Adolescent Finswimmers: Early Myocardial Adaptations in Different Swimming Styles
Abstract
:1. Introduction
2. Method
2.1. Participants
2.2. Data Collection
2.3. Data Analyses
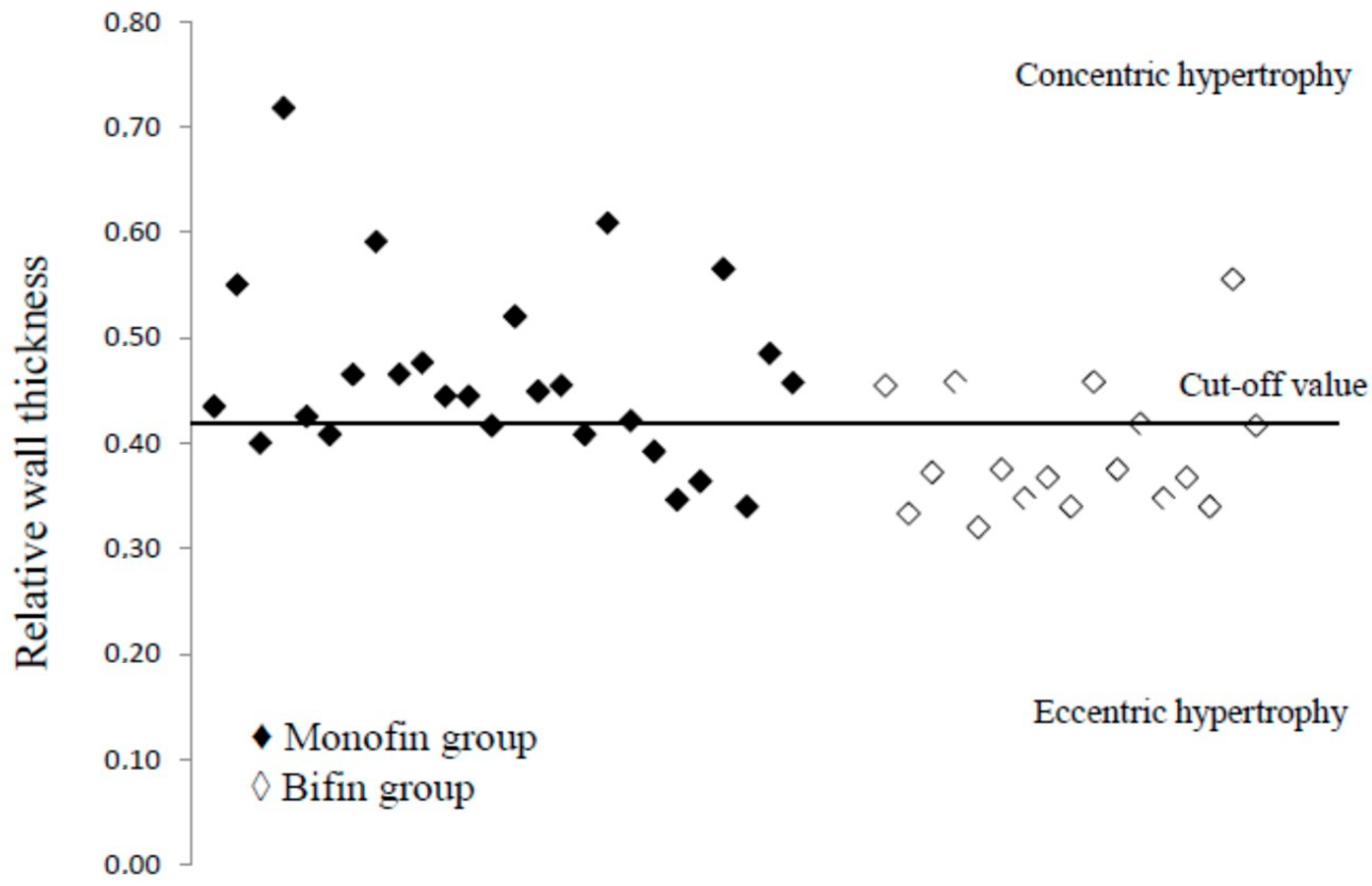
3. Results
4. Discussion
5. Limitations of Our Study
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Grazioli, G.; Sanz, M.; Montserrat, S.; Vidal, B.; Sitges, M. Echocardiography in the evaluation of athletes. F1000 Res. 2015, 4, 151. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, A.; Caso, P.; Sarubbi, B.; Limongelli, G.; Liccardo, B.; Cice, G.; D’Andrea, L.; Scherillo, M.; Cotrufo, M.; Calabro, R. Right ventricular myocardial adaptation to different training protocols in top-level athletes. Echocardiography 2003, 20, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Ayabakan, C.; Figen, A.; Sami, M.; Birol, Ç.; Ilhan, O.; Ali, Ö. Athlete’s heart in prepubertal male swimmers. Cardiol. Young 2006, 16, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Haykowsky, M.J.; Smith, D.J.; Malley, L.; Norris, S.R.; Smith, E.R. Effects of short-term altitude training and tapering on left ventricular morphology in elite swimmers. Can. J. Cardiol. 1998, 14, 678–681. [Google Scholar] [PubMed]
- Triposkiadis, F.; Ghiokas, S.; Skoularigis, I.; Kotsakis, A.; Giannakoulis, I.; Thanopoulos, V. Cardiac adaptation to intensive training in prepubertal swimmers. Eur. J. Clin. Investig. 2002, 32, 16–23. [Google Scholar] [CrossRef]
- Stavrou, V.; Voutselas, V.; Karetsi, E.; Gourgoulianis, K.I. Acute responses of breathing techniques in maximal inspiratory pressure. Sport Sci. Health 2017, 14, 1–5. [Google Scholar] [CrossRef]
