Will Nigerians Win the War Against Urinary Schistosomiasis? Prevalence, Intensity, Risk Factors and Knowledge Assessment among Some Rural Communities in Southwestern Nigeria
Abstract
:1. Introduction
2. Results
2.1. Demographic Characteristics of Participants
2.2. Prevalence and Intensity of Urinary Schistosomiasis in Relation to Sex and Age among the Study Population
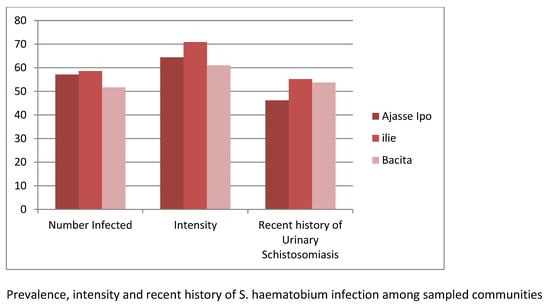
2.3. Prevalence and Intensity of Urinary Schistosomiasis in Relation to Study Communities
2.4. Prevalence and Intensity of Urinary Schistosomiasis in Relation to Socioeconomic Factors Affecting the Transmission of Urinary Schistosomiasis among the Study Population
2.5. Knowledge of Respondents on Urinary Schistosomiasis among the Study Population
3. Discussion
4. Materials and Methods
4.1. Study Area
4.2. Study Population, Design and Sample Size
4.3. Urine Collection and Examination for Schistosoma haematobium Egg
4.4. Examination of Urine for Microhaematuria and Proteinuria
4.5. Focus Group Discussions (FGDs) and Questionnaire Administration
4.6. Statistical Analysis
4.7. Ethical Clearance
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Van der Werf, M.J.; de Vlas, S.J.; Brooker, S.; Looman, C.W.N.; Nagelkerke, N.J.D.; Habbema, J.D.F.; Engels, D. Quantification of clinical morbidity associated with schistosome infection in sub-Saharan Africa. Acta Trop. 2003, 86, 125–139. [Google Scholar] [CrossRef]
- Steinmann, P.; Keiser, J.; Bos, R.; Tanner, M.; Utzinger, J. Schistosomiasis and water resources development: Systematic review, meta-analysis, and estimates of people at risk. Lancet Infect. Dis. 2006, 6, 411–425. [Google Scholar] [CrossRef]
- Okon, O.E.; Udoutun, M.F.; Oku, E.E.; Nta, A.I.; Etim, S.E.; Abraham, J.T.; Akpan, P.A. Prevalence of urinary schistosomiasis in Abini community, Biase local government area, Cross River state, Nigeria. Niger. J. Parasitol. 2007, 28, 28–31. [Google Scholar] [CrossRef]
- Hotez, P.J.; Kamath, A. Neglected tropical diseases in sub-Saharan Africa: Review of their prevalence, distribution, and disease burden. PLoS Negl. Trop. Dis. 2009, 3, e412. [Google Scholar] [CrossRef]
- Abe1, E.M.; Guan, W.; Guo, Y.; Kassegne, K.; Qin, Z.; Xu, J.; Chen, J.; Ekpo, U.F.; Li, S.; Zhou, X. Differentiating snail intermediate hosts of Schistosoma spp. using molecular approaches: Fundamental to successful integrated control mechanism in Africa. Infect. Dis. Poverty 2018, 7, 29. [Google Scholar] [CrossRef]
- Mahmoud, A.A.F. Schistosomiasis and other trematode infections. In Harrison’s Principles of Internal Medicine, 16th ed.; Kasper, D.L., Braunwald, E., Fauci, A.S., Eds.; McGraw-Hill: New York, NY, USA, 2006. [Google Scholar]
- World Health Organization. Investing to Overcome the Global Impact of Neglected Tropical Diseases, Third WHO Report on Neglected Tropical Diseases; World Health Organization: Geneva, Switzerland, 2015; pp. 154–191. [Google Scholar]
- World Health Organization. A Review of Human Carcinogens. Biological Agents. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; World Health Organization: Lyon, France, 2012; Volume 100B. [Google Scholar]
- Van Tong, H.; Brindley, P.J.; Meyer, C.G.; Velavan, T.P. Parasite infection, carcinogenesis and human malignancy. EBioMedicine 2017, 15, 12–23. [Google Scholar] [CrossRef]
- Mafiana, C.F.; Ekpo, U.F.; Ojo, D.A. Urinary schistosomiasis in preschool children in settlements around Oyan reservoir in Ogun state, Nigeria: Implication for control. Trop. Med. Int. Health 2003, 8, 78–82. [Google Scholar] [CrossRef] [Green Version]
- Okoli, C.G.; Iwuala, M.O.E. The prevalence, intensity and clinical signs of urinary schistosomiasis in Imo state, Nigeria. J. Helminthol. 2004, 78, 337–342. [Google Scholar] [CrossRef]
