Phenotypic Plasticity Promotes Overwintering Survival in A Globally Invasive Crop Pest, Drosophila suzukii
Abstract
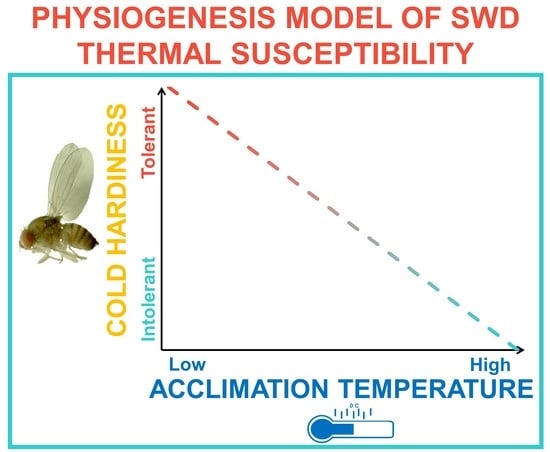
:1. Introduction
2. Materials and Methods
2.1. Insect Colony
2.2. Static Susceptibility in Adults
2.3. Pupal Susceptibility
2.4. Dynamic Acclimation
2.5. Statistical Analysis
3. Results
3.1. Adult Susceptibility
3.2. Pupal Susceptibility
3.3. Dynamic Acclimation
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cini, A.; Ioriatti, C.; Anfora, G. A review of the invasion of Drosophila suzukii in Europe and a draft research agenda for integrated pest management. Bull. Insectol. 2012, 65, 149–160. [Google Scholar]
- Asplen, M.K.; Anfora, G.; Biondi, A.; Choi, D.-S.; Chu, D.; Daane, K.M.; Gibert, P.; Gutierrez, A.P.; Hoelmer, K.A.; Hutchison, W.D.; et al. Invasion biology of spotted wing Drosophila (Drosophila suzukii): A global persepcetive and future priorities. J. Pest Sci. 2015, 88, 469–494. [Google Scholar] [CrossRef]
- Deprá, M.; Poppe, J.L.; Schmitz, H.J.; De Toni, D.C.; Valente, V.L.S. The first records of the invasive pest Drosophila suzukii in the South American continent. J. Pest Sci. 2014, 87, 379–383. [Google Scholar] [CrossRef]
- dos Santos, L.A.; Mendes, M.F.; Krüger, A.P.; Blauth, M.L.; Gottschalk, M.S.; Garcia, F.R.M.; Alvarez, E.; Jara, C.; Godoy-Herrera, R.; Yemshanov, D. Global potential distribution of Drosophila suzukii (Diptera, Drosophilidae). PLoS ONE 2017, 12, e0174318. [Google Scholar] [CrossRef] [PubMed]
- Kanzawa, T. Studies on Drosophila suzukii Mats. Yamanashi Kofu Agric. Exp. Stn. 1939, 29, 49. [Google Scholar]
- Rota-Stabelli, O.; Blaxter, M.; Anfora, G. Drosophila suzukii. Curr. Biol. 2013, 23, R8–R9. [Google Scholar] [CrossRef] [PubMed]
- Atallah, J.; Teixeira, L.; Salazar, R.; Zaragoza, G.; Kopp, A. The making of a pest: The evolution of a fruit-penetrating ovipositor in Drosophila suzukii and related species. Proc. R. Soc. Lond. B Biol. Sci. 2014, 281. [Google Scholar] [CrossRef] [PubMed]
- Karageorgi, M.; Bräcker, L.B.; Lebreton, S.; Minervino, C.; Cavey, M.; Siju, K.P.; Grunwald Kadow, I.C.; Gompel, N.; Prud’homme, B. Evolution of Multiple Sensory Systems Drives Novel Egg-Laying Behavior in the Fruit Pest Drosophila suzukii. Curr. Biol. 2017, 27, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Bolda, M.P.; Goodhue, R.E.; Zalom, F.G. Spotted Wing Drosophila: Potential Economic Impact of a Newly Established Pest. Updat. Univ. Calif. Giannini Found. Agric. Econ. 2010, 13, 5–8. [Google Scholar]
- Walsh, D.B.; Bolda, M.P.; Goodhue, R.E.; Dreves, A.J.; Lee, J.; Bruck, D.J.; Walton, V.M.; O’Neal, S.D.; Zalom, F.G. Drosophila suzukii (Diptera: Drosophilidae): Invasive Pest of Ripening Soft Fruit Expanding its Geographic Range and Damage Potential. J. Integr. Pest Manag. 2011, 2, G1–G7. [Google Scholar] [CrossRef]
- Hamby, K.A.; Bellamy, D.E.; Chiu, J.C.; Lee, J.C.; Walton, V.M.; Wiman, N.G.; York, R.M.; Biondi, A. Biotic and abiotic factors impacting development, behavior, phenology, and reproductive biology of Drosophila suzukii. J. Pest Sci. 2016, 89, 605–619. [Google Scholar] [CrossRef]
- Wiman, N.G.; Walton, V.M.; Dalton, D.T.; Anfora, G.; Burrack, H.J.; Chiu, J.C.; Daane, K.M.; Grassi, A.; Miller, B.; Tochen, S.; et al. Integrating Temperature-Dependent Life Table Data into a Matrix Projection Model for Drosophila suzukii Population Estimation. PLoS ONE 2014, 9, e106909. [Google Scholar] [CrossRef] [PubMed]
- Elsensohn, J.E.; Loeb, G.M. Non-crop host sampling yields insights into small-scale population dynamics of Drosophila suzukii (Matsumura). Insects 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Rossi-Stacconi, M.V.; Kaur, R.; Mazzoni, V.; Ometto, L.; Grassi, A.; Gottardello, A.; Rota-Stabelli, O.; Anfora, G. Multiple lines of evidence for reproductive winter diapause in the invasive pest Drosophila suzukii: Useful clues for control strategies. J. Pest Sci. 2016, 89, 689–700. [Google Scholar] [CrossRef]
- Thistlewood, H.M.A.; Gill, P.; Beers, E.H.; Shearer, P.W.; Walsh, D.B.; Rozema, B.M.; Acheampong, S.; Castagnoli, S.; Yee, W.L.; Smytheman, P.; et al. Spatial Analysis of Seasonal Dynamics and Overwintering of Drosophila suzukii (Diptera: Drosophilidae) in the Okanagan-Columbia Basin, 2010–2014. Environ. Entomol. 2018, 47, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Tobari, Y.N.; Tsuno, K.; Watanabe, T.K. Association of chromosome and enzyme polymorphisms in natural and cage populations of Drosophila melanogaster. Genetics 1984, 106, 267–277. [Google Scholar] [PubMed]
- Machado, H.E.; Bergland, A.O.; O’Brien, K.R.; Behrman, E.L.; Schmidt, P.S.; Petrov, D.A. Comparative population genomics of latitudinal variation in Drosophila simulans and Drosophila melanogaster. Mol. Ecol. 2016, 25, 723–740. [Google Scholar] [CrossRef] [PubMed]
- Mukai, T.; Yamaguchi, O. The genetic structure of natural populations of Drosophila melanogaster. XI. Genetic variability in a local population. Genetics 1974, 76, 339–366. [Google Scholar] [PubMed]
- Fabian, D.K.; Kapun, M.; Nolte, V.; Kofler, R.; Schmidt, P.S.; Schlötterer, C.; Flatt, T. Genome-wide patterns of latitudinal differentiation among populations of Drosophila melanogaster from North America. Mol. Ecol. 2012, 21, 4748–4769. [Google Scholar] [CrossRef] [PubMed]
- Jakobs, R.; Gariepy, T.D.; Sinclair, B.J. Adult plasticity of cold tolerance in a continental-temperate population of Drosophila suzukii. J. Insect Physiol. 2015, 79, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M.T. Cold and heat tolerance of drosophilid flies with reference to their latitudinal distributions. Oecologia 2004, 140, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Dalton, D.T.; Walton, V.M.; Shearer, P.W.; Walsh, D.B.; Caprile, J.; Isaacs, R. Laboratory survival of Drosophila suzukii under simulated winter conditions of the Pacific Northwest and seasonal field trapping in five primary regions of small and stone fruit production in the United States. Pest Manag. Sci. 2011, 67, 1368–1374. [Google Scholar] [CrossRef] [PubMed]