- CMAS; World Underwater Federation. Finswimming CMAS Rules, Version 2010/01, (23/04/10, BOB /SPO/No 26). 2010. Available online: http://www.cmas.org/comspo/nap/ (accessed on 10 January 2013).
- Mosteller, R.D. Simplified Calculation of Body Surface Area. N. Engl. J. Med. 1987, 317, 1098. [Google Scholar] [CrossRef] [PubMed]
- Tanner, J.; Whitehouse, R.H. Clinical longitudinal standards for height, weight, height velocity, weight velocity and the stages of puberty. Arch. Dis. Child. 1976, 51, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; van der Grinten, C.P.; Gustafsson, P.; et al. ATS/ERS Task Force. Standardisation of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef] [PubMed]
- Lopez, L.; Colan, S.; Frommelt, P.; Ensing, G.; Kendall, K.; Younoszai, A.; Lai, W.; Geva, T. Recommendations for Quantification Methods during the Performance of a Pediatric Echocardiogram: A Report from the Pediatric Measurements Writing Group of the American Society of Echocardiography Pediatric and Congenital Heart Disease Council. J. Am. Soc. Echocardiogr. 2010, 23, 465–495. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American society of echocardiography and the European association of cardiovascular imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–271. [Google Scholar] [CrossRef] [PubMed]
- Lenth, R.V. Java Applets for Power and Sample Size [Computer Software]. 2006–2009. Available online: http://www.stat.uiowa.edu/~rlenth/Power (accessed on 12 May 2018).
