Effects of the Environmental Temperature on Aedes aegypti and Aedes albopictus Mosquitoes: A Review
Abstract
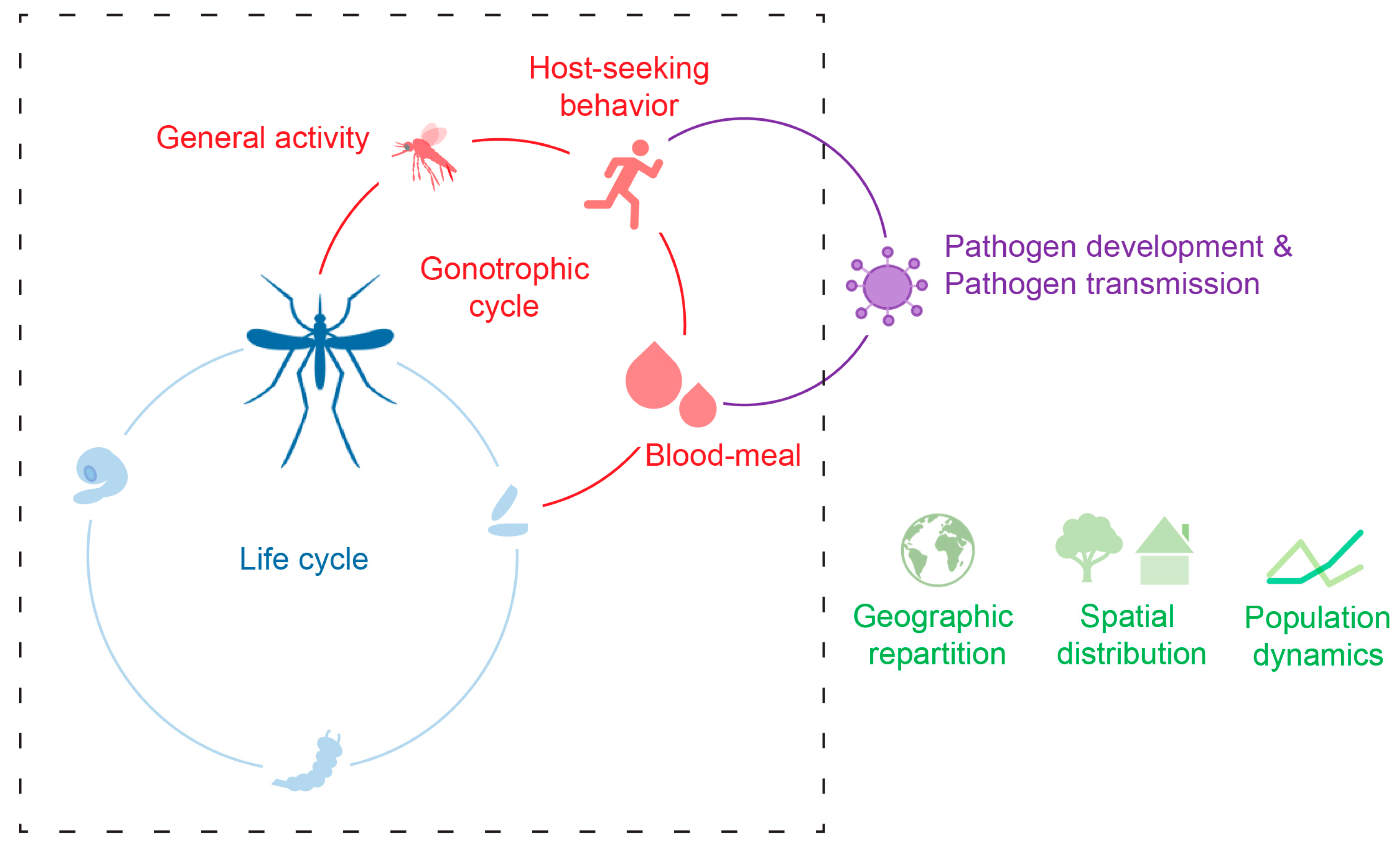
:1. Introduction
2. Fight Activity, Host-Seeking, and Blood-Feeding
3. Ecology and Dispersion
3.1. Thermal Optimum and Lower/Upper Zero Developmental and Survival Temperatures
3.2. Phenology and Population Dynamics
3.3. Spatial Distribution and Ta Variability
3.4. Vector Distribution in the Context of Climate Change
4. Pathogen Transmission
4.1. Dengue Virus Complex
4.2. Chikungunya Virus
4.3. Zika Virus
4.4. West Nile Virus
4.5. Yellow Fever Virus
4.6. Other Pathogens
5. Conclusions, Future Directions and Knowledge Gaps
Author Contributions
Funding
Conflicts of Interest
References
- Denlinger, D.L.; Yocum, G.D. Physiology of heat sensitivity. In Temperature Sensitivity in Insects and Application in Integrated Pest Management; Hallman, G.J., Denlinger, D.L., Eds.; Westview Press: Boulder, CO, USA; Oxford, UK, 1998; pp. 7–53. [Google Scholar]
- Heinrich, B. The Hot-Blooded Insects: Strategies and Mechanisms of Thermoregulation; Harvard University Press: Cambridge, MA, USA, 1993; p. 600. [Google Scholar]
- Huey, R.B.; Stevenson, R.D. Integrating thermal physiology and ecology of ectotherms: A discussion of approaches. Am. Zool. 1979, 19, 357–366. [Google Scholar] [CrossRef]
- Benoit, J.B.; Lopez-Martinez, G.; Patrick, K.R.; Phillips, Z.P.; Krause, T.B.; Denlinger, D.L. Drinking a hot blood meal elicits a protective heat shock response in mosquitoes. Proc. Natl. Acad. Sci. USA 2011. [Google Scholar] [CrossRef] [PubMed]
- Lahondère, C.; Lazzari, C.R. Mosquitoes cool down during blood feeding to avoid overheating. Curr. Biol. 2012, 22, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Angilletta, M.J. Thermal Adaptation: A Theoretical and Empirical Synthesis; Oxford University Press: New York, NY, USA, 2009; p. 304. [Google Scholar]
- World Health Statistics (WHO). Monitoring Health for the SDGs, Sustainable Development Goals; Licence: CC BY-NC-SA 3.0 IGO; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- World Health Organization. Handbook for Integrated Vector Management; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- Matthews, G. Integrated Vector Management: Controlling Vectors of Malaria and Other Insect Vector Borne Diseases; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Janzen, D.H. On ecological fitting. Oikos 1985, 45, 308–310. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2014: Synthesis Report; Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Pachauri, R.K., Meyer, L.A., Eds.; IPCC: Geneva, Switzerland, 2014; 151p. [Google Scholar]
- Christophers, S.R. Aedes aegypti (L.) the Yellow Fever Mosquito; Cambridge University Press: London, UK, 1960. [Google Scholar]
- Delatte, H.; Desvars, A.; Bouétard, A.; Bord, S.; Gimonneau, G.; Vourc’h, G.; Fontenille, D. Blood-feeding behavior of Aedes albopictus, a vector of Chikungunya on La Réunion. Vector-Borne Zoonotic 2010, 10, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Harrington, L.C.; Edman, J.D.; Scott, T.W. Why do female Aedes aegypti (Diptera: Culicidae) feed preferentially and frequently on human blood? J. Med. Entomol. 2001, 38, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D.J. Observations on Aedes aegypti, L. (Dipt. Culic.) under controlled atmospheric conditions. B Entomol. Res. 1933, 24, 363–372. [Google Scholar] [CrossRef]
- Otto, M.; Neumann, R.O. Studien über Gelbfieber in Brasilien. Z. Hyg. InfektKr. 1905, 51, 357–506. [Google Scholar] [CrossRef]
- Rowley, W.A.; Graham, C.L. The effect of temperature and relative humidity on the flight performance of female Aedes aegypti. J. Insect Physiol. 1968, 14, 1251–1257. [Google Scholar] [CrossRef]
- Bowen, M.F. The sensory physiology of host-seeking behavior in mosquitoes. Annu. Rev. Entomol. 1991, 36, 139–158. [Google Scholar] [CrossRef] [PubMed]