- Tochen, S.; Dalton, D.T.; Wiman, N.; Hamm, C.; Shearer, P.W.; Walton, V.M. Temperature-Related Development and Population Parameters for Drosophila suzukii (Diptera: Drosophilidae) on Cherry and Blueberry. Environ. Entomol. 2014, 43, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Zerulla, F.N.; Schmidt, S.; Streitberger, M.; Zebitz, C.P.W.; Zelger, R. On the overwintering ability of Drosophila suzukii in South Tyrol. J. Berry Res. 2015, 5, 41–48. [Google Scholar] [CrossRef]
- Wallingford, A.K.; Lee, J.C.; Loeb, G.M. The influence of temperature and photoperiod on the reproductive diapause and cold tolerance of spotted-wing drosophila, Drosophila suzukii. Entomol. Exp. Appl. 2016, 159, 327–337. [Google Scholar] [CrossRef]
- Grassi, A.; Gottardello, A.; Dalton, D.T.; Tait, G.; Rendon, D.; Ioriatti, C.; Gibeaut, D.; Rossi Stacconi, M.V.; Walton, V.M. Seasonal Reproductive Biology of Drosophila suzukii (Diptera: Drosophilidae) in Temperate Climates. Environ. Entomol. 2018, 47, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Gibert, P.; Moreteau, B.; David, J.R. Developmental constraints on an adaptive plasticity: Reaction norms of pigmentation in adult segments of Drosophila melanogaster. Evol. Dev. 2000, 2, 249–260. [Google Scholar] [CrossRef] [PubMed]
- David, J.; Moreteau, B.; Gauthier, J.; Pétavy, G.; Stockel, A.; Imasheva, A. Reaction norms of size characters in relation to growth temperature in Drosophila melanogaster: An isofemale lines analysis. Genet. Sel. Evol. 1994, 26, 229–251. [Google Scholar] [CrossRef]
- Hoffmann, A.A.; Sorensen, J.G.; Loeschchke, V. Adaptation of Drosophila to temperature extremes: Bringing together quantitative and molecular approaches. J. Therm. Biol. 2003, 28, 175–216. [Google Scholar] [CrossRef]
- Overgaard, J.; Malmendal, A.; Sørensen, J.G.; Bundy, J.G.; Loeschcke, V.; Chr Nielsen, N.; Holmstrup, M. Metabolomic profiling of rapid cold hardening and cold shock in Drosophila melanogaster. J. Insect Physiol. 2007, 53, 1218–1232. [Google Scholar] [CrossRef] [PubMed]
- Stephens, A.R.; Asplen, M.K.; Hutchison, W.D.; Venette, R.C. Cold Hardiness of Winter-Acclimated Drosophila suzukii (Diptera: Drosophilidae) Adults. Environ. Entomol. 2015, 44, 1619–1626. [Google Scholar] [CrossRef] [PubMed]
- Shearer, P.W.; West, J.D.; Walton, V.M.; Brown, P.H.; Svetec, N.; Chiu, J.C. Seasonal cues induce phenotypic plasticity of Drosophila suzukii to enhance winter survival. BMC Ecol. 2016, 16. [Google Scholar] [CrossRef] [PubMed]
- Wallingford, A.K.; Loeb, G.M. Developmental Acclimation of Drosophila suzukii (Diptera: Drosophilidae) and Its Effect on Diapause and Winter Stress Tolerance. Environ. Entomol. 2016, 45, 1081–1089. [Google Scholar] [CrossRef] [PubMed]
- Toxopeus, J.; Jakobs, R.; Ferguson, L.V.; Gariepy, T.D.; Sinclair, B.J. Reproductive arrest and stress resistance in winter-acclimated Drosophila suzukii. J. Insect Physiol. 2016, 89, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Tonina, L.; Mori, N.; Giomi, F.; Battisti, A. Development of Drosophila suzukii at low temperatures in mountain areas. J. Pest Sci. 2016, 89, 667–678. [Google Scholar] [CrossRef]
- Tucic, N. Genetic capacity for adaptation to cold resistance at different developmental stages of Drosophila melanogaster. Evolution (N. Y.) 1979, 33, 3–358. [Google Scholar] [CrossRef]
- Kimura, M.T. Adaptations to temperate climates and evolution of overwintering strategies in the Drosophila melanogaster species group. Evolution (N. Y.) 1988, 42, 1288–1297. [Google Scholar]
- Chen, C.; Walker, V. Increase in cold-shock tolerance by selection of cold resistant lines in Drosophila melanogaster. Ecol. Entomol. 1993, 18, 184–190. [Google Scholar] [CrossRef]
- Ohtsu, T.; Kimura, M.T.; Hori, S.H. The influence of eclosion timing on winter survival and triacylglycerol accumulation in four temperate species of Drosophila. Physiol. Entomol. 1995, 20, 248–252. [Google Scholar] [CrossRef]
- Lee, R.E. Principles of Insect Low Temperature Tolerance. In Insects at Low Temperature; Springer: Boston, MA, USA, 1991; pp. 17–46. [Google Scholar]
- Kelty, J.D.; Lee, R.E., Jr. Rapid cold-hardening of Drosophila melanogaster (Diptera: Drosophilidae) during ecologically based thermoperiodic cycles. J. Exp. Biol. 2001, 204, 1659–1666. [Google Scholar] [PubMed]
- Denlinger, D.L.; Lee, R.E. Low Temperature Biology of Insects, 1st ed.; Cambridge University Press: New York, NY, USA, 2010; ISBN 978-0-521-88635-2. [Google Scholar]
- Strachan, L.A.; Tarnowski-Garner, H.E.; Marshall, K.E.; Sinclair, B.J. The evolution of cold tolerance in Drosophila larvae. Physiol. Biochem. Zool. 2011, 84, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, B.J. Insect cold tolerance: How many kinds of frozen? Eur. J. Entomol. 1999, 96, 157–164. [Google Scholar]
- Aly, M.F.K.; Kraus, D.A.; Burrack, H.J. Effects of Postharvest Cold Storage on the Development and Survival of Immature Drosophila suzukii (Diptera: Drosophilidae) in Artificial Diet and Fruit. J. Econ. Entomol. 2016, 147, tow289. [Google Scholar] [CrossRef] [PubMed]
- Enriquez, T.; Colinet, H. Basal tolerance to heat and cold exposure of the spotted wing drosophila, Drosophila suzukii. PeerJ 2017, 5, e3112. [Google Scholar] [CrossRef] [PubMed]
- Lankinen, P.; Riihimaa, A.J. Weak circadian eclosion rhythmicity in Chymomyza costata (Diptera: Drosophilidae), and its independence of diapause type. J. Insect Physiol. 1992, 38, 803–811. [Google Scholar] [CrossRef]
- Kostal, V.; Berkova, P.; Simek, P. Remodelling of membrane phospholipids during transition to diapause and cold-acclimation in the larvae of Chymomyza costata (Drosophilidae). Comp. Biochem. Physiol. Part B 2003, 135, 407–419. [Google Scholar] [CrossRef]
- Shimada, K.; Riihimaa, A. Cold acclimation, inoculative freezing and slow cooling: Essential factors contributing to the freezing-tolerance in diapausing larvae of Chymomyza costata (Diptera: Drosophilidae). Cryo-Letters 1988, 9, 5–10. [Google Scholar]
- Kellermann, V.; Loeschcke, V.; Hoffmann, A.A.; Kristensen, T.N.; Fløjgaard, C.; David, J.R.; Svenning, J.-C.; Overgaard, J. Phylogenetic constraints in key functional traits behind species’ climate niches: Patterns of desiccation and cold resistance across 95 Drosophila species. Evolution (N. Y.) 2012, 66, 3377–3389. [Google Scholar] [CrossRef]
- Hori, Y.; Kimura, A.T. Relationship between Cold Stupor and Cold Tolerance in Drosophila (Diptera: Drosophilidae). Physiol. Chem. Ecol. 1998, 27, 1297–1302. [Google Scholar] [CrossRef]
- Hoffmann, A.A.; Shirriffs, J.; Scott, M. Relative importance of plastic vs genetic factors in adaptive differentiation: Geographical variation for stress resistance in Drosophila melanogaster from eastern Australia. Funct. Ecol. 2005, 19, 222–227. [Google Scholar] [CrossRef]
- Everatt, M.J.; Bale, J.S.; Convey, P.; Worland, M.R.; Hayward, S.A.L. The effect of acclimation temperature on thermal activity thresholds in polar terrestrial invertebrates. J. Insect Physiol. 2013, 59, 1057–1064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kostal, V. Eco-physiological phases of insect diapause. J. Insect Physiol. 2006, 52, 113–127. [Google Scholar] [CrossRef] [PubMed]
- Andrewartha, H.G. Diapause in relation to the ecology of insects. Biol. Rev. 1952, 27, 50–107. [Google Scholar] [CrossRef]
- Andersen, J.L.; Manenti, T.; Sørensen, J.G.; MacMillan, H.A.; Loeschcke, V.; Overgaard, J. How to assess Drosophila cold tolerance: Chill coma temperature and lower lethal temperature are the best predictors of cold distribution limits. Funct. Ecol. 2015, 29, 55–65. [Google Scholar] [CrossRef]
- Jensen, D.; Overgaard, J.; Sorensen, J.G. The influence of developmental stage on cold shock resistance and ability to cold-harden in Drosophila melanogaster. J. Insect Physiol. 2007, 53, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Overgaard, J.; Kristensen, T.N.; Mitchell, K.A.; Hoffmann, A.A. Thermal tolerance in widespread and tropical Drosophila species: Does phenotypic plasticity increase with latitude? Am. Nat. 2011, 178 (Suppl. 1), S80–S96. [Google Scholar] [CrossRef]
- Bale, J.S.; Hayward, S.A.L. Insect overwintering in a changing climate. J. Exp. Biol. 2010, 213, 980–994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bale, J.S.; Masters, G.J.; Hodkinson, I.D.; Awmack, C.; Bezemer, T.M.; Brown, V.K.; Butterfield, J.; Buse, A.; Coulson, J.C.; Farrar, J.; et al. Herbivory in global climate change research: Direct effects of rising temperature on insect herbivores. Glob. Chang. Biol. 2002, 8, 1–16. [Google Scholar] [CrossRef]
- Rosenzweig, C.; Iglesius, A.; Yang, X.B.; Epstein, P.R.; Chivian, E. Climate change and extreme weather events-Implications for food production, plant diseases, and pests. Glob. Chang. Hum. Heal. 2001, 2, 90–104. [Google Scholar] [CrossRef]
- Pecl, G.T.; Araújo, M.B.; Bell, J.D.; Blanchard, J.; Bonebrake, T.C.; Chen, I.-C.; Clark, T.D.; Colwell, R.K.; Danielsen, F.; Evengård, B.; et al. Biodiversity redistribution under climate change: Impacts on ecosystems and human well-being. Science 2017, 355. [Google Scholar] [CrossRef] [PubMed]
- Langille, A.B.; Arteca, E.M.; Newman, J.A. The impacts of climate change on the abundance and distribution of the Spotted Wing Drosophila (Drosophila suzukii ) in the United States and Canada. PeerJ 2017, 5, e3192. [Google Scholar] [CrossRef] [PubMed]
- Wallingford, A.K.; Hesler, S.P.; Cha, D.H.; Loeb, G.M. Behavioral response of spotted-wing drosophila, Drosophila suzukii Matsumura, to aversive odors and a potential oviposition deterrent in the field. Pest Manag. Sci. 2016, 72, 701–706. [Google Scholar] [CrossRef] [PubMed]
- David, J.R.; Gibert, P.; Moreteau, B.; Gilchrist, G.W.; Huey, R.B. The fly that came in from the cold: Geographic variation of recovery time from low-temperature exposure in Drosophila subobscura. Funct. Ecol. 2003, 17, 425–430. [Google Scholar] [CrossRef]
- Hanley, J.A.; Negassa, A.; Forrester, J.E. Statistical Analysis of Correlated Data Using Generalized Estimating Equations: An Orientation. Am. J. Epidemiol. 2003, 157, 364–375. [Google Scholar] [CrossRef] [PubMed]
- Plantamp, C.; Salort, K.; Gibert, P.; Dumet, A.; Mialdea, G.; Mondy, N.; Voituron, Y. All or nothing: Survival, reproduction and oxidative balance in Spotted Wing Drosophila (Drosophila suzukii) in response to cold. J. Insect Physiol. 2016, 89, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Colinet, H.; Hoffmann, A.A. Comparing phenotypic effects and molecular correlates of developmental, gradual and rapid cold acclimation responses in Drosophila melanogaster. Funct. Ecol. 2012, 26, 84–93. [Google Scholar] [CrossRef]
- Rako, L.; Hoffmann, A.A. Complexity of the cold acclimation response in Drosophila melanogaster. J. Insect Physiol. 2006, 52, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M.T.; Beppu, K. Climatic adaptations in the Drosophila immigrans species group: Seasonal migration and thermal tolerance. Ecol. Entomol. 1993, 18, 141–149. [Google Scholar] [CrossRef]
- Klick, J.; Yang, W.Q.; Walton, V.M.; Dalton, D.T.; Hagler, J.R.; Dreves, A.J.; Lee, J.C.; Bruck, D.J. Distribution and activity of Drosophila suzukii in cultivated raspberry and surrounding vegetation. J. Appl. Entomol. 2016, 140, 37–46. [Google Scholar] [CrossRef]
- Diepenbrock, L.M.; Swoboda-Bhattarai, K.A.; Burrack, H.J. Ovipositional preference, fidelity, and fitness of Drosophila suzukii in a co-occurring crop and non-crop host system. J. Pest Sci. 2016, 89, 761–769. [Google Scholar] [CrossRef]
- Iglesias, L.E.; Liburd, O.E. The effect of border sprays and between-row soil tillage on Drosophila suzukii in organic blackberry production. J. Appl. Entomol. 2017, 141, 19–27. [Google Scholar] [CrossRef]
- Liburd, O.E.; Iglesias, L.E.; Nyoike, T.W. Integrated pest management strategies to combat the invasive spotted wing drosophila: Drosophila suzukii (Matsumura) Diptera: Drosophilidae. In Proceedings of the NABREW Conference, Atlantic City, NJ, USA, 24 June 2014. [Google Scholar] [CrossRef]