- Morganroth, J.; Maron, B.J.; Henry, W.L.; Epstein, S.E. Comparative left ventricular dimensions in trained athletes. Ann. Intern. Med. 1975, 82, 521–524. [Google Scholar] [CrossRef] [PubMed]
- George, K.; Whyte, P.G.; Green, J.D.; Oxborough, D.; Shave, R.E.; Gaze, D.; Somauroo, J. The endurance athletes heart: Acute stress and chronic adaptation. Br. J. Sports Med. 2012, 46, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Marion, K.; Guillaume, G.; Pascale, C.; Charlie, B.; Antonb, S. Muscle activity during fin swimming. Procedia Eng. 2010, 2, 3029–3034. [Google Scholar] [CrossRef]
- Wasfy, M.M.; Weiner, R.B.; Wang, F.; Berkstresser, B.; Lewis, G.D.; DeLuca, J.R.; Hutter, A.M.; Picard, M.H.; Baggish, A.L. Endurance exercise-induced cardiac remodeling: Not all sports are created equal. J. Am. Soc. Echocardiogr. 2015, 28, 1434–1440. [Google Scholar] [CrossRef] [PubMed]
- Ghorayeb, N.; Batlouni, M.; Pinto, I.M.; Dioguardi, G.S. Left ventricular hypertrophy of athletes: Adaptative physiologic response of the heart. Arq. Bras. Cardiol. 2005, 85, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Fagard, R. Athlete’s Heart. Heart 2003, 89, 1455–1461. [Google Scholar] [CrossRef] [PubMed]
- Stavrou, V.; Toubekis, A.; Karetsi, E. Changes in respiratory parameters and fin-swimming performance following a 16-week training period with intermittent breath holding. J. Hum. Kinet. 2015, 49, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Stavrou, V.; Vavougios, G.; Karetsi, E.; Adam, G.; Daniil, Z.; Gourgoulianis, K.I. Evaluation of respiratory parameters in finswimmers regarding gender, swimming style and distance. Respir. Physiol. Neurobiol. 2018, 254, 30–31. [Google Scholar] [CrossRef] [PubMed]
- Venckunas, T.; Lionikas, A.; Marcinkeviciene, J.E.; Raugaliene, R.; Alekrinskis, A.; Stasiulis, A. Echocardiographic Parameters in Athletes of Different Sports. J. Sports Sci. Med. 2008, 7, 151–156. [Google Scholar] [PubMed]
- Sitges, M.; Merino, B.; Butakoff, C.; Garza, M.S.; Paré, C.; Montserrat, S.; Vidal, B.; Azqueta, M.; Sarquella, G.; Gutierrez, J.A.; et al. Characterizing the spectrum of right ventricular remodelling in response to chronic training. Int. J. Cardiovasc. Imaging 2017, 33, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, R.; Baggish, A.L. Cardiovascular adaptation in athletes. Trends Cardiovasc. Med. 2016, 26, 46–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haykowsky, M.; Taylor, D.; Teo, K.; Quinney, A.; Humen, D. Left ventricular wall stress during leg-press exercise performed with a brief valsalva maneuver. Chest 2001, 119, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Cain, P.A.; Ahl, R.; Hedstrom, E.; Ugander, M.; Allansdotter-Johnsson, A.; Friberg, P.; Arheden, H. Age and gender specific normal values of left ventricular mass, volume and function for gradient echo magnetic resonance imaging: A cross sectional study. BMC Med. Imaging 2009, 9, 2. [Google Scholar] [CrossRef] [PubMed]
- Cain, P.A.; Ahl, R.; Hedstrom, E.; Ugander, M.; Allansdotter-Johnsson, A.; Friberg, P.; Marild, S.; Arheden, H. Physiological determinants of the variation in left ventricular mass from early adolescence to late adulthood in healthy subjects. Clin. Physiol. Funct. Imaging 2005, 25, 332–339. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Total (n = 43) | MFgroup (n = 26) | BFgroup (n = 17) | p-Value |
|---|---|---|---|---|
| Age, years | 15.6 ± 2.1 | 15.9 ± 2.1 | 15.1 ± 2.1 | NS |
| Body mass index, kg/m2 | 20.4 ± 2.2 | 20.7 ± 2.2 | 19.8 ± 2.1 | NS |
| Body surface area, m2 | 1.5 ± 0.04 | 1.6 ± 0.04 | 1.5 ± 0.03 | NS |
| Body fat, % | 11.2 ± 0.6 | 11.4 ± 0.6 | 10.9 ± 0.7 | NS |
| Tanner score | 4.1 ± 0.7 | 4.0 ± 0.7 | 4.3 ± 0.8 | NS |
| Training age, years | 4.1 ± 1.5 | 4.5 ± 1.8 | 3.4 ± 0.5 | 0.017 |
| Training, day/h | 2.1 ± 0.2 | 2.1 ± 0.2 | 2.0 ± 0.1 | NS |
| Training, week/h | 13.0 ± 1.0 | 13.1 ± 3.1 | 13.2 ± 2.9 | NS |
| Gym, week/h | 3.0 ± 1.1 | 3.2 ± 0.9 | 2.8 ± 1.3 | NS |
| Best trial | ||||
| 50-m, second | 23.4 ± 2.6 | 22.0 ± 1.8 | 26.5 ± 1.1 | <0.001 |
| 100-m, second | 55.7 ± 5.7 | 51.5 ± 2.4 | 59.4 ± 5.1 | 0.003 |
| 200-m, second | 113.4 ± 10.1 | 106.6 ± 4.5 | 124.6 ± 3.6 | 0.001 |
| Hemodynamic, LV and Respiratory Parameters | Total (n = 43) | MFgroup (n = 26) | BFgroup (n = 17) | p-Value |
|---|---|---|---|---|
| Hemodynamic | ||||
| HRresting, bpm−1 | 78.8 ± 9.2 | 80.1 ± 7.8 | 76.9 ± 10.9 | NS |
| SBPresting, mmHg | 111.5 ± 8.4 | 113.4 ± 8.8 | 108.4 ± 7.1 | NS |
| DBPresting, mmHg | 71.4 ± 4.6 | 72.1 ± 4.7 | 70.2 ± 4.1 | NS |
| Left ventricular volume and function | ||||
| EDV, mL | 101.2 ± 20.9 | 97.3 ± 18.7 | 107.3 ± 23.1 | NS |
| ESV, mL | 38.1 ± 12.9 | 41.0 ± 14.5 | 33.6 ± 8.4 | 0.040 |
| SV, mL | 62.2 ± 17.1 | 56.6 ± 16.2 | 70.7 ± 14.9 | 0.006 |
| EF, % | 62.8 ± 9.4 | 59.5 ± 10.2 | 67.8 ± 5.0 | 0.001 |
| Intracardiac dimensions | ||||
| IVSd, cm | 1.0 ± 0.2 | 1.0 ± 0.2 | 1.0 ± 0.1 | NS |
| IVSd indexed, cm/m2 | 0.63 ± 0.1 | 0.63 ± 0.1 | 0.62 ± 0.1 | NS |
| LVIDd, cm | 4.5 ± 0.8 | 4.3 ± 0.9 | 4.7 ± 0.4 | NS |
| LVIDd indexed, cm/m2 | 2.94 ± 0.3 | 2.87 ± 0.3 | 3.06 ± 0.3 | NS |
| LVPWd, cm | 0.99 ± 0.2 | 1.0 ± 0.2 | 0.9 ± 0.1 | 0.015 |
| LVPWd, cm/m2 | 0.63 ± 0.1 | 0.66 ± 0.1 | 0.59 ± 0.1 | 0.03 |
| Left ventricle myocardial mass | ||||
| LV mass, g | 158.3 ± 37.7 | 160 ± 39.9 | 155.4 ± 34.9 | NS |
| LV mass indexed, g/m2 | 101.3 ± 23.6 | 102 ± 24.8 | 100.3 ± 22.5 | NS |
| Pulmonary function tests | ||||
| FEV1, %pred. | 116 ± 7.5 | 112.8 ± 5.9 | 120.9 ± 6.9 | <0.001 |
| FVC, %pred. | 114.9 ± 7.9 | 114 ± 7.7 | 116 ± 8.3 | NS |
| PEF, %pred. | 106.7 ± 5.4 | 107.7 ± 6.2 | 105 ± 3.5 | NS |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stavrou, V.; Tsarouhas, K.; Karetsi, E.; Michos, P.; Daniil, Z.; I. Gourgoulianis, K. Adolescent Finswimmers: Early Myocardial Adaptations in Different Swimming Styles. Sports 2018, 6, 78. https://0-doi-org.brum.beds.ac.uk/10.3390/sports6030078
Stavrou V, Tsarouhas K, Karetsi E, Michos P, Daniil Z, I. Gourgoulianis K. Adolescent Finswimmers: Early Myocardial Adaptations in Different Swimming Styles. Sports. 2018; 6(3):78. https://0-doi-org.brum.beds.ac.uk/10.3390/sports6030078
Chicago/Turabian StyleStavrou, Vasileios, Konstantinos Tsarouhas, Eleni Karetsi, Panagiotis Michos, Zoe Daniil, and Konstantinos I. Gourgoulianis. 2018. "Adolescent Finswimmers: Early Myocardial Adaptations in Different Swimming Styles" Sports 6, no. 3: 78. https://0-doi-org.brum.beds.ac.uk/10.3390/sports6030078