- Oladejo, S.O.; Ofoezie, I.E. Unabated schistosomiasis transmission in Erinle River Dam, Osun State, Nigeria: Evidence of neglect of environmental effects of development projects. Trop. Med. Int. Health 2006, 11, 845–850. [Google Scholar] [CrossRef]
- Opara, K.N.; Udoidung, N.I.; Ukpong, I.G. Genitourinary schistosomiasis among pre-primary schoolchildren in rural community within the Cross-River Basin, Nigeria. J. Helminthol. 2007, 81, 393–394. [Google Scholar] [CrossRef] [PubMed]
- Awosolu, O.B.; Akinnifesi, O.J.; Salawu, A.S.; Omotayo, Y.F.; Obimakinde, E.T.; Olise, C. Prevalence and intensity of urinary schistosomiasis among school age children in Ikota, Southwestern Nigeria. Braz. J. Biol. Sci. 2019, 6, 391–399. [Google Scholar] [CrossRef] [Green Version]
- Hotez, P.J.; Alvarado, M.; Basáñez, M.G.; Bolliger, I.; Bourne, R.; Boussinesq, M.; Brooker, S.J.; Brown, A.S.; Buckle, G.; Budke, C.M.; et al. The Global Burden of Disease Study 2010: Interpretation and implications for the neglected tropical diseases. PLoS Negl. Trop. Dis. 2014, 8, e2865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McManus, D.P.; Dunne, D.W.; Sacko, M.; Utzinger, J.; Vennervald, B.J.; Zhou, X.N. Schistosomiasis. Nat. Rev. Dis. Primer 2018, 4, 13. [Google Scholar] [CrossRef] [PubMed]
- Global Burden of Disease. DALYs and Hale Collaborators. Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease study 2017. Lancet 2018, 392, 1736–1788. [Google Scholar]
- Kapito-Tembo, A.P.; Mwapasa, V.; Meshnick, S.R.; Samanyika, Y.; Banda, D. Prevalence Distribution and Risk Factors for Schistosoma hematobium Infection among School Children in Blantyre, Malawi. PLoS Negl. Trop. Dis. 2009, 3, e361. [Google Scholar] [CrossRef]
- Chitsulo, L.; Engels, D.; Montresor, A.; Savioli, L. The global status of schistosomiasis and its control. Acta Trop. 2000, 77, 41–51. [Google Scholar] [CrossRef] [Green Version]
- Ezeh, C.O.; Onyekwelu, K.C.; Akinwale, O.P.; Shan, L.; Wei, H. Urinary schistosomiasis in Nigeria: A 50 year review of prevalence, distribution and disease burden. Parasite 2019, 26, 19. [Google Scholar] [CrossRef] [Green Version]
- Amoo, A.O.J.; Hassan, A.O. A survey of the Prevalence of Urinary Schistosomiasis among pupils in Osogbo, Osun State. Niger. J. Genitourin. Med. 2005, 5, 4–7. [Google Scholar]
- Bolaji, O.S.; Adeyeba, O.A.; Ojurongbe, O.; Ukaga, C.N.; Ojo, J.A. Epidemiological Studies on Urinary Schistosomiasis in Osun State, Nigeria. Int. J. Pharm. Res. Sch. 2014, 3, 1. [Google Scholar]
- Hassan, A.O.; Amoo, A.O.J.; Akinwale, O.P.; Deji-Agboola, A.M.; Adeleke, M.A.; Gyang, P.V. Current status of urinary schistosomiasis in communities around the Erinle and Eko-Ende Dams and the implications for schistosomiasis control in Nigeria, Southern. Afr. J. Infect. Dis. 2014, 29, 137–140. [Google Scholar] [CrossRef] [Green Version]
- Bolaji, O.S.; Elkanah, F.A.; Ojo, J.A.; Ojurongbe, O.; Adeyeba, O.A. Prevalence and Intensity of Schistosoma haematobium among school children in Ajase-Ipo, Kwara State, Nigeria. Asian J. Biomed. Pharm. Sci. 2015, 5, 6–11. [Google Scholar]
- Uchen, O.; Oladoyin, V.; Idowu, M.; Adeyera, O.; Olabisi, O.; Oluwatosin, O.; Leigh, G. Urinary schistosomiasis among vulnerable children in a rehabilitation home in Ibadan, Oyo state, Nigeria Obioma. BMC Infect. Dis. 2017, 17, 487. [Google Scholar] [CrossRef]
- Ofoezie, I.E. Human health and sustainable water resources development in Nigeria: Schistosomiasis in artificial lakes. Nat. Resour. Forum 2002, 26, 150–160. [Google Scholar] [CrossRef]
- Abdulkadir, A.; Ahmed, M.; Abubakar, B.M.; Suleiman, I.E.; Yusuf, I.; Imam, I.M.; Sule, A.A.; Tela, U.M.; Dogo, H.M.; Yakasai, A.M.; et al. Prevalence of urinary schistosomiasis in Nigeria, 1994–2015: Systematic review and meta-analysis. Afr. J. Urol. 2017, 23, 228–239. [Google Scholar] [CrossRef]