- Corfas, R.A.; Vosshall, L.B. The cation channel TRPA1 tunes mosquito thermotaxis to host temperatures. eLife 2015, 4, e11750. [Google Scholar] [CrossRef] [PubMed]
- Zermoglio, P.F.; Robuchon, E.; Leonardi, M.S.; Chandre, F.; Lazzari, C.R. What does heat tell a mosquito? Characterization of the orientation behaviour of Aedes aegypti towards heat sources. J. Insect Physiol. 2017, 100, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Bishop, A.; Gilchrist, B.M. Experiments upon the feeding of Aedes aegypti through animal membranes with a view to applying this method to the chemotherapy of malaria. Parasitology 1946, 37, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Connor, M.E. Suggestions for Developing a Campaign to Control Yellow Fever. Am. J. Trop. Med. Hyg. 1924, 1, 277–307. [Google Scholar] [CrossRef]
- Cossio, V. Observations sobre al Aedes aegypti (Stegomyia) mosquito de la febbre amarilla en Montevideo. Bol. Cons. Nat. Hig. Uruguay 1931, 23, 1664. [Google Scholar]
- Marchoux, E.; Salimbeni, A.T.; Simond, P.L. La Fièvre Jaune: Rapport de la Mission Française; Annales de l’Institut Pasteur: Paris, France, 1903. [Google Scholar]
- Scott, T.W.; Clark, G.G.; Amerasinghe, P.H.; Lorenz, L.H.; Reiter, P.; Edman, J.D. Detection of multiple blood feeding patterns in Aedes aegypti (Diptera: Culicidae) during a single gonotrophic cycle using a histological technique. J. Med. Entomol. 1993, 30, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Scott, T.W.; Amerasinghe, P.H.; Morrison, A.C.; Lorenz, L.H.; Clark, G.G.; Strickman, D.; Kittayapong, P.; Edman, J.D. Longitudinal studies of Aedes aegypti (Diptera: Culicidae) in Thailand and Puerto Rico: Blood feeding frequency. J. Med. Entomol. 2000, 37, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Yasuno, M.; Pant, C. Seasonal changes in biting and larval infestation rates of Aedes aegypti in Bangkok, Thailand in 1969. Bull. WHO 1970, 43, 319–325. [Google Scholar] [PubMed]
- Delatte, H.; Gimonneau, G.; Triboire, A.; Fontenille, D. Influence of temperature on immature development, survival, longevity, fecundity, and gonotrophic cycles of Aedes albopictus, vector of chikungunya and dengue in the Indian Ocean. J. Med. Entomol. 2009, 46, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Löwenberg Neto, P.; Navarro-Silva, M.A. Development, longevity, gonotrophic cycle and oviposition of Aedes albopictus Skuse (Diptera: Culicidae) under cyclic temperatures. Neotrop. Entomol. 2004, 33, 29–33. [Google Scholar] [CrossRef]
- Carrington, L.B.; Armijos, M.V.; Lambrechts, L.; Barker, C.M.; Scott, T.W. Effects of fluctuating daily temperatures at critical thermal extremes on Aedes aegypti life-history traits. PLoS ONE 2013, 8, e58824. [Google Scholar] [CrossRef] [PubMed]
- Carrington, L.B.; Seifert, S.N.; Willits, N.H.; Lambrechts, L.; Scott, T.W. Large diurnal temperature fluctuations negatively influence Aedes aegypti (Diptera: Culicidae) life history traits. J. Med. Entomol. 2013, 50, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Couret, J.; Benedict, M.Q. A meta-analysis of the factors influencing development rate variation in Aedes aegypti (Diptera: Culicidae). BMC Ecol. 2014, 14, 3. [Google Scholar] [CrossRef] [PubMed]
- Couret, J.; Dotson, E.; Benedict, M.Q. Temperature, larval diet, and density effects on development rate and survival of Aedes aegypti (Diptera: Culicidae). PLoS ONE 2014, 9, e87468. [Google Scholar] [CrossRef] [PubMed]
- Bar-Zeev, M. The effect of temperature on the growth rate and survival of the immature stages of Aedes aegypti (L.). Bull. Entomol. Res. 1958, 49, 157–163. [Google Scholar] [CrossRef]
- Bar-Zeev, M. The effect of extreme temperatures on different stages of Aedes aegypti (L.). Bull. Entomol. Res. 1957, 48, 593–599. [Google Scholar] [CrossRef]