- Pelton, E.; Gratton, C.; Isaacs, R.; Van Timmeren, S.; Blanton, A.; Guédot, C. Earlier activity of Drosophila suzukii in high woodland landscapes but relative abundance is unaffected. J. Pest Sci. 2016, 89, 725–733. [Google Scholar] [CrossRef]
- Hoffmann, A.A.; Scott, M.; Partridge, L.; Hallas, R. Overwintering in Drosophila melanogaster: Outdoor field cage experiments on clinal and laboratory selected populations help to elucidate traits under selection. J. Evol. Biol. 2003, 16, 614–623. [Google Scholar] [CrossRef] [PubMed]
- Leather, S.R.; Walters, K.F.A.; Bale, J.S. The Ecology of Insect Overwintering, 1st ed.; Cambridge University Press: New York, NY, USA, 1993. [Google Scholar]
- Mukerji, M.K.; Braun, M.P. Effect of low temperatures on mortality of grasshopper eggs (Orthoptera: Acrididae). Can. Entomol. 1988, 120, 1147–1148. [Google Scholar] [CrossRef]
- Lamb, R.J.; Turnock, W.J.; Hayhoe, H.N. Winter survival and outbreaks of bertha armyworm, Mamestra conjigurarta (Lepidoptera: Noctuidae) on canola. Can. Entomol. 1985, 117, 727–736. [Google Scholar] [CrossRef]
- Kobayashi, S.; Ide, M.; Higashi, O. Overwintering of the rice weevil, Lissorhoptrus oryzophilus Kuschel (Coleoptera: Curculionidae) under snow and freezing conditions at a high elevation. Jpn. J. Appl. Entomol. Zool. 1988, 29, 45–49. [Google Scholar] [CrossRef]
- Boulétreau-Merle, J.; Fouillet, P. How to overwinter and be a founder: Egg-retention phenotypes and mating status in Drosophila melanogaster. Evol. Ecol. 2002, 16, 309–332. [Google Scholar] [CrossRef]
- Ryan, G.D.; Emiljanowicz, L.; Wilkinson, F.; Kornya, M.; Newman, J.A. Thermal Tolerances of the Spotted-Wing Drosophila Drosophila suzukii (Diptera: Drosophilidae). J. Econ. Entomol. 2016, 109, 746–752. [Google Scholar] [CrossRef] [PubMed]







| Acclimation | Phenotype | Total No. Insects Tested per Treatment N = (#Replicates) | |||||
|---|---|---|---|---|---|---|---|
| 4.4 °C | 1.7 °C | −1.1 °C | −3.9 °C | −6.7 °C | −9.4 °C | ||
| Yes | WM | 78 (3) | 71 (3) | 69 (3) | 75 (3) | 72 (3) | 75 (3) |
| Yes | SM | 131 (5) | 142 (5) | 130 (5) | 138 (5) | 124 (5) | 125 (5) |
| No | SM | 129 (5) | 127 (5) | 127 (5) | 135 (5) | 108 (5) | 125 (5) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stockton, D.G.; Wallingford, A.K.; Loeb, G.M. Phenotypic Plasticity Promotes Overwintering Survival in A Globally Invasive Crop Pest, Drosophila suzukii. Insects 2018, 9, 105. https://0-doi-org.brum.beds.ac.uk/10.3390/insects9030105
Stockton DG, Wallingford AK, Loeb GM. Phenotypic Plasticity Promotes Overwintering Survival in A Globally Invasive Crop Pest, Drosophila suzukii. Insects. 2018; 9(3):105. https://0-doi-org.brum.beds.ac.uk/10.3390/insects9030105
Chicago/Turabian StyleStockton, Dara G., Anna K. Wallingford, and Gregory M. Loeb. 2018. "Phenotypic Plasticity Promotes Overwintering Survival in A Globally Invasive Crop Pest, Drosophila suzukii" Insects 9, no. 3: 105. https://0-doi-org.brum.beds.ac.uk/10.3390/insects9030105