- Dabo, A.; Badawi, H.M.; Bary, B.; Doumbo, O.K. Urinary schistosomiasis among preschool-aged children in Sahelian rural communities in Mali. Parasites Vectors 2011, 4, 21. [Google Scholar] [CrossRef] [Green Version]
- Alabi, P.; Oladejo, S.O.; Odaibo, A.B. Prevalence and intensity of urinary schistosomiasis in Ogun state, Southwest, Nigeria. J. Public Health Epidemiol. 2018, 10, 413–417. [Google Scholar] [CrossRef]
- Oniya, M.O.; Olofintoye, L.K. The prevalence of urinary schistosomiasis in two endemic local government areas in Ondo State. Niger. J. Parasitol. 2009, 30, 147–151. [Google Scholar]
- Dahab, T.O.; El-Bingawi, H.M. Epidemiological survey: Schistosoma haematobium in schoolchildren of White Nile areas, Khartoum. Sudan Med. J. 2012, 48, 135–140. [Google Scholar]
- Negussu, N.; Wali, M.; Ejigu, M.; Debebe, F.; Aden, S. Prevalence and distribution of schistosomiasis in Afder and Gode zone of Somali region, Ethiopia. J. Glob. Infect. Dis. 2013, 5, 149–152. [Google Scholar] [CrossRef]
- Bello, Y.M.; Adamu, T.; Abubakar, U.; Muhammad, A.A. Urinary schistosomiasis in some villages around the Goronyo Dam, Sokoto State, Nigeria. Niger. J. Parasitol. 2003, 24, 109–114. [Google Scholar] [CrossRef]
- Geleta, S.; Alemu, A.; Getie, S.; Mekonnen, Z.; Erko, B. Prevalence of urinary schistosomiasis and associated risk factors among Abobo Primary School children in Gambella Regional State, southwestern Ethiopia: A cross sectional study. Parasites Vectors 2015, 8, 215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onifade, O.E.; Oniya, M.O. Prevalence of Urinary Schistosomiasis and Efficacy of Praziquantel; a Case Study of School Pupils in Oke-Igbo, Ondo State, Nigeria. South Asian J. Parasitol. 2018, 1, 1–10. [Google Scholar]
- Federal Ministry of Health. Report on Epidemiological Mapping of Schistosomiasis and Soil Transmitted Helminthiasis in 19 States and the FCT, Nigeria. 2015. Available online: www.health.gov.ng/doc/SchistoSTH (accessed on 5 December 2019).
- Duwa, M.R.; Oyeyi, T.I.; Bassey, S.E. Prevalence and intensity of urinary schistosomiasis among primary school pupils in Minjibir local government area of Kano State, Nigeria. Bayero J. Pure Appl. Sci. 2009, 2, 75–78. [Google Scholar]
- Ivoke, N.; Ivoke, O.N.; Nwani, C.D.; Ekeh, F.N.; Asogwa, C.N.; Atama, C.I. Prevalence and transmission dynamics of Schistosoma haematobium infection in a rural community of southwestern Ebonyi State, Nigeria. Trop. Biomed. 2014, 31, 77–88. [Google Scholar]
- Dawaki, S.; Al-Mekhlafi, H.M.; Ithoi, I.; Ibrahim, J.; Abdulsalam, A.M.; Ahmed, A.; Sady, H.; Atroosh, W.M.; Al-Areeqi, M.A.; Elyana, F.N.; et al. Prevalence and risk factors of schistosomiasis among Hausa communities in Kano State, Nigeria. Rev. Inst. Med. Trop. Sao Paulo 2016, 58, 54. [Google Scholar] [CrossRef] [Green Version]
- Kazibwe, F.; Makanga, B.; Rubaire, A.C.; Ouma, J.; Kariuki, C.; Kabatereine, N.B. Transmission studies of intestinal schistosomiasis in Lake Albert, Uganda and experimental compatibility of local Biomphalaria spp. Parasitol. Int. 2010, 59, 49–533. [Google Scholar] [CrossRef]
- Hassan, A.O.; Amoo, A.O.J.; Akinwale, O.P.; Deji-Agboola, A.M.; Adeleke, M.A.; Gyang, P.V. Human water contact activities and urinary schistosomiasis around Erinle and Eko-Ende dams. Glob. Adv. Res. J. Med. Med. Sci. 2012, 1, 77–84. [Google Scholar]
- Mazigo, H.D.; Nuwaha, F.; Kinung’hi, S.M.; Morona, D.; de Moira, A.P.; Wilson, S. Epidemiology and control of human schistosomiasis in Tanzania. Parasites Vectors 2012, 5, 274. [Google Scholar] [CrossRef] [Green Version]
- Sokolow, S.H.; Jones, I.J.; Jocque, M.; La, D.; Cords, O.; Knight, A.; Lund, A.; Wood, C.L.; Lafferty, K.D.; Hoover, C.M.; et al. Nearly 400 million people are at higher risk of schistosomiasis because dams block the migration of snail-eating river prawns. Philos. Trans. R. Soc. B 2017, 372, 20160127. [Google Scholar] [CrossRef] