Epigenetic Analyses of Alcohol Consumption in Combustible and Non-Combustible Nicotine Product Users
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Franceschi, S.; Talamini, R.; Barra, S.; E Barón, A.; Negri, E.; Bidoli, E.; Serraino, D.; La Vecchia, C. Smoking and drinking in relation to cancers of the oral cavity, pharynx, larynx, and esophagus in northern Italy. Cancer Res. 1990, 50, 6502–6507. [Google Scholar]
- Zheng, T.; Boyle, P.; Hu, H.; Duan, J.; Jiang, P.; Ma, D.; Shui, L.; Niu, S.; MacMahon, B. Tobacco smoking, alcohol consumption, and risk of oral cancer: A case-control study in Beijing, People’s Republic of China. Cancer Causes Control. 1990, 1, 173–179. [Google Scholar] [CrossRef]
- Zheng, T.; Boyal, P.; Zhang, B.; Zhang, Y.; Owens, P.; Lan, Q. Tobacco Use and Risk of Oral Cancer. In Tobacco and Public Health: Science and Policy; Boyle, P., Gray, N., Henningfield, J., Seffrin, J., Eds.; Oxford University Press: Oxford, UK, 2004; pp. 399–432. [Google Scholar]
- Marrero, J.A.; Fontana, R.J.; Fu, S.; Conjeevaram, H.S.; Su, G.; Lok, A.S. Alcohol, tobacco and obesity are synergistic risk factors for hepatocellular carcinoma. J. Hepatol. 2005, 42, 218–224. [Google Scholar] [CrossRef]
- Thom, T.; Haase, N.; Rosamond, W.; Howard, V.J.; Rumsfeld, J.; Manolio, T.; Zheng, Z.J.; Flegal, K.; O’Donnell, C.; Kittner, S.; et al. Heart disease and stroke statistics—2006 update: A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2006, 113, e85–e151. [Google Scholar]
- Falk, D.E.; Yi, H.-Y.; Hiller-Sturmhöfel, S. An epidemiologic analysis of co-occurring alcohol and tobacco use and disorders: Findings from the National Epidemiologic Survey on Alcohol and Related Conditions. Alcohol Res. Health 2006, 29, 162. [Google Scholar]
- Miller, N.S.; Gold, M.S. Comorbid cigarette and alcohol addiction: Epidemiology and treatment. J. Addict. Dis. 1998, 17, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Mason, B.J.; Lehert, P. Effects of nicotine and illicit substance use on alcoholism treatment outcomes and acamprosate efficacy. J. Addict. Med. 2009, 3, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Daeppen, J.-B.; Smith, T.L.; Danko, G.P.; Gordon, L.; Landi, N.A.; Nurnberger, J.; Bucholz, K.K.; Raimo, E.; Schuckit, M.A. Clinical correlates of cigarette smoking and nicotine dependence in alcohol-dependent men and women. Alcohol Alcohol. 2000, 35, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Barrett, S.P.; Tichauer, M.; Leyton, M.; Pihl, R.O. Nicotine increases alcohol self-administration in non-dependent male smokers. Drug Alcohol Depend. 2006, 81, 197–204. [Google Scholar] [CrossRef]
- Montanari, C.; Secci, M.E.; Driskell, A.; McDonald, K.O.; Schratz, C.L.; Gilpin, N.W. Chronic nicotine increases alcohol self-administration in adult male Wistar rats. Psychopharmacology 2020, 238, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Glautier, S.; Clements, K.; White, J.A.; Taylor, C.; Stolerman, I.P. Alcohol and the reward value of cigarette smoking. Behav. Pharmacol. 1996, 7, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Mintz, J.; Boyd, G.; Rose, J.E.; Charuvastra, V.; Jarvik, M.E. Alcohol increases cigarette smoking: A laboratory demonstration. Addict. Behav. 1985, 10, 203–207. [Google Scholar] [CrossRef]
- Oliver, J.A.; Blank, M.D.; Van Rensburg, K.J.; MacQueen, D.A.; Brandon, T.H.; Drobes, D.J. Nicotine interactions with low-dose alcohol: Pharmacological influences on smoking and drinking motivation. J. Abnorm. Psychol. 2013, 122, 1154–1165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perkins, K.A.; Sexton, J.E.; DiMarco, A.; Grobe, J.E.; Scierka, A.; Stiller, R.L. Subjective and cardiovascular responses to nicotine combined with alcohol in male and female smokers. Psychopharmacology 1995, 119, 205–212. [Google Scholar] [CrossRef]
- Perkins, K.A. Chronic tolerance to nicotine in humans and its relationship to tobacco dependence. Nicotine Tob. Res. 2002, 4, 405–422. [Google Scholar] [CrossRef] [PubMed]
- McKee, S.A.; Weinberger, A.H. How can we use our knowledge of alcohol-tobacco interactions to reduce alcohol use? Annu. Rev. Clin. Psychol. 2013, 9, 649–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiappetta, V.; García-Rodríguez, O.; Jin, C.J.; Secades-Villa, R.; Blanco, C. Predictors of quit attempts and successful quit attempts among individuals with alcohol use disorders in a nationally representative sample. Drug Alcohol Depend. 2014, 141, 138–144. [Google Scholar] [CrossRef]
- DiFranza, J.R.; Guerrera, M.P. Alcoholism and smoking. J. Stud. Alcohol 1990, 51, 130–135. [Google Scholar] [CrossRef]
- Weinberger, A.H.; Pilver, C.E.; Hoff, R.A.; Mazure, C.M.; McKee, S.A. Changes in smoking for adults with and without alcohol and drug use disorders: Longitudinal evaluation in the US population. Am. J. Drug Alcohol Abus. 2013, 39, 186–193. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; (DSM-5); American Psychiatric Publishing: Washington, DC, USA, 2013. [Google Scholar]
- Bradford, L.E.; Rebuli, M.; Ring, B.J.; Jaspers, I.; Clement, K.C.; Loughlin, C.E. Danger in the vapor? ECMO for adolescents with status asthmaticus after vaping. J. Asthma 2019, 57, 1168–1172. [Google Scholar] [CrossRef]
- Hasin, D.S.; O’Brien, C.P.; Auriacombe, M.; Borges, G.; Bucholz, K.; Budney, A.; Compton, W.M.; Crowley, T.; Ling, W.; Petry, N.M.; et al. DSM-5 criteria for substance use disorders: Recommendations and rationale. Am. J. Psychiatry 2013, 170, 834–851. [Google Scholar] [CrossRef] [Green Version]
- Chou, S.P.; Goldstein, R.; Smith, S.; Huang, B.; June Ruan, W.; Zhang, H.; Jung, J.; Saha, T.; Pickering, R.; Grant, B. The epidemiology of DSM-5 nicotine use disorder: Results from the National Epidemiologic Survey on Alcohol and Related Conditions-III. J. Clin. Psychiatry 2016, 77. [Google Scholar] [CrossRef]
- Chung, T.; Martin, C.S.; Maisto, S.A.; Cornelius, J.R.; Clark, D.B. Greater prevalence of proposed DSM-5 nicotine use disorder compared to DSM-IV nicotine dependence in treated adolescents and young adults. Addiction 2011, 107, 810–818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masonbrink, A.; Richardson, T.; Hall, M.; Catley, D.; Wilson, K. Trends in incidence of nicotine use disorder among adolescents in the pediatric hospital, 2012–2019. Hosp. Pediatr. 2020, 11, 25–29. [Google Scholar] [CrossRef]
- Levy, D.T.; Tam, J.; Sanchez-Romero, L.M.; Li, Y.; Yuan, Z.; Jeon, J.; Meza, R. Public health implications of vaping in the USA: The smoking and vaping simulation model. Popul. Health Metr. 2021, 19, 1–18. [Google Scholar] [CrossRef]
- Cropsey, K.L.; Trent, L.R.; Clark, C.B.; Stevens, E.N.; Lahti, A.C.; Hendricks, P.S. How Low Should You Go? Determining the optimal cutoff for exhaled carbon monoxide to confirm smoking abstinence when using cotinine as reference. Nicotine Tob. Res. 2014, 16, 1348–1355. [Google Scholar] [CrossRef] [Green Version]
- Dawes, K.; Andersen, A.; Papworth, E.; Hundley, B.; Hutchens, N.; El Manawy, H.; Becker, A.; Sampson, L.; Philibert, W.; Gibbons, F.X.; et al. Refinement of cg05575921 demethylation response in nascent smoking. Clin. Epigenet. 2020, 12, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sakai, L.M.; Esposito, T.J.; Ton-That, H.H.; Omi, E.C.; Kovacs, E.J.; Schermer, C.R. Comparison of objective screening and self-report for alcohol and drug use in traumatically injured patients. Alcohol. Treat. Q. 2012, 30, 433–442. [Google Scholar] [CrossRef]
- Rubinstein, M.L.; Delucchi, K.; Benowitz, N.L.; Ramo, D.E. Adolescent exposure to toxic volatile organic chemicals from E-cigarettes. Pediatrics 2018, 141, e20173557. [Google Scholar] [CrossRef] [Green Version]
- Goniewicz, M.L.; Smith, D.M. Are some E-cigarette users “blowing smoke”? Assessing the accuracy of self-reported smoking abstinence in exclusive E-cigarette users. Nicotine Tob. Res. 2018, 21, 699–700. [Google Scholar] [CrossRef] [PubMed]
- Goniewicz, M.; Havel, C.M.; Peng, M.W.; Jacob, P.; Dempsey, D.; Yu, L.; Zielińska-Danch, W.; Koszowski, B.; Czogala, J.; Sobczak, A.; et al. Elimination kinetics of the tobacco-specific biomarker and lung carcinogen 4-(Methylnitrosamino)-1-(3-Pyridyl)-1-Butanol. Cancer Epidemiol. Biomark. Prev. 2009, 18, 3421–3425. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, S.; Jain, R.; Jhanjee, S.; Rao, R.; Mishra, A. Alcohol biomarkers and their relevance in detection of alcohol consumption in clinical settings. Int. Arch. Subst. Abus. Rehabil. 2019, 1, 1–8. [Google Scholar]
- Florescu, A.; Ferrence, R.; Einarson, T.; Selby, P.; Soldin, O.; Koren, G. Methods for quantification of exposure to cigarette smoking and environmental tobacco smoke: Focus on developmental toxicology. Ther. Drug Monit. 2009, 31, 14–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandberg, A.; Sköld, C.M.; Grunewald, J.; Eklund, A.; Wheelock, M. Assessing recent smoking status by measuring exhaled carbon monoxide levels. PLoS ONE 2011, 6, e28864. [Google Scholar] [CrossRef] [Green Version]
- Hecht, E.; Vogt, T.M. Marijuana smoking: Effect on expired air carbon monoxide levels. Int. J. Addict. 1985, 20, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Florath, I.; Saum, K.-U.; Brenner, H. Self-reported smoking, serum cotinine, and blood DNA methylation. Environ. Res. 2016, 146, 395–403. [Google Scholar] [CrossRef]
- Philibert, R.; Miller, S.; Noel, A.; Dawes, K.; Papworth, E.; Black, D.; Beach, S.R.H.; Long, J.; Mills, J.A.; Dogan, M. A four marker digital PCR toolkit for detecting heavy alcohol consumption and the effectiveness of its treatment. J. Insur. Med. 2019, 48, 90–102. [Google Scholar] [CrossRef]
- Dawes, K.; Andersen, A.; Vercande, K.; Papworth, E.; Philibert, W.; Beach, S.R.; Gibbons, F.X.; Gerrard, M.; Philibert, R. Saliva DNA methylation detects nascent smoking in adolescents. J. Child Adolesc. Psychopharmacol. 2019, 29, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Philibert, R.; Dogan, M.; Noel, A.; Miller, S.; Krukow, B.; Papworth, E.; Cowley, J.; Long, J.; Beach, S.R.H.; Black, D. Dose response and prediction characteristics of a methylation sensitive digital PCR assay for cigarette consumption in adults. Front. Genet. 2018, 9, 137. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Jia, M.; Zhang, Y.; Breitling, L.P.; Brenner, H. DNA methylation changes of whole blood cells in response to active smoking exposure in adults: A systematic review of DNA methylation studies. Clin. Epigenet. 2015, 7, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Ramadoss, P.; Marcus, C.; Perdew, G.H. Role of the aryl hydrocarbon receptor in drug metabolism. Expert Opin. Drug Metab. Toxicol. 2005, 1, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Andersen, A.; Reimer, R.; Dawes, K.; Becker, A.; Hutchens, N.; Miller, S.; Dogan, M.; Hundley, B.; A Mills, J.; Long, J.D.; et al. DNA methylation differentiates smoking from vaping and non-combustible tobacco use. Epigenetics 2021, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Philibert, R.; Mills, J.A.; Long, J.D.; Salisbury, S.E.; Comellas, A.; Gerke, A.; Dawes, K.; Weg, M.V.; Hoffman, E.A. The reversion of cg05575921 methylation in smoking cessation: A potential tool for incentivizing healthy aging. Genes 2020, 11, 1415. [Google Scholar] [CrossRef]
- Miller, S.; A Mills, J.; Long, J.; Philibert, R. A comparison of the predictive power of DNA methylation with carbohydrate deficient transferrin for heavy alcohol consumption. Epigenetics 2020, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Michaud, M.S.; Langevin, S.M.; McClean, M.D.; Eliot, M.; Kelsey, K.T. Smokeless tobacco and risk of head and neck cancer: Evidence from a case-control study in New England. Int. J. Cancer 2012, 132, 1911–1917. [Google Scholar] [CrossRef] [Green Version]
- Hershberger, A.; Argyriou, E.; Cyders, M. Electronic nicotine delivery system use is related to higher odds of alcohol and marijuana use in adolescents: Meta-analytic evidence. Addict. Behav. 2020, 105, 106325. [Google Scholar] [CrossRef]
- Lanza, H.I.; Teeter, H. Electronic nicotine delivery systems (E-cigarette/vape) use and co-occurring health-risk behaviors among an ethnically diverse sample of young adults. Subst. Use Misuse 2017, 53, 154–161. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef]
- Hamilton, C.M.; Strader, L.C.; Pratt, J.G.; Maiese, D.; Hendershot, T.; Kwok, R.; Hammond, J.A.; Huggins, W.; Jackman, D.; Pan, H.; et al. The PhenX Toolkit: Get the most from your measures. Am. J. Epidemiol. 2011, 174, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Philibert, R.; Penaluna, B.; White, T.; Shires, S.; Gunter, T.; Liesveld, J.; Erwin, C.; Hollenbeck, N.; Osborn, T. A pilot examination of the genome-wide DNA methylation signatures of subjects entering and exiting short-term alcohol dependence treatment programs. Epigenetics 2014, 9, 1212–1219. [Google Scholar] [CrossRef] [Green Version]
- Philibert, R.; Dogan, M.; Beach, S.R.H.; Mills, J.A.; Long, J. AHRR methylation predicts smoking status and smoking intensity in both saliva and blood DNA. Am. J. Med. Genet. Part B Neuropsychiatry Genet. 2019, 183, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Andersen, A.M.; Philibert, R.A.; Gibbons, F.X.; Simons, R.L.; Long, J. Accuracy and utility of an epigenetic biomarker for smoking in populations with varying rates of false self-report. Am. J. Med. Genet. Part B Neuropsychiatry Genet. 2017, 174, 641–650. [Google Scholar] [CrossRef]
- De Boor, C. A Practical guide to splines. Math. Comput. 1980, 34, 325. [Google Scholar] [CrossRef]
- Akaike, H. Akaike’s Information Criterion; Springer: New York, NY, USA, 2011. [Google Scholar]
- Mishra, S. Handling imbalanced data: SMOTE vs. random undersampling. Int. Res. J. Eng. Technol. 2017, 4, 317–320. [Google Scholar]
- More, A.; Rana, D.P. Review of random forest classification techniques to resolve data imbalance. In Proceedings of the 1st International Conference on Intelligent Systems and Information Management (ICISIM), Aurangabad, India, 5–6 October 2017; IEEE: Piscataway, NJ, USA, 2017; pp. 72–78. [Google Scholar]

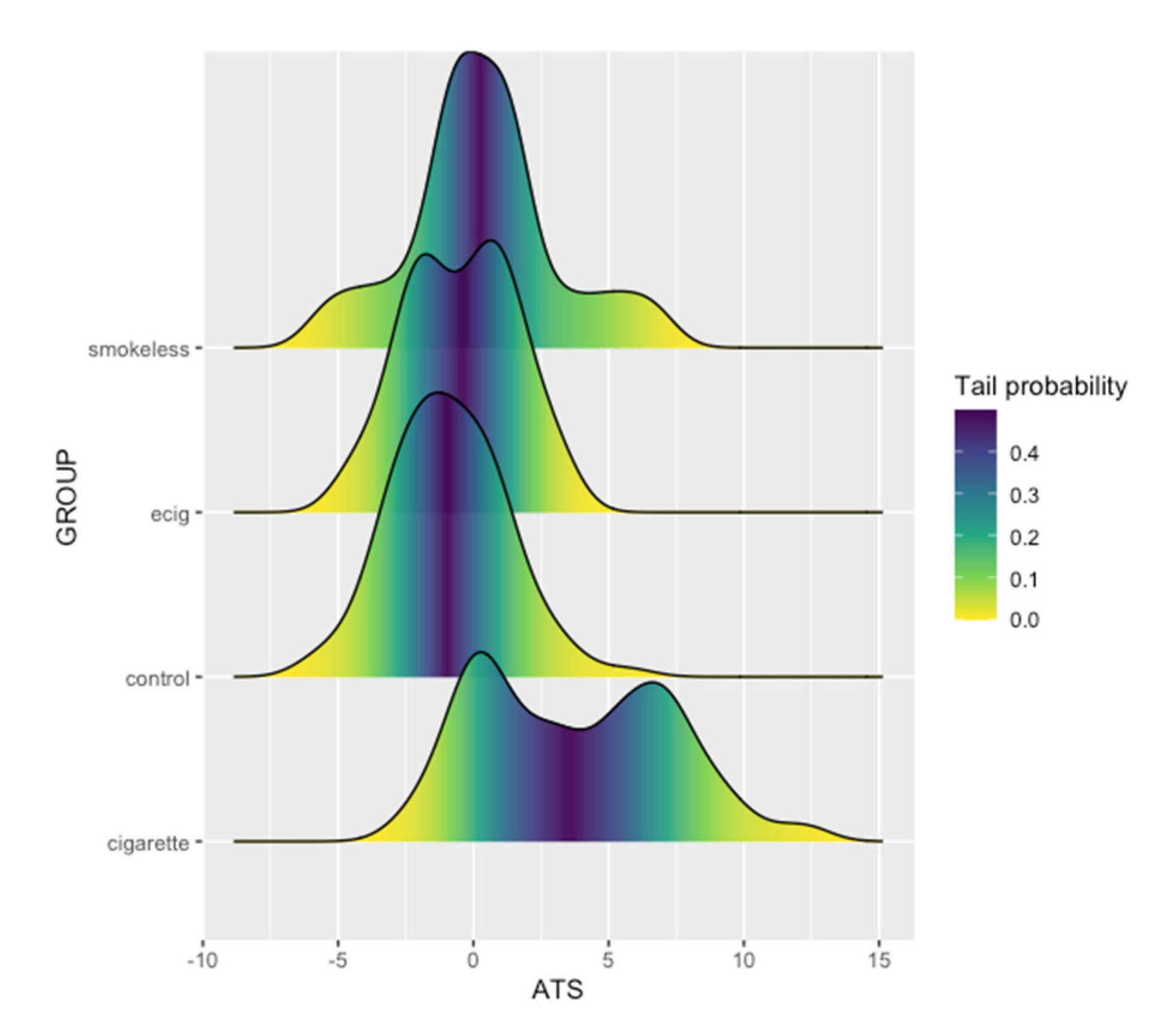
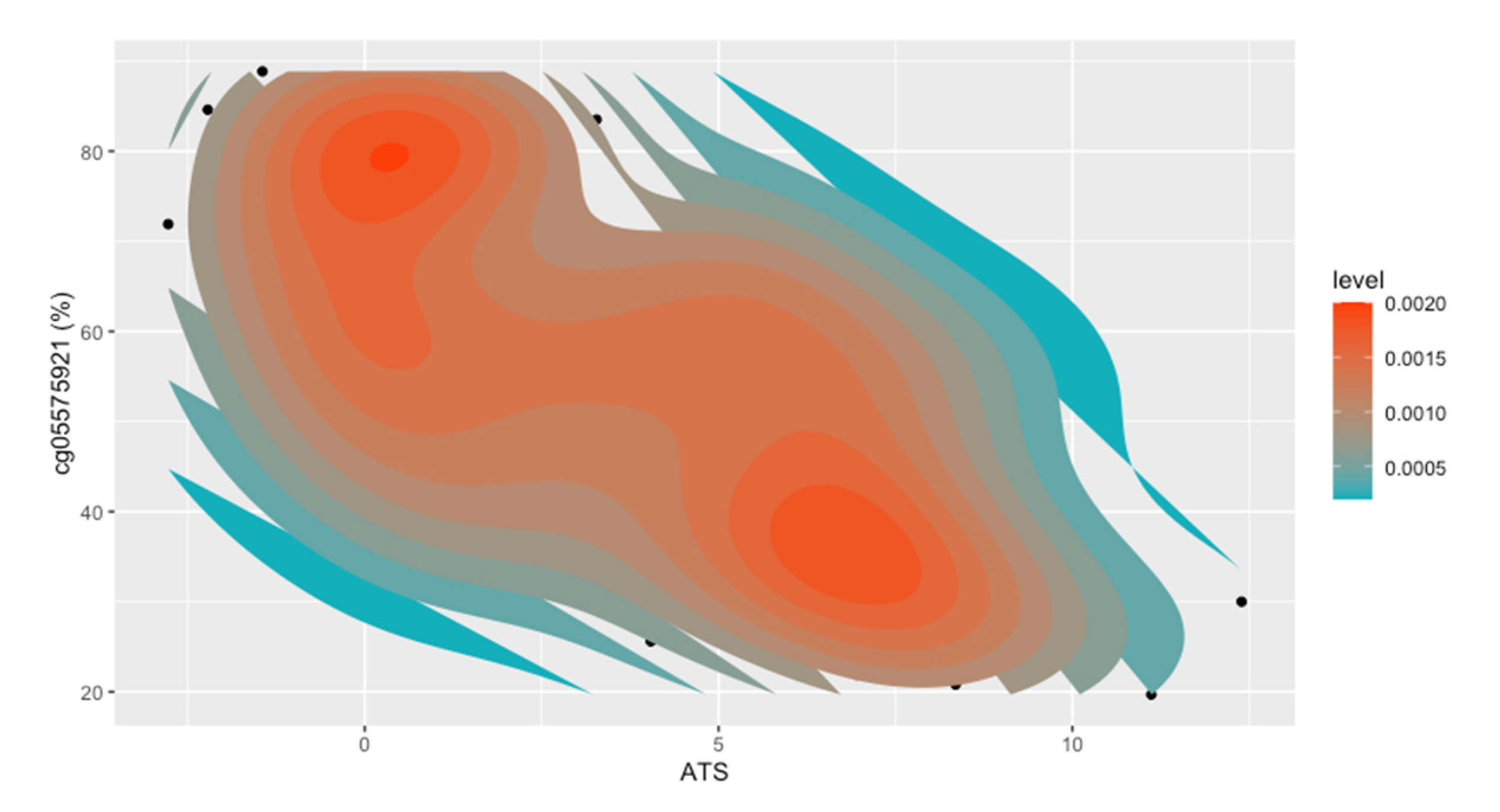
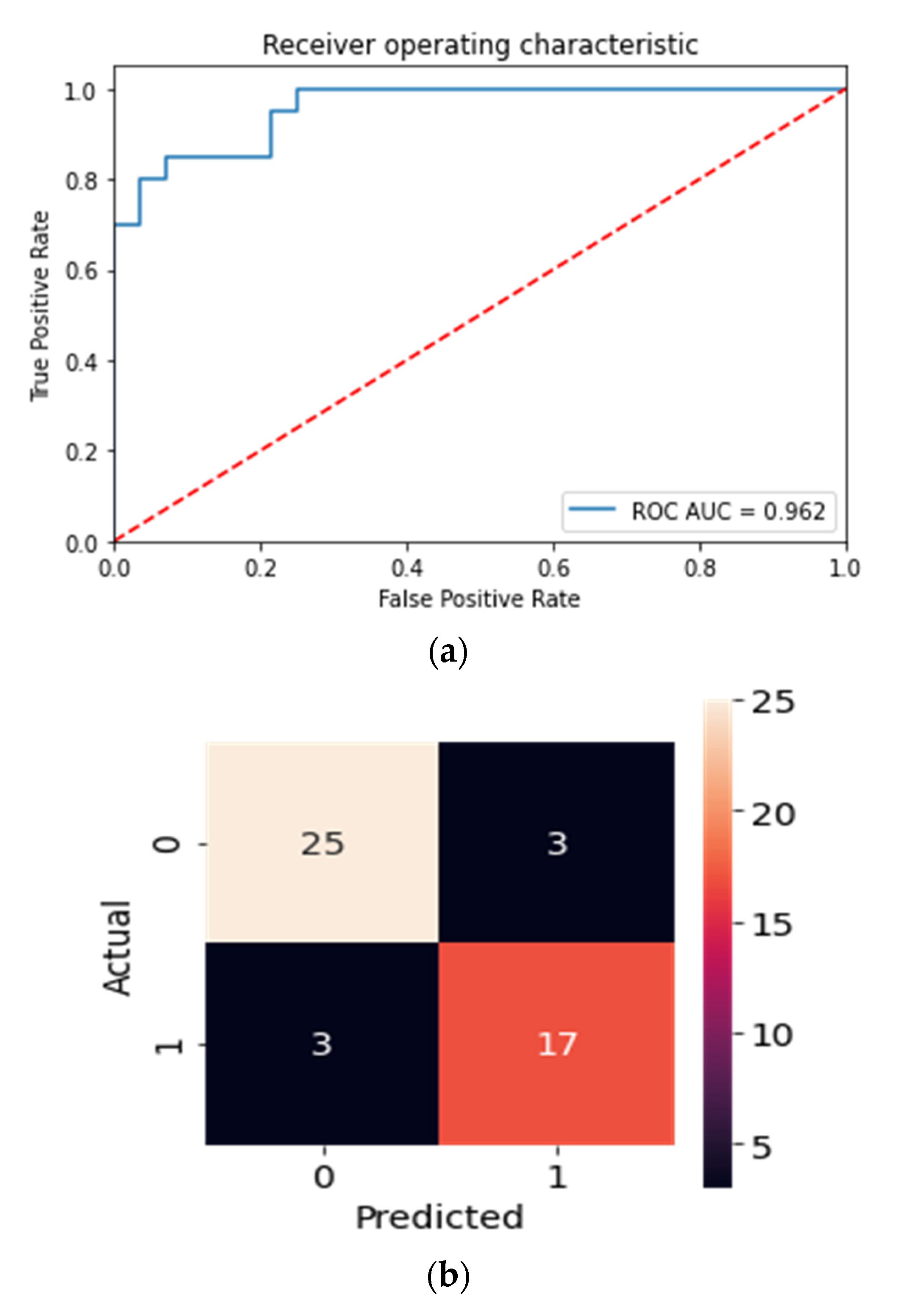
| Smokers | ENDS | Smokeless | Control | |
|---|---|---|---|---|
| n = 108 | n = 35 | n = 19 | n = 262 | |
| Age | 40.9 ± 13.0 | 22.7 ± 4.8 | 36.1 ± 12.3 | 29.6 ± 11.4 |
| Gender | ||||
| Male | 42 (39) | 13 (37) | 18 (95) | 79 (30) |
| Female | 66 (61) | 22 (63) | 1 (5) | 182 (69) |
| Other | - | - | - | 1 (<1) |
| Ethnicity | ||||
| White | 89 (82) | 30 (86) | 18 (95) | 226 (86) |
| African American | 9 (8) | 2 (6) | - | 6 (2) |
| American Indian | 1 (1) | - | - | 2 (<1) |
| Asian | 3 (3) | 1 (3) | - | 14 (5) |
| Bi-racial | 5 (5) | 2 (6) | 1 (5) | 7 (3) |
| Other | 1 (1) | - | - | 7 (3) |
| Substance Dependence | ||||
| Nicotine | 90 (83) | 20 (60) | 12 (63) | - |
| Alcohol | 15 (14) | 3 (9) | 3 (16) | - |
| Both | 15 (14) | 2 (6) | 3 (16) | - |
| Drinks per Week | ||||
| None | 24 (22) | 6 (17) | 2 (11) | 81 (31) |
| 1 to 7 | 53 (49) | 16 (46) | 7 (37) | 169 (65) |
| 8 to 14 | 15 (14) | 6 (17) | 6 (32) | 7 (3) |
| >14 | 12 (11) | 5 (14) | 4 (21) | 3 (1) |
| cg05575921 | 54.5 ± 19.1 | 84.1 ± 5.1 | 84.3 ± 4.6 | 86.8 ± 2.8 |
| Cotinine ng/mL | 93.6 ± 32.4 | 78.4 ± 45.2 | 108.1 ± 39 | - |
| ATS | 3.7 ± 3.6 | −0.5 ± 2.0 | 0.3 ± 2.8 | −0.9 ± 2.1 |
| ATS > 5 | 45 (42) | - | 2 (11) | 3 (1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dawes, K.; Sampson, L.; Reimer, R.; Miller, S.; Philibert, R.; Andersen, A. Epigenetic Analyses of Alcohol Consumption in Combustible and Non-Combustible Nicotine Product Users. Epigenomes 2021, 5, 18. https://0-doi-org.brum.beds.ac.uk/10.3390/epigenomes5030018
Dawes K, Sampson L, Reimer R, Miller S, Philibert R, Andersen A. Epigenetic Analyses of Alcohol Consumption in Combustible and Non-Combustible Nicotine Product Users. Epigenomes. 2021; 5(3):18. https://0-doi-org.brum.beds.ac.uk/10.3390/epigenomes5030018
Chicago/Turabian StyleDawes, Kelsey, Luke Sampson, Rachel Reimer, Shelly Miller, Robert Philibert, and Allan Andersen. 2021. "Epigenetic Analyses of Alcohol Consumption in Combustible and Non-Combustible Nicotine Product Users" Epigenomes 5, no. 3: 18. https://0-doi-org.brum.beds.ac.uk/10.3390/epigenomes5030018








