of enzymatic reactions, as well as decreased sensitivity of the target site so that the insecticide does not bind and activate in the usual way [
14,
15].
The insecticide resistance status of
Ae.
aegypti in Indonesia has not been monitored on a regular basis, despite the strong dependence on insecticides for vector control. Pyrethroid resistance assays of three strains of
Ae.
aegypti from different areas in Indonesia demonstrated that a Bandung strain was resistant to permethrin and deltamethrin with RR
90 (resistance ratios for 90% mortality) of 79.3 and 23.7, respectively [
16]. Relatively weak resistance to permethrin (RR
90 of 11.1) was detected in a strain from Palembang which was still susceptible to deltamethrin (RR
90 of 2.2). In addition, a strain from Surabaya remained relatively susceptible to permethrin and deltamethrin with RR
90 of 8.6 and 2.5, respectively [
16]. Resistance to pyrethroids was studied in two laboratory reared strains (Namru and IPB) and a field collected strain (ITB) [
17] and, in this case, all three strains exhibited some resistance to permethrin 92%, cypermethrin 92% and d-allethrin 93% indicated by high values of LT
90. Strains resistant to permethrin from Larentuka and Semarang, Indonesia, were reported by Brengues
et al. [
18] and used in an investigation of pyrethroid resistance mechanisms.
Pyrethroids primarily affect both the peripheral and central nervous systems of insects by binding to a target site in the voltage-gated sodium channel or voltage-sensitive sodium channel (
Vssc) within the nerve membrane [
19]. They can be classified into two groups, Type I and Type II, based on their chemistry and effects. Type I pyrethroids typically exhibit low potency, knockdown and high repellency. They lack an α-cyano group at the phenoxybenzyl alcohol position and prolong opening of the sodium channel causing repetitive firing of the neuron [
20]. Type II pyrethroids show a much higher potency with acute lethal effects. They contain an α-cyano-3-phenoxybenzyl alcohol moiety and prolong opening of the sodium channel causing membrane depolarization [
20].
The intensive and sustained use of pyrethroids has led to the development of knockdown resistance (
kdr) in many insect species [
21].
Kdr is caused by a reduction in the sensitivity of sodium channels to pyrethroids, due to reduced binding of the insecticide at the target site [
21,
22]. The
kdr phenotype is associated with a range of non-synonymous, single point mutations within the
Vssc gene (
para) which code for these channels in the transmembrane protein [
23]. At least seven mutations have been detected in the
Vssc gene of
Ae.
aegypti: V1023I (
i.e., Valine to Isoleucine amino acid change), V1023G, F1565C, I1018M, I1018V, S996P and D1794Y [
24]. The most widely studied have been V1023I and F1565C. Du
et al. [
24] tested all seven
Vssc mutations reported in
Ae. aegypti, by developing a functional expression system for the sodium channel in
Xenopus oocytes, but concluded that only three of these, V1023G, I1018M and F1565C are directly implicated in pyrethroid resistance.
V1023G has been shown to confer resistance to type I and II pyrethroids [
25] and has been detected in
Ae. aegypti from southeast Asia [
26,
27]. F1565C is thought to confer resistance to type I pyrethroids [
25] and has been detected in
Ae.
aegypti from Thailand, Vietnam, the Caribbean [
28,
29] and recently in Brazil [
30]. Mutation I1011M [
24] only confers resistance to type I pyrethroids and is present in
Ae.
aegypti from South America and Martinique [
18,
31]. V1023I, the widely monitored mutation [
29,
32,
33,
34,
35,
36], did not alter channel sensitivity in the study by Du
et al. [
24].
In many insects, some
kdr mutations are known to act in conjunction with others. An example is the first
kdr mutation discovered in the house fly [
26] which causes higher levels of resistance when another mutation is present (M918T super
kdr) [
19]. A recent study by Hirata
et al. [
37] suggested that S996P alone has no effect on the
Vssc and pyrethroid resistance. However, it was shown to have a slight synergistic effect when it occurs in conjunction with V1023G and increases resistance to deltamethrin (Type II), but not to permethrin (Type I) [
37]. V1023G alone reduced sensitivity of the
Vssc to permethrin by 100-fold, whereas F1565C alone produced a 25-fold reduction. The same trend occurred for deltamethrin for V1023G, though the magnitude of the effect was smaller (2-fold). F1565C alone had no effect on the sensitivity of the
Vssc to deltamethrin which agrees with the result of Du
et al. [
24]. Interestingly, the triple mutation (S996P, V1023G, F1565C), when created artificially and expressed in
Xenopus oocytes for voltage clamp testing, appears to have synergistic effects, increasing the resistance effect of permethrin by 1100-fold and deltamethrin by 90-fold. V1023I did not alter channel sensitivity [
24], but effects may be different if it occurs as a triple mutation as in the case above.
Brito
et al. [
34] crossed V1023I, F1565C homozygous resistant and homozygous susceptible lines of
Ae.
aegypti and found evidence that
kdr is a recessive trait. Similar crosses made by Chang
et al. [
31] with V1023G, D1794Y homozygotes with susceptible lines suggested that the trait is incompletely recessive.
A number of techniques exist for detecting
kdr mutations including allele-specific PCR [
23,
27,
30,
34,
36,
38]; Hot Ligation, a method that detects ligation between detector and reporter oligonucleotides when the detector is annealed to the SNP site using a thermal stable ligase and cycles of denaturing and hybridization to produce detectable qualities of ligated detector and reporter [
29,
35,
39]; and Tetraplex, with two outer flanking primer to amplify a large fragment of target gene as a control and two inner allele specific primers in opposite directions to each other to generate smaller PCR products by forming PCR primer pairs with the outer primers [
29,
35]. All of these assays might be useful for routine diagnostic use. However, for large scale screening of samples, we need an assay that can detect these mutations of interest in a robust but faster manner than these conventional PCR assays.
Research relating to point mutations in the target site of pyrethroid insecticides in
Ae.
aegypti is very limited in Indonesian material [
18] and the
kdr mutation status of
Ae.
aegypti is not known. Therefore we aimed to (1) develop high-resolution melt (HRM) genotyping assays to detect those mutations with the most evidence for causing pyrethroid resistance, F1565C, V1023G and S996P, in the voltage-sensitive sodium channel gene of
Ae.
aegypti (
Figure 1) and use the assays to determine the frequency of these mutations in the mosquitoes around Yogyakarta; We also aimed to (2) investigate the association of the mutations both singly and in combination with resistance (as defined in bioassays); (3) determine the resistance status and
kdr allelic composition of a
Wolbachia-infected laboratory population with virus-blocking properties which has been outcrossed to field mosquitoes from Yogyakarta and which will be used to replace the natural population of
Ae.
aegypti as a dengue suppression technique; (4) provide information to assist with management of insecticide resistance; and (5) provide specific comparative resistance information to inform the release of
Wolbachia mosquitoes in this city.
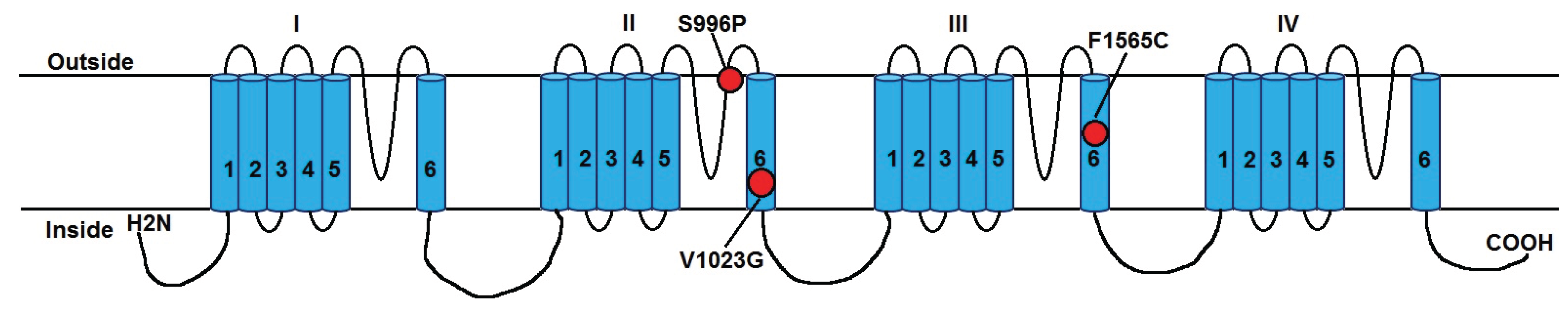
Figure 1.
Positions of pyrethroid resistance-associated
Vssc mutations of
Aedes aegypti that are detected in this study. The schematic diagram shows the sodium channel protein indicating the four internally homologous domains (I–IV), each having six hydrophobic transmembrane helices (1–6). The mutations are numbered according to amino acid positions in the sodium channel gene of
Aedes aegypti (diagram based on that of Du
et al. [
24]).
Figure 1.
Positions of pyrethroid resistance-associated
Vssc mutations of
Aedes aegypti that are detected in this study. The schematic diagram shows the sodium channel protein indicating the four internally homologous domains (I–IV), each having six hydrophobic transmembrane helices (1–6). The mutations are numbered according to amino acid positions in the sodium channel gene of
Aedes aegypti (diagram based on that of Du
et al. [
24]).
2. Experimental Section
2.1. Mosquito Samples
Larvae of
Ae.
aegypti were collected using ovitrap buckets, 12.50 cm in diameter and 13.00 cm in height. About 900 mL fresh water was added to a level of 9 cm and a flannel oviposition strip (5 cm × 12 cm) was placed vertically with a part of it in the water. Fish food was added to attract female mosquitoes. Ovitraps were set up at ten “outer city” sites (Site 1 to Site 10) in Yogyakarta during November to December 2011 and these samples were described as Season 1. Season 2 comprised samples from five of the same “outer city” sites (Site 2, 3, 6, 7 and 8) collected in July 2012 and three “city” sites (Site 11, 12, and 13) collected in November 2012 (
Figure 2). To ensure that there was a representation of mosquitoes from throughout each site, larvae were sourced from at least 100 indoor and 100 outdoor ovitraps placed at a site. Pupae were collected, separated into males and females, placed in cages and allowed to emerge as adults.
Figure 2.
Map of Yogyakarta and its surrounding area showing the sites where samples of Aedes aegypti were collected.
Figure 2.
Map of Yogyakarta and its surrounding area showing the sites where samples of Aedes aegypti were collected.
Three mosquito samples were used for bioassays of insecticides: Site 6, Site 8 and the
wMelYog strain. For Site 6 and Site 8, samples were F
2 from field-collected mosquitoes. The
wMelYog strain is a laboratory strain made by crossing two uninfected field strains (derived from Site 6 and Site 8) with the
Wolbachia-infected strain,
wMel [
40,
41] and undertaking repeated backcrossing for 6 generations to ensure that the infection was on the genetic background of the target strain.
2.2. Deltamethrin and Permethrin Bioassay
The standard WHO mosquito bioassay protocol was followed using bioassay tubes and insecticide-impregnated papers containing a diagnostic dose [
42]. At least 150 non-blood fed, virgin females aged 3–5 days post emergence (four treatment replicates of 25 mosquitoes per tubes and two control replicates of 25 mosquitoes per tube) were evaluated for each insecticide from each site. The bioassay was repeated at a second time point.
Mosquitoes were exposed in a tube for 1 h to filter paper impregnated with deltamethrin (0.05%) or permethrin (0.75%) in a silicone oil solvent. The paper was obtained from Universiti Sains Malaysia (USM), Penang, Malaysia, a WHO collaborator. Control mosquitoes were exposed to papers impregnated with solvent (silicone oil) only. Knockdown was recorded after 1 h of exposure. All surviving mosquitoes were provided with 10% sugar solution, and final mortality was recorded at 24 h post-exposure.
2.4. Tetra-Primer Assay to Genotype F1565C Mutation
The tetra-primer PCR procedure [
29] was used to identify whether the F1565C mutation was present in the sodium channel gene of
Ae.
aegypti from Yogyakarta. PCR primer pairs designed by Harris
et al. [
29] were used to amplify exon 31 at domain III, subunit 6 of the voltage-sensitive sodium channel. They consist of a pair of flanking primers (AaEx31P and AaEx31Q) and two internal primers (AaEx31wt and AaEx31mut). The flanking primers were required to amplify a control band of 350 bp. Primer AaEx31wt was paired with AaEx31Q to genotype the “wild type” (phenylalanine allele) of 231 bp, whereas the combination of primer AaEx31mut and AaEx31P was used to genotype the mutant cysteine allele (AaEx31mut) of 163 bp. Forty-six samples were genotyped using this method.
Each PCR reaction was carried out in a total volume of 25 μL containing 2.5 μL 10× Reaction Buffer (MgCl2 free) (NEB); 1.25 μL 50 mM MgCl2; 4.00 μL 2.5 mM dNTPs (Bioline); 1.50 μL BSA (NEB) (10 mg/mL); 1.25 μL 10 μM Primer Forward; 1.25 μL 10 μM Primer Reverse; 0.50 μL Taq polymerase (NEB) (5 u/μL); and 5 μL of 1 in 10 diluted DNA template. Cycling conditions were as follows: initial denaturation of 95 °C for 5 min followed by 35 cycles of 94 °C for 30 s, 63 °C for 30 s, and 72 °C for 30 s, then a final elongation at 72 °C for 10 min. Each DNA template was assayed separately with all three primer combinations to yield either a 350 bp, 231 bp or 163 bp product. To visualize amplification, a 4 μL aliquot of each of the three PCR products were combined and mixed with 4 μL loading buffer and run on a 2% agarose gel where a single lane represents one individual typed for all three primer combinations. A 100-bp ladder (Hyperladder V, Bioline, Taunton, MA, USA) was used for sizing.
2.5. PCR Assay to Identify V1023G and S996P in Domain IIS6 of Vssc
Primers designed by Martins
et al. [
32] were used to identify mutations V1023G and S996P in the
Vssc IIS6 region in
Ae.
aegypti from Yogyakarta. The primers were designed based on the alignment of the IIS6 region, partially covering exons 20 and 21, obtained from AaNav cDNA (GenBank accession No. AF534112) and the
Drosophila melanogaster orthologous genomic DNA sequence (GenBank accession No. M32078): 5'-ACAATGTGGATCGCTTCCC-3' and 5'-TGGACAAAAGCAAGGCTAAG-3' [
32].
Ninety mosquito samples from Yogyakarta with similar numbers each from Season 1 and Season 2 were amplified. The PCR was conducted in 40 μL [10× Reaction Buffer (MgCl2 free) (NEB) 4.0 μL; MgCl2 (50 mM) 1.2 μL; dNTPs (Bioline) (2.5 mM) 3.2 μL; 4.0 μL of each primer Forward and Reverse (10 μM); Taq polymerase (NEB) (5 u/μL) 1.0 μL, 4 μL DNA template (1 in 10 dilution) and 18.6 μL of ddH20). Reactions were performed for 3 min at 94 °C for initial denaturation, followed by 35 cycles of 30 s at 94 °C for denaturation, 30 s at 60 °C for annealing, and 60 s at 72 °C for polymerase extension. To confirm amplification, aliquots of 10 μL of the PCR products were loaded onto 2.0% agarose gels.
2.6. DNA Sequencing
To confirm the results from the above PCR assays, 96 of the amplified samples (80 for V1023G and 16 for F1565C) were sequenced by Macrogen Inc. (Seoul, South Korea) in order to identify all mutations in Ae. aegypti in this region and to determine how the intron varies if multiple mutations from different exons are present. The results were used to ascertain if a set of primers could be designed to amplify the mutations in a real time PCR HRM assay. Sequencing data were analyzed using Geneious 7.0.5 Software (Biomatters Ltd., Auckland, New Zealand).
2.7. HRM Assays to Genotype F1565, V1023 and S996P Polymorphisms in the Para Gene
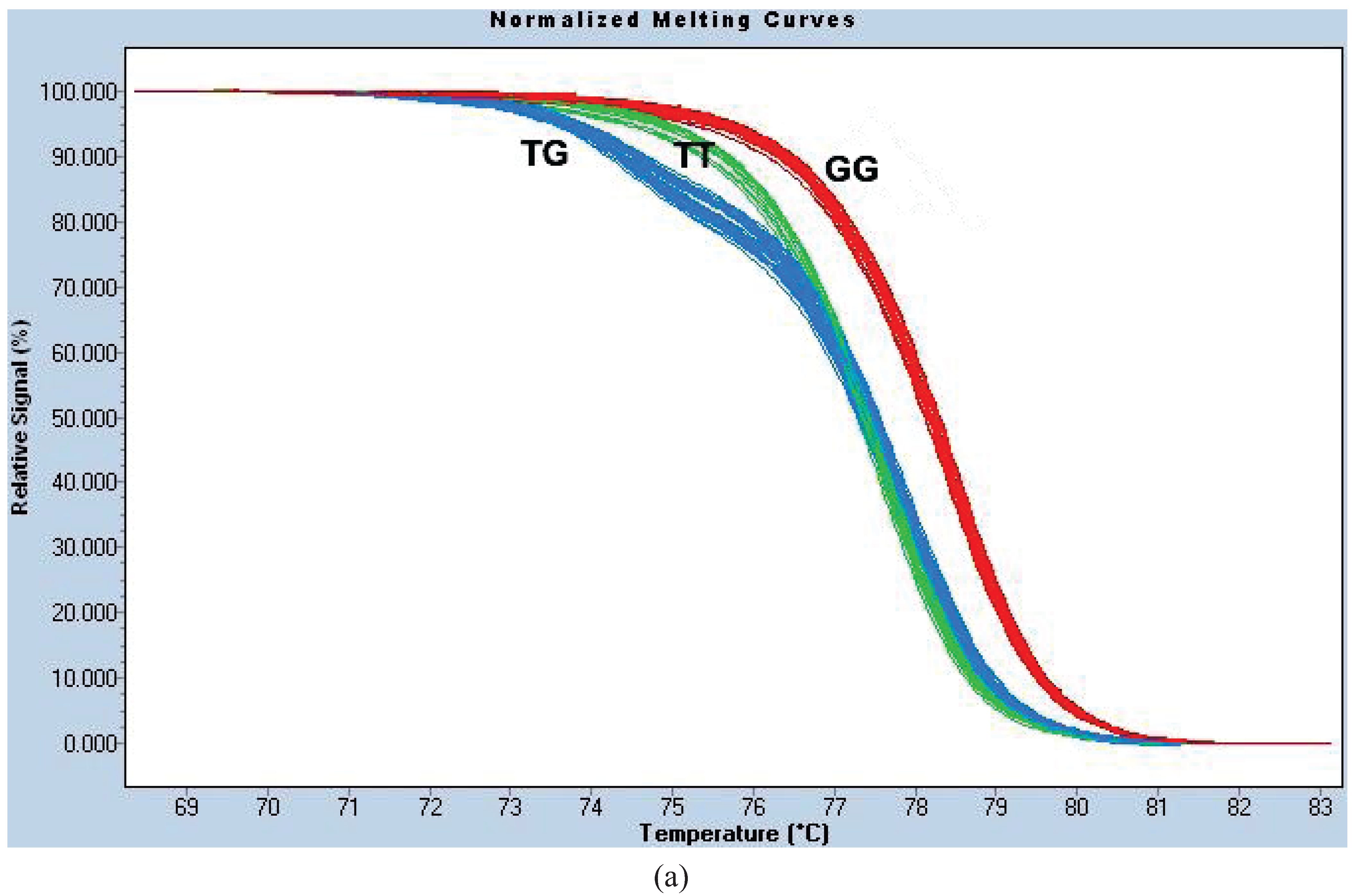
High-resolution melt (HRM) assays were developed to genotype the F1565, V1023 and S996P polymorphisms in the para gene. The overall approach is as follows: Universal primers were designed flanking each target di-allelic polymorphism. Fluorescent (ResoLight™ Dye) PCR was performed in the LightCycler® (Roche, Basel, Switzerland) 480 real time PCR machine. Genotypes of each target site were distinguished on the basis of their characteristic melt profiles.
The first target polymorphism, “site 1565”, is in the 24th codon of exon 31 in the
para gene. Two non-synonymous polymorphisms are known: TTC (Phenylalanine) and TGC (Cysteine) (see Harris
et al., 2010 [
29]). Genotyping primers, 5'-TACCTCTACTTTGTGTTCTTCATCATC-3' and 5'-GATTCAGCGTGAAGAACGACCCG-3', were placed adjacent to the target polymorphism. These primers produce an amplicon of 52 bp (
Figure A1c). An additional primer pair, (5'-GTGGGAAAGCAGCCGATTCGCG-3' and 5'-CTAGGCCGTGGAATAGCTTTCAGC-3') was designed to yield a 245 bp product (encompassing target site 1565) for validation by Sanger sequencing. All primers for site 1565 are within exon 31.
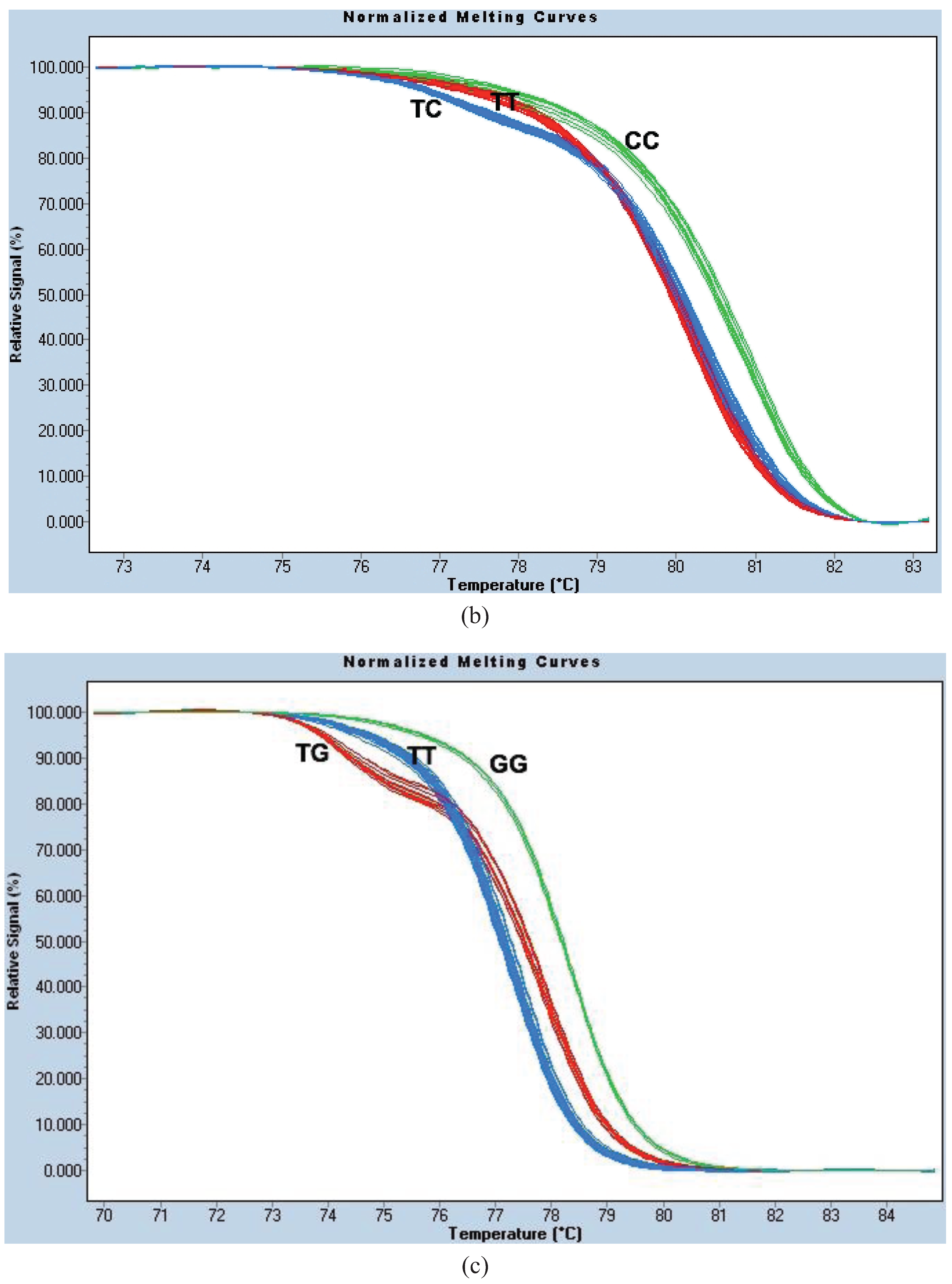
The second target polymorphism, “site 1023”, is in the first codon of exon 21 of the
para gene (VectorBase IDs: AAEL006109 and AAEL006109-PA). There are three known non-synonymous polymorphisms: GTA (Valine), GGA (Glycine) and ATA (Isoleucine) [
23]. The ATA (Isoleucine) allele was not found in the 75
para sequences from 10 “outer city” sites (all sampled during November to December 2011 and five re-sampled in July 2012) and 3 “city” sites (all sampled in November 2012) from Yogyakarta mosquito populations. Our assay was therefore designed to differentiate between the GTA (Valine) and the GGA (Glycine) alleles. Genotyping primers (5'-GACAAATTGTTTCCCACCCGCACAG-3' and 5'-AAGCAAGGCTAAGAAAAGGTTAAG-3') flank the target site, producing an amplicon of 52 bp (
Figure A1a). The priming site sequences were conserved among the 75 natural sequences from Yogyakarta.
The third target polymorphism, “site 996” is located in the P-region which links the membrane spanning segments S5 and S6 in Domain II of the
para gene. The non-synonymous mutation occurs in the first codon position, changing serine (TCC) to proline (CCC) [
43]. Genotyping primers used to amplify this site were 5'-CGGGTATTATGCGGCGAGTGGATC-3' and 5'-CCCACAAGCATACAATCCCACATGG-3' (
Figure A1b). Amplicon size was 53 bp.
For site 1565, the 10 μL PCR reaction contained 2 μL of 1 in 10 diluted template DNA, 0.4 μL each of the primers at 10 μM, 1 μL of the ThermoPol reaction buffer (NEB Inc., Ipswich, MA, USA; Cat. No. B9004S), 0.064 μL of dNTP’s at 25 mM (Bioline, Alexandria, NSW, Australia; Cat. No. BIO-39029), 0.4 μL of MgCl2 (50 mM) (Bioline, Alexandria, NSW, Australia; Cat. No. BIO-21047), 0.25 μL of the LightCycler® 480 High Resolution Melting Master (Roche, Mannheim, Germany; Cat. No. 04909631001), 0.01 μL of IMMOLASE™ DNA polymerase (10 u/μL) (Bioline, Alexandria, NSW, Australia; Cat. No. BIO-21047) and 5.476 μL of ddH2O (Honeywell, Burdick and Jackson; Muskegon, MI, USA; Cat. No. 365-4).
PCR amplification was carried out using the Roche LightCycler
® 480 system (384-well format). Thermo cycling steps were: 95 °C for 10 min, 20 cycles of 95 °C for 5 s, 65 °C (reduce 0.5 °C each cycle) for 15 s, 72 °C for 15 s, followed by an additional 20 cycles of 95 °C for 5 s, 55 °C for 15 s, and 72 °C for 15 s. Fluorescence information was captured at the end of each 72 °C step. PCR products were then subjected to HRM analysis. The HRM step involved heating the PCR products to 95 °C for 1 min, cooling to 40 °C for 20 s and then increasing the temperature to 65 °C. As the temperature increased from 65 to 95 °C, fluorescence data were recorded continuously. Melt curves were generated in the Gene Scanning module of the Roche LightCycler
® 480 software package. The parameter settings for melt curve normalization were: Pre-Melt Slider = 69.8–73.2 °C, Post-Melt Slider = 81.86–84.89 °C, Temperature Shift threshold = 0% and Sensitivity = 0.30 (
Figure A1c). We termed this the “HRM1565 assay”.
For site 1023, the PCR reaction conditions were identical to those for 1565. The melt curves were normalized using the following settings: Pre-Melt Slider = 68.35–70.16 °C, Post-Melt Slider = 81.74–83.16 °C, Temperature Shift threshold = 0% and Sensitivity = 0.40. We termed this genotyping method the “HRM1023 assay” (
Figure A1a).
For site 996, we used the same PCR protocol and thermocycling conditions as for HRM1023. The parameter settings for melt curve normalization were: Pre-Melt Slider = 73.69–76.18 °C, Post-Melt Slider = 83.53–84.86 °C, Temperature Shift threshold = 0% and Sensitivity = 0.40. This genotyping method is known as the “HRM996 assay” (
Figure A1b).
Sanger sequencing of the HRM-genotyped individuals confirmed the accuracy of each of the assays. Assays were then used for screening of field samples and mosquitoes from bioassays with permethrin and deltamethrin.
2.8. Statistical Analysis
All data from the tetraprimer, HRM1565, HRM1023 and HRM996 screens of mosquitoes from Yogyakarta Season 1 and Season 2 were analyzed for site and season differences in allele frequencies using contingency tables with significance tested through the chi-square statistic. Permutation tests were used to determine significance where appropriate (i.e., where expected values in cells were particularly low). For sites sampled in both seasons, a log linear analysis was performed to compare the variation in resistance allele frequencies between sites and seasons. All analyses were performed in IBM SPSS Statistics (IBM Corp., Armonk, NY, USA; 2013). The 95% binomial confidence intervals for allele frequencies were also computed.
To test for
kdr genotype and insecticide resistance associations, mosquito resistance status from the bioassays was treated as columns and
kdr genotypes from HRM assays were treated as rows. Genotypes of susceptible homozygotes and heterozygotes were collapsed into one group as the heterozygote is also expected to have a phenotype close to susceptible because of the incomplete recessive effect of
kdr mutants on resistance (as opposed to these mutations being incompletely dominant). Odds ratios (with 95% confidence intervals) [
44] were calculated to indicate the odds of an individual being resistant if it carried a copy of the putative resistance mutation. The ratio is generated from a 2 × 2 table (two genotypes
vs. two effects) [OR = (axd)/(bxc)] [
45]. An odds ratio of 1 indicates that there is no relationship between resistance and the genotype under investigation. If 95% confidence intervals of the odds ratio do not span the value “1” then this suggests that the genotype is associated with resistance. We also analyzed significance by testing the association between the putative resistance genotypes and the resistance phenotype using Fisher’s exact tests [
45]. Odds ratios were also used to determine whether mutations in combination in an individual were more likely to be associated with resistance than mutations occurring singly.
4. Discussion
Knockdown resistance in
Ae. aegypti against pyrethroid insecticides may be conferred by one or more mutations present in the target site, the
Vssc locus [
18]. Genotyping of mutations directly related to insecticide resistance could provide a useful surveillance tool for monitoring resistance and helping to target chemical applications for vector control. Tetra-primer PCR assays for
Ae. aegypti larvae were used to confirm a mutation at position 1565 in IIIS6 region of the
Vssc in samples from Yogyakarta, Indonesia. The resistance allele frequency of 1565C observed (2.9% in Yogyakarta Season 1 and 10.9% in Yogyakarta Season 2) was lower than the frequency recorded elsewhere in South East Asian countries including Vietnam (21.6%) [
26], Thailand (20%–100%) [
38] and Myanmar (21.2%) [
46]. The homozygous genotype, 1565C, was rare in mosquitoes sampled from Yogyakarta.
Sequence analysis of IIS6 region of
Vssc from 75 larvae of
Ae. aegypti collected in Yogyakarta revealed the presence of two mutations, V1023G and S996P. We detected a high frequency of the 1023G homozygotes (83%), and a moderate frequency of 996P homozygotes (17%). The V1023G mutation has been reported in other southeast Asian countries including Thailand [
27,
38,
47] (allele frequency of 23%), Vietnam [
26] (at a very low frequency), Myanmar [
46] (80% homozygous) and Singapore [
48] (44% homozygous). The simultaneous occurrence of both
kdr mutations, V1023G and S996P, appears to be widely distributed in this region. The V1023G mutation has been found previously in Indonesia in the Semarang strain as first reported by Brengues
et al. [
18].
The large differences detected between the susceptible 1023V homozygotes and the heterozygote VG/homozygote GG sequences may be evidence of a genetic sweep of the 1023G resistance allele and proximate intron sequences in Yogyakarta. The sweep may be relatively recent because high rates of recombination have been observed in codon 1023 [
23], but sequence variation within each of the three phenotypes is low. Similar evidence for a genetic sweep was detected in
Ae. aegypti in Latin America and involved the alternative mutation 1023I [
23].
Based on sequencing results, we have successfully developed RT-PCR HRM assays to genotype the V1023G, S996P and F1565C mutations. The assays initially were used to screen samples of
Ae.
aegypti from Yogyakarta Season 1 and Yogyakarta Season 2. In both seasons a high frequency of V1023G, a medium frequency of S996P and a very low frequency of F1565C were detected. This trend in frequencies is similar to that found in Myanmar [
46], but not in Vietnam [
26] or Singapore where the F1565C mutation can occur at high frequency [
48], or in Thailand where there is fixation in some regions [
38].