- Teng, H.J.; Apperson, C.S. Development and survival of immature Aedes albopictus and Aedes triseriatus (Diptera: Culicidae) in the laboratory: Effects of density, food, and competition on response to temperature. J. Med. Entomol. 2000, 37, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Mori, A.; Oda, T.; Wada, Y. Studies on the egg diapause and overwintering of Aedes albopictus in Nagasaki. Trop. Med. 1981, 23, 79–90. [Google Scholar]
- Higa, Y.; Toma, T.; Araki, Y.; Onodera, I.; Miyagi, I. Seasonal changes in oviposition activity, hatching and embryonation rates of eggs of Aedes albopictus (Diptera: Culicidae) on three islands of the Ryukyu Archipelago, Japan. Med. Entomol. Zool. 2007, 58, 1–10. [Google Scholar] [CrossRef]
- Hawley, W.A.; Pumpuni, C.B.; Brady, R.H.; Craig, G.B., Jr. Overwintering survival of Aedes albopictus (Diptera: Culicidae) eggs in Indiana. J. Med. Entomol. 1989, 26, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Hanson, S.M.; Craig, G.B., Jr. Cold acclimation, diapause, and geographic origin affect cold hardiness in eggs of Aedes albopictus (Diptera: Culicidae). J. Med. Entomol. 1994, 31, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Soper, F.L. Dynamics of Aedes aegypti distribution and density. Seasonal fluctuations in the Americas. Bull. WHO 1967, 36, 536. [Google Scholar] [PubMed]
- Rozeboom, L.E. Overwintering of Aedes aegypti in Stillwater. Proc. Okla. Acad. Sci. 1938, 19, 81–82. [Google Scholar]
- Lima, A.; Lovin, D.D.; Hickner, P.V.; Severson, D.W. Evidence for an overwintering population of Aedes aegypti in Capitol Hill neighborhood, Washington, DC. Am. J. Trop. Med. Hyg. 2016, 94, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Tsunoda, T.; Cuong, T.C.; Dong, T.D.; Yen, N.T.; Le, N.H.; Phong, T.V.; Minakawa, N. Winter refuge for Aedes aegypti and Ae. albopictus mosquitoes in Hanoi during Winter. PLoS ONE 2014, 9, e95606. [Google Scholar] [CrossRef] [PubMed]
- Lambrechts, L.; Paaijmans, K.P.; Fansiri, T.; Carrington, L.B.; Kramer, L.D.; Thomas, M.B.; Scott, T.W. Impact of daily temperature fluctuations on dengue virus transmission by Aedes aegypti. Proc. Natl. Acad. Sci. USA 2011, 108, 7460–7465. [Google Scholar] [CrossRef] [PubMed]
- Elbers, A.R.W.; Koenraadt, C.J.; Meiswinkel, R. Mosquitoes and Culicoides biting midges: Vector range and the influence of climate change. Rev. Sci. Tech. Off. Int. Epizoot. 2015, 34, 123–137. [Google Scholar] [CrossRef]
- Suwonkerd, W.; Tsuda, Y.; Takagi, M.; Wada, Y. Seasonal occurrence of Aedes aegypti and Ae. albopictus in used tires in 1992–1994, Chiangmai, Thailand. Trop. Med. 1997, 38, 101–105. [Google Scholar]
- Mogi, M. Overwintering strategies of mosquitoes (Diptera: Culicidae) on warmer islands may predict impact of global warming on Kyushu, Japan. J. Med. Entomol. 1996, 33, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Tsunoda, T.; Chaves, L.F.; Nguyen, G.T.T.; Nguyen, Y.T.; Takagi, M. Winter Activity and Diapause of Aedes albopictus (Diptera: Culicidae) in Hanoi, Northern Vietnam. J. Med. Entomol. 2015, 52, 1203–1212. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, Y.; Suwonkerd, W.; Chawprom, S.; Prajakwong, S.; Takagi, M. Different spatial distribution of Aedes aegypti and Aedes albopictus along an urban–rural gradient and the relating environmental factors examined in three villages in northern Thailand. J. Am. Mosq. Control 2006, 22, 222–228. [Google Scholar] [CrossRef]
- Weaver, S.C.; Reisen, W.K. Present and future arboviral threats. Antivir. Res. 2010, 85, 328–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jansen, C.C.; Beebe, N.W. The dengue vector Aedes aegypti: What comes next. Microbes Infect. 2010, 12, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Chaves, L.F.; Morrison, A.C.; Kitron, U.D.; Scott, T.W. Nonlinear impacts of climatic variability on the density-dependent regulation of an insect vector of disease. Glob. Chang. Biol. 2012, 18, 457–468. [Google Scholar] [CrossRef]