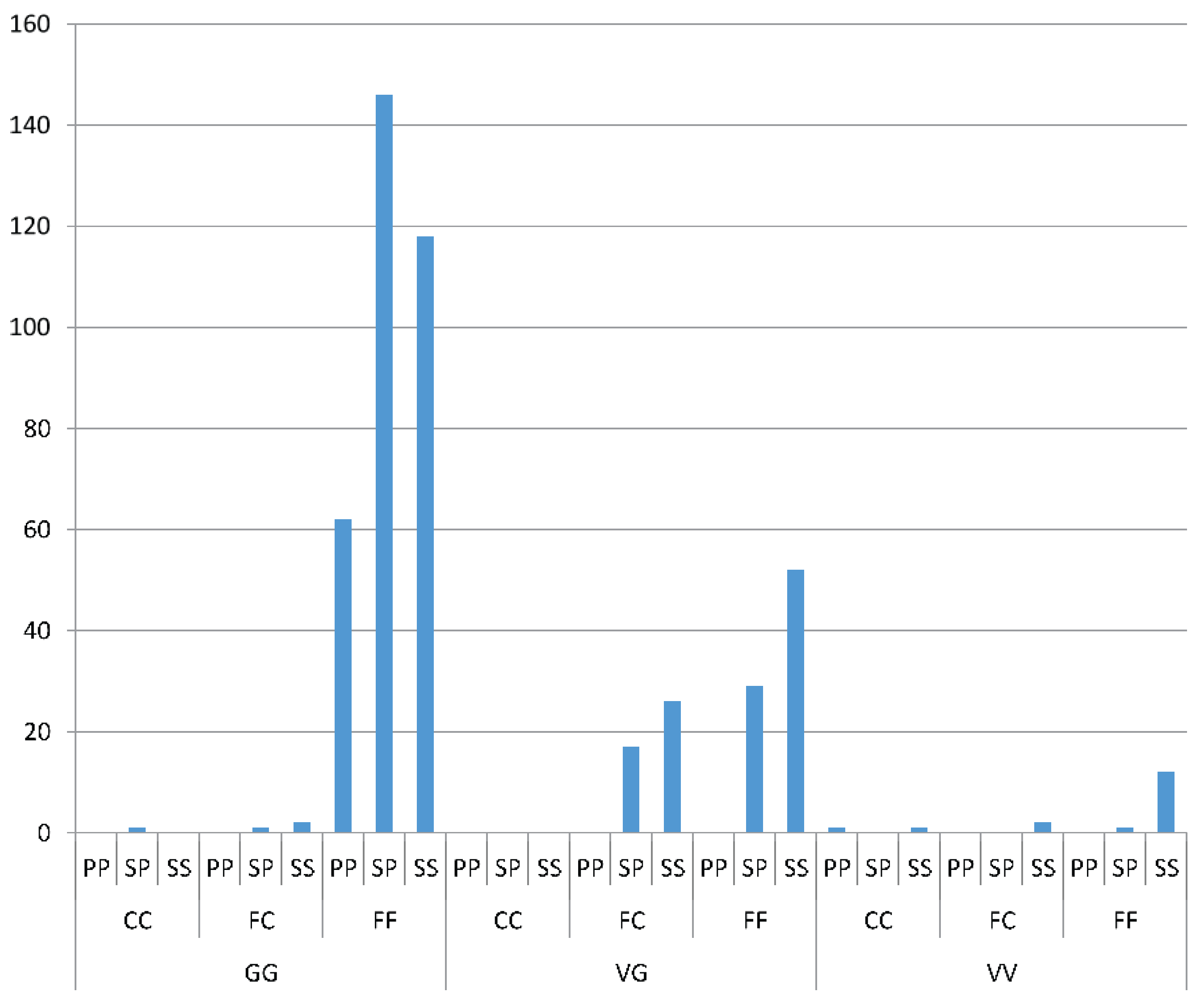
Odds ratio calculations to link mutations to insecticide resistance in three Yogyakarta populations indicated that the 1023G homozygote was more highly associated with both deltamethrin and permethrin resistance than susceptible heterozygotes, showing odds ratio values ranging from 5.68 to 22.00. Our result is in agreement with the findings of Du
et al. [
24], who were able to demonstrate that V1023G is one of the six mutations located in IIS6 that reduced the channel sensitivity to both deltamethrin (Type II) and permethrin (Type I) when expressed in
Xenopus oocytes.
While 1023G is clearly associated with resistance, it does not completely explain the resistance phenotype in the Yogyakarta samples as there were 1023V homozygotes and heterozygotes which survived in the assay, as well as 1023G homozygotes which did not survive. Alternative mechanisms of resistance and/or differences in genetic background may be involved, and/or the WHO doses may also not be infallibly diagnostic of resistance. A previous study has shown that
Ae. aegypti of the Indonesian Bandung strain were resistant to permethrin and deltamethrin with a RR
90 of 79.3 and 23.7 respectively and suggested that detoxifying enzymes were involved as indicated by a high level of enzyme activity (oxidases, esterase A, esterase B) measured in a biochemical assay [
16].
We found a suggestion of a synergistic effect of the S996P mutation when it occurred in conjunction with V1023G, particularly in relation to the Type II pyrethroid, deltamethrin which is similar to the findings of Hirata
et al. [
37]. We did not find the S996P mutation without V1023G as a homozygote in any individuals in the bioassay, but one individual was found in the field. When looking at S996P in isolation, in terms of frequency of the putative resistant allele, there was also suggestive evidence of an association with resistance to deltamethrin. Within the strain
wMelYog and the mosquitoes from Site 8, there were high odds of a mosquito being resistant if it had the S996P mutation. Why Site 6 did not show a similar trend is unclear, but it would be worth investigating effects of different concentrations of insecticide to improve the diagnostic dose. The changes in S996P allele frequency between sites in Season 2 may point to ongoing selection.
Overall, the study demonstrates the presence of putative resistance alleles in
Ae.
aegypti in Yogyakarta. It also shows that at least one allele is associated with pyrethroid resistance and is, therefore, useful in ongoing monitoring of mosquito populations destined for release. The study also highlights the lack of strong differences in allele frequencies between sites around Yogyakarta, although there was a tendency for the inner city sites to have a higher frequency of resistance alleles for V1023G and S996P. These results suggest that in future releases of
Wolbachia-infected mosquitoes, source populations with a suitable resistance background might be obtained from various sites around the city in a similar proposal as that made by Hoffmann
et al. [
40] for collection and dispersal of
Wolbachia-infected mosquitoes from field-established “nurseries”.