- Chaves, L.F.; Scott, T.W.; Morrison, A.C.; Takada, T. Hot temperatures can force delayed mosquito outbreaks via sequential changes in Aedes aegypti demographic parameters in autocorrelated environments. Acta Trop. 2014, 129, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Lewontin, R.; Levins, R. Schmalhausen’s law. Capital. Natl. Soc. 2000, 11, 103–108. [Google Scholar] [CrossRef]
- Chaves, L.F. Globally invasive, withdrawing at home: Aedes albopictus and Aedes japonicus facing the rise of Aedes flavopictus. Int. J. Biometeorol. 2016, 60, 1727–1738. [Google Scholar] [CrossRef] [PubMed]
- Chaves, L.F. Climate change and the biology of insect vectors of human pathogens. Glob. Clim. Chang. Terr. Invertebr. 2017, 126–147. [Google Scholar] [CrossRef]
- Higa, Y.; Thi Yen, N.; Kawada, H.; Hai Son, T.; Thuy Hoa, N.; Takagi, M. Geographic distribution of Aedes aegypti and Aedes albopictus collected from used tires in Vietnam. J. Am. Mosq. Control 2010, 26, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, M.U.; Sinka, M.E.; Duda, K.A.; Mylne, A.Q.; Shearer, F.M.; Barker, C.M.; Moore, C.G.; Carvalho, R.G.; Coelho, G.E.; Van Bortel, W.; et al. The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. eLife 2015, 4, e08347. [Google Scholar] [CrossRef] [PubMed]
- Romi, R.; Di Luca, M.; Marjori, G. Current status of Aedes albopictus and Aedes atropalpus in Italy. J. Am. Mosq. Control 1999, 15, 425–427. [Google Scholar]
- Benedict, M.Q.; Levine, R.S.; Hawley, W.A.; Lounibos, L.P. Spread of the tiger: Global risk of invasion by the mosquito Aedes albopictus. Vector-Borne Zoonotic 2007, 7, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Kearney, M.; Porter, W.P.; Williams, C.; Ritchie, S.; Hoffmann, A.A. Integrating biophysical models and evolutionary theory to predict climatic impacts on species’ ranges: The dengue mosquito Aedes aegypti in Australia. Funct. Ecol. 2009, 23, 528–538. [Google Scholar] [CrossRef]
- Alto, B.W.; Juliano, S.A. Precipitation and temperature effects on populations of Aedes albopictus (Diptera: Culicidae): Implications for range expansion. J. Med. Entomol. 2001, 38, 646–656. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.M.; Macoris, M.D.L.D.G.; Galvani, K.C.; Andrighetti, M.T.M.; Wanderley, D.M.V. Assessing the effects of temperature on the population of Aedes aegypti, the vector of dengue. Epidemiol. Infect. 2009, 137, 1188–1202. [Google Scholar] [CrossRef] [PubMed]
- O’meara, G.F.; Evans, L.F., Jr.; Gettman, A.D.; Cuda, J.P. Spread of Aedes albopictus and decline of Ae. aegypti (Diptera: Culicidae) in Florida. J. Med. Entomol. 1995, 32, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Lounibos, L.P.; Suárez, S.; Menéndez, Z.; Nishimura, N.; Escher, R.L.; OConnell, S.M.; Rey, J.R. Does temperature affect the outcome of larval competition between Aedes aegypti and Aedes albopictus? J. Vector Ecol. 2002, 27, 86–95. [Google Scholar] [PubMed]
- Kobayashi, M.; Nihei, N.; Kurihara, T. Analysis of northern distribution of Aedes albopictus (Diptera: Culicidae) in Japan by geographical information system. J. Med. Entomol. 2002, 39, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Mogi, M.; Tuno, N. Impact of climate change on the distribution of Aedes albopictus (Diptera: Culicidae) in northern Japan: Retrospective analyses. J. Med. Entomol. 2014, 51, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Epstein, P.R.; Diaz, H.F.; Elias, S.; Grabherr, G.; Graham, N.E.; Martens, W.J.; MosIey-Thompson, E.; Susskind, J. Biological and physical signs of climate change: Focus on mosquito-borne diseases. Bull. Am. Meteorol. Soc. 1998, 79, 409–417. [Google Scholar] [CrossRef]
- Ostfeld, R.S. Climate change and the distribution and intensity of infectious diseases. Ecology 2009, 90, 903–905. [Google Scholar] [CrossRef] [PubMed]
- Patz, J.A.; Martens, W.J.; Focks, D.A.; Jetten, T.H. Dengue fever epidemic potential as projected by general circulation models of global climate change. Environ. Health Perspect. 1998, 106, 147. [Google Scholar] [CrossRef] [PubMed]
- Sutherst, R.W. Implications of global change and climate variability for vector-borne diseases: Generic approaches to impact assessments. Int. J. Parasitol. 1998, 28, 935–945. [Google Scholar] [CrossRef]
- Rochlin, I.; Ninivaggi, D.V.; Hutchinson, M.L.; Farajollahi, A. Climate change and range expansion of the Asian tiger mosquito (Aedes albopictus) in Northeastern USA: Implications for public health practitioners. PLoS ONE 2013, 8, e60874. [Google Scholar] [CrossRef] [PubMed]
- Kramer, L.D.; Ebel, G.D. Dynamics of flavivirus infection in mosquitoes. Adv. Virus Res. 2003, 60, 187–232. [Google Scholar] [PubMed]
- Gratz, N.G. Critical review of the vector status of Aedes albopictus. Med. Vet. Entomol. 2004, 18, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Vega-Rúa, A.; Zouache, K.; Girod, R.; Failloux, A.B.; Lourenço-de-Oliveira, R. High vector competence of Aedes aegypti and Aedes albopictus from ten American countries as a crucial factor of the spread of Chikungunya. J. Virol. 2014, JVI-00370. [Google Scholar] [CrossRef] [PubMed]
- Brady, O.J.; Golding, N.; Pigott, D.M.; Kraemer, M.U.; Messina, J.P.; Reiner, R.C., Jr.; Scott, T.W.; Smith, D.L.; Gething, P.W.; Hay, S.I. Global temperature constraints on Aedes aegypti and Aedes albopictus persistence and competence for dengue virus transmission. Parasite Vector 2014, 7, 338. [Google Scholar] [CrossRef] [PubMed]
- Patz, J.A.; Githeko, A.K.; McCarty, J.P.; Hussein, S.; Confalonieri, U.; De Wet, N. Climate change and infectious diseases. Clim. Chang. Hum. Health Risks Responses 2003, 6, 103–137. [Google Scholar]
- Lafferty, K.D. The ecology of climate change and infectious diseases. Ecology 2009, 90, 888–900. [Google Scholar] [CrossRef] [PubMed]
- McMichael, A.J.; Woodruff, R.E. Climate change and infectious diseases. Soc. Ecol. Infect. Dis. 2008, 378–407. [Google Scholar] [CrossRef]
- World Health Organization. Dengue and Severe Dengue. Available online: http://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue (accessed on 13 September 2018).
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O. The global distribution and burden of dengue. Nature 2013, 496, 504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brady, O.J.; Gething, P.W.; Bhatt, S.; Messina, J.P.; Brownstein, J.S.; Hoen, A.G.; Moyes, C.L.; Farlow, A.W.; Scott, T.W.; Hay, S.I. Refining the global spatial limits of dengue virus transmission by evidence-based consensus. PLoS NTDs 2012, 6, e1760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watts, D.M.; Burke, D.S.; Harrison, B.A.; Whitmire, R.E.; Nisalak, A. Effect of temperature on the vector efficiency of Aedes aegypti for dengue 2 virus. Am. J. Trop. Med. Hyg. 1987, 36, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Rohani, A.; Wong, Y.C.; Zamre, I.; Lee, H.L.; Zurainee, M.N. The effect of extrinsic incubation temperature on development of dengue serotype 2 and 4 viruses in Aedes aegypti (L.). SE Asian J. Trop. Med. 2009, 40, 942. [Google Scholar]
- Alto, B.W.; Bettinardo, D. Temperature and dengue virus infection in mosquitoes: Independent effects of the immature and adult stages. Am. J. Trop. Med. Hyg. 2013, 88, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Whitehorn, J.; Kien, D.T.; Nguyen, N.M.; Nguyen, H.L.; Kyrylos, P.P.; Carrington, L.B.; Tran, C.N.; Quyen, N.T.; Thi, L.V.; Le Thi, D.; et al. Comparative susceptibility of Aedes albopictus and Aedes aegypti to dengue virus infection after feeding on blood of viremic humans: Implications for public health. J. Infect. Dis. 2015, 212, 1182–1190. [Google Scholar] [CrossRef] [PubMed]
- Thu, H.M.; Aye, K.M.; Thein, S. The effect of temperature and humidity on dengue virus propagation in Aedes aegypti mosquitoes. Southeast Asian J. Trop. Med. Public Health 1998, 29, 280–284. [Google Scholar] [PubMed]
- World Health Organization. Chikungunya. Available online: http://www.who.int/news-room/fact-sheets/detail/chikungunya (accessed on 12 April 2017).
- Renault, P.; Solet, J.L.; Sissoko, D.; Balleydier, E.; Larrieu, S.; Filleul, L.; Lassalle, C.; Thiria, J.; Rachou, E.; de Valk, H.; et al. A major epidemic of chikungunya virus infection on Reunion Island, France, 2005–2006. Am. J. Trop. Med. Hyg. 2007, 77, 727–731. [Google Scholar] [CrossRef] [PubMed]
- Yoon, I.K.; Alera, M.T.; Lago, C.B.; Tac-An, I.A.; Villa, D.; Fernandez, S.; Thaisomboonsuk, B.; Klungthong, C.; Levy, J.W.; Velasco, J.M.; et al. High rate of subclinical chikungunya virus infection and association of neutralizing antibody with protection in a prospective cohort in the Philippines. PLoS NTDs 2015, 9, e0003764. [Google Scholar] [CrossRef] [PubMed]
- Becker, N. Influence of climate change on mosquito development and mosquito-borne diseases in Europe. Parasitol. Res. 2008, 103, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Paupy, C.; Delatte, H.; Bagny, L.; Corbel, V.; Fontenille, D. Aedes albopictus, an arbovirus vector: From the darkness to the light. Microbes Infect. 2009, 11, 1177–1185. [Google Scholar] [CrossRef] [PubMed]
- Zouache, K.; Fontaine, A.; Vega-Rua, A.; Mousson, L.; Thiberge, J.M.; Lourenco-De-Oliveira, R.; Caro, V.; Lambrechts, L.; Failloux, A.B. Three-way interactions between mosquito population, viral strain and temperature underlying chikungunya virus transmission potential. Proc. R. Soc. Lond. B Biol. Sci. 2014, 281, 20141078. [Google Scholar] [CrossRef] [PubMed]
- Li, C.X.; Guo, X.X.; Deng, Y.Q.; Xing, D.; Sun, A.J.; Liu, Q.M.; Wu, Q.; Zhang, Y.M.; Zhang, H.D.; Cao, W.C.; et al. Vector competence and transovarial transmission of two Aedes aegypti strains to Zika virus. Emerg. Microbes Infec. 2017, 6, e23. [Google Scholar] [CrossRef] [PubMed]
- Niyas, K.P.; Abraham, R.; Unnikrishnan, R.N.; Mathew, T.; Nair, S.; Manakkadan, A.; Issac, A.; Sreekumar, E. Molecular characterization of Chikungunya virus isolates from clinical samples and adult Aedes albopictus mosquitoes emerged from larvae from Kerala, South India. Virol. J. 2010, 7, 189. [Google Scholar] [CrossRef] [PubMed]
- Mavale, M.; Parashar, D.; Sudeep, A.; Gokhale, M.; Ghodke, Y.; Geevarghese, G.; Arankalle, V.; Mishra, A.C. Venereal transmission of chikungunya virus by Aedes aegypti mosquitoes (Diptera: Culicidae). Am. J. Trop. Med. Hyg. 2010, 83, 1242–1244. [Google Scholar] [CrossRef] [PubMed]
- Cauchemez, S.; Ledrans, M.; Poletto, C.; Quenel, P.D.; De Valk, H.; Colizza, V.; Boëlle, P.Y. Local and regional spread of chikungunya fever in the Americas. Euro Surveillance: Bulletin Europeen sur les Maladies Transmissibles=Eur. Commun. Dis. Bull. 2014, 19, 20854. [Google Scholar] [CrossRef] [Green Version]
- Kendrick, K.; Stanek, D.; Blackmore, C.; Centers for Disease Control and Prevention (CDC). Notes from the field: Transmission of chikungunya virus in the continental United States—Florida, 2014. MMWR Morb. Mortal. Wkly. Rep. 2014, 63, 1137. [Google Scholar] [PubMed]
- Center for Disease Control and Prevention. Chikungunya Virus Home: Geographic Distribution. Available online: https://www.cdc.gov/chikungunya/geo/index.html (accessed on 29 May 2018).
- Barba-Spaeth, G.; Dejnirattisai, W.; Rouvinski, A.; Vaney, M.C.; Medits, I.; Sharma, A.; Simon-Lorière, E.; Sakuntabhai, A.; Cao-Lormeau, V.M.; Haouz, A.; et al. Structural basis of potent Zika–dengue virus antibody cross-neutralization. Nature 2016, 536, 48. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Zika Virus. Available online: http://www.who.int/news-room/fact-sheets/detail/zika-virus (accessed on 20 July 2018).
- Benelli, G.; Romano, D. Mosquito vectors of Zika virus. Entomol. Gen. 2017, 36, 309–318. [Google Scholar] [CrossRef]
- Hayes, E.B. Zika virus outside Africa. Emerg. Infect. Dis. 2009, 15, 1347. [Google Scholar] [CrossRef] [PubMed]
- Haddow, A.D.; Schuh, A.J.; Yasuda, C.Y.; Kasper, M.R.; Heang, V.; Huy, R.; Guzman, H.; Tesh, R.B.; Weaver, S.C. Genetic characterization of Zika virus strains: Geographic expansion of the Asian lineage. PLoS NTDs 2012, 6, e1477. [Google Scholar] [CrossRef] [PubMed]
- Petersen, L.R.; Jamieson, D.J.; Powers, A.M.; Honein, M.A. Zika virus. New Engl. J. Med. 2016, 374, 1552–1563. [Google Scholar] [CrossRef] [PubMed]
- Haddow, A.D.; Schuh, A.J.; Yasuda, C.Y.; Kasper, M.R.; Heang, V.; Huy, R.; Guzman, H.; Tesh, R.B.; Weaver, S.C. On the seasonal occurrence and abundance of the Zika virus vector mosquito Aedes aegypti in the contiguous United States. PLoS Curr. 2016, 8. [Google Scholar] [CrossRef]
- Mordecai, E.A.; Cohen, J.M.; Evans, M.V.; Gudapati, P.; Johnson, L.R.; Lippi, C.A.; Miazgowicz, K.; Murdock, C.C.; Rohr, J.R.; Ryan, S.J.; et al. Detecting the impact of temperature on transmission of Zika, dengue, and chikungunya using mechanistic models. PLoS NTDs 2017, 11, e0005568. [Google Scholar] [CrossRef] [PubMed]
- Azar, S.R.; Roundy, C.M.; Rossi, S.L.; Huang, J.H.; Leal, G.; Yun, R.; Fernandez-Salas, I.; Vitek, C.J.; Paploski, I.A.; Stark, P.M.; et al. Differential vector competency of Aedes albopictus populations from the Americas for Zika virus. Am. J. Trop. Med. Hyg. 2017, 97, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Gendernalik, A.; Weger-Lucarelli, J.; Luna, S.M.G.; Fauver, J.R.; Rückert, C.; Murrieta, R.A.; Burgren, N.; Samaras, D.; Nguyen, C.; Kading, R.C.; Ebel, G.D. American Aedes vexans mosquitoes are competent vectors of Zika virus. Am. J. Trop. Med. Hyg. 2017, 96, 1338–1340. [Google Scholar] [CrossRef] [PubMed]
- Ayres, C.F. Identification of Zika virus vectors and implications for control. Lancet Infect. Dis. 2016, 16, 278–279. [Google Scholar] [CrossRef]
- Gardner, L.M.; Chen, N.; Sarkar, S. Global risk of Zika virus depends critically on vector status of Aedes albopictus. Lancet Infect. Dis. 2016, 16, 522–523. [Google Scholar] [CrossRef]
- World Health Organization. West Nile Virus. Available online: http://www.who.int/news-room/fact-sheets/detail/west-nile-virus (accessed on 3 October 2017).
- Klenk, K.; Snow, J.; Morgan, K.; Bowen, R.; Stephens, M.; Foster, F.; Gordy, P.; Beckett, S.; Komar, N.; Gubler, D.; et al. Alligators as West Nile virus amplifiers. Emerg. Infect. Dis. 2004, 10, 2150. [Google Scholar] [CrossRef] [PubMed]
- Campbell, G.L.; Marfin, A.A.; Lanciotti, R.S.; Gubler, D.J. West Nile virus. Lancet Infect. Dis. 2002, 2, 519–529. [Google Scholar] [CrossRef]
- Turell, M.J.; O’Guinn, M.L.; Dohm, D.J.; Jones, J.W. Vector competence of North American mosquitoes (diptera: Culicidae) for West Nile virus. J. Med. Entomol. 2001, 38, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Turell, M.J.; Dohm, D.J.; Sardelis, M.R.; O’guinn, M.L.; Andreadis, T.G.; Blow, J.A. An update on the potential of North American mosquitoes (Diptera: Culicidae) to transmit West Nile virus. J. Med. Entomol. 2005, 42, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Richards, S.L.; Mores, C.N.; Lord, C.C.; Tabachnick, W.J. Impact of extrinsic incubation temperature and virus exposure on vector competence of Culex pipiens quinquefasciatus Say (Diptera: Culicidae) for West Nile virus. Vector-Borne Zoonotic 2007, 7, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Dohm, D.J.; O’Guinn, M.L.; Turell, M.J. Effect of environmental temperature on the ability of Culex pipiens (Diptera: Culicidae) to transmit West Nile virus. J. Med. Entomol. 2002, 39, 221–225. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Yellow Fever Virus. Available online: http://www.who.int/news-room/fact-sheets/detail/yellow-fever (accessed on 1 May 2018).
- Rogers, D.J.; Wilson, A.J.; Hay, S.I.; Graham, A.J. The global distribution of yellow fever and dengue. Adv. Parasit. 2006, 62, 181–220. [Google Scholar]
- Aitken, T.H.; Tesh, R.B.; Beaty, B.J.; Rosen, L. Transovarial transmission of yellow fever virus by mosquitoes (Aedes aegypti). Am. J. Trop. Med. Hyg. 1979, 28, 119–121. [Google Scholar] [CrossRef] [PubMed]
- Center for Disease Control and Prevention. Transmission of Yellow Fever Virus. Available online: https://www.cdc.gov/yellowfever/transmission/index.html (accessed on 13 August 2015).
- Gubler, D.J. The changing epidemiology of yellow fever and dengue, 1900 to 2003: Full circle? Comp. Immunol. Microb. 2004, 27, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Davis, N.C. The Effect of Various Temperatures in modifying the Extrinsic Incubation Period of the Yellow Fever Virus in Aedes aegypti. Am. J. Hyg. 1932, 16, 163–176. [Google Scholar] [CrossRef]
- Moore, C.G.; Mitchell, C.J. Aedes albopictus in the United States: Ten-year presence and public health implications. Emerg. Infect. Dis. 1997, 3, 329. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.J.; Niebylski, M.L.; Smith, G.C.; Karabatsos, N.; Martin, D.; Mutebi, J.P.; Craig, G.B.; Mahler, M.J. Isolation of eastern equine encephalitis virus from Aedes albopictus in Florida. Science 1992, 257, 526–527. [Google Scholar] [CrossRef] [PubMed]
- Komar, N.; Dohm, D.J.; Turell, M.J.; Spielman, A. Eastern equine encephalitis virus in birds: Relative competence of European starlings (Sturnus vulgaris). Am. J. Trop. Med. Hyg. 1999, 60, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, R.; Sudia, W. The Effects of Temperature upon the Extrinsic Incubation of Eastern Equine Encephalitis in Mosquitoes. Am. J. Hyg. 1955, 62, 295–305. [Google Scholar] [PubMed]
- Lednicky, J.; De Rochars, V.M.B.; Loeb, J.; Telisma, T.; Chavannes, S.; Anilis, G.; Cella, E. Ciccozzi, M.; Okech, B.; Salemi, M.; et al. Mayaro virus in child with acute febrile illness, Haiti, 2015. Emerg. Infect. Dis. 2016, 22, 2000. [Google Scholar] [CrossRef] [PubMed]
- Da Costa Carvalho, M.D.G.; Fournier, M.V. Effect of heat shock on gene expression of Aedes albopictus cells infected with Mayaro virus. Res. Virol. 1991, 142, 25–31. [Google Scholar] [CrossRef]
- Hotez, P.J.; Murray, K.O. Dengue, West Nile virus, chikungunya, Zika—And now Mayaro? PLoS Negl. Trop. Dis. 2017, 11, e005462. [Google Scholar] [CrossRef] [PubMed]
- Kartman, L. Factors influencing infection of the mosquito with Dirofilaria immitis (Leidy, 1856). Exp. Parasitol. 1953, 2, 27–78. [Google Scholar] [CrossRef]
- McGreevy, P.B.; Kolstrup, N.; Tao, J.; McGreevy, M.M.; de C. Marshall, T.F. Ingestion and development of Wuchereria bancrofti in Culex quinquefasciatus, Anopheles gambiae and Aedes aegypti after feeding on humans with varying densities of microfilariae in Tanzania. Trans. R. Soc. Trop. Med. Hyg. 1982, 76, 288–296. [Google Scholar] [CrossRef]
- Simón, F.; López-Belmonte, J.; Marcos-Atxutegi, C.; Morchón, R.; Martín-Pacho, J.R. What is happening outside North America regarding human dirofilariasis? Vet. Parasitol. 2005, 133, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Ledesma, N.; Harrington, L. Mosquito vectors of dog heartworm in the United States: Vector status and factors influencing transmission efficiency. Top. Companion Anim. Med. 2011, 26, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Ledesma, N.; Harrington, L. Fine-scale temperature fluctuation and modulation of Dirofilaria immitis larval development in Aedes aegypti. Vet. Parasitol. 2015, 209, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Mas-Coma, S.; Valero, M.A.; Bargues, M.D. Effects of climate change on animal and zoonotic helminthiases. Rev. Sci. Tech. 2008, 27, 443–457. [Google Scholar] [CrossRef] [PubMed]
- Beck-Johnson, L.M.; Nelson, W.A.; Paaijmans, K.P.; Read, A.F.; Thomas, M.B.; Bjørnstad, O.N. The effect of temperature on Anopheles mosquito population dynamics and the potential for malaria transmission. PLoS ONE 2013, 8, e79276. [Google Scholar] [CrossRef] [PubMed]
- Paaijmans, K.P.; Blanford, S.; Bell, A.S.; Blanford, J.I.; Read, A.F.; Thomas, M.B. Influence of climate on malaria transmission depends on daily temperature variation. Proc. Natl. Acad. Sci. USA 2010, 107, 15135–15139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanderberg, J.P.; Yoeli, M. Effects of temperature on sporogonic development of Plasmodium berghei. J. Parasitol. 1966, 52, 559–564. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reinhold, J.M.; Lazzari, C.R.; Lahondère, C. Effects of the Environmental Temperature on Aedes aegypti and Aedes albopictus Mosquitoes: A Review. Insects 2018, 9, 158. https://0-doi-org.brum.beds.ac.uk/10.3390/insects9040158
Reinhold JM, Lazzari CR, Lahondère C. Effects of the Environmental Temperature on Aedes aegypti and Aedes albopictus Mosquitoes: A Review. Insects. 2018; 9(4):158. https://0-doi-org.brum.beds.ac.uk/10.3390/insects9040158
Chicago/Turabian StyleReinhold, Joanna M., Claudio R. Lazzari, and Chloé Lahondère. 2018. "Effects of the Environmental Temperature on Aedes aegypti and Aedes albopictus Mosquitoes: A Review" Insects 9, no. 4: 158. https://0-doi-org.brum.beds.ac.uk/10.3390/insects9040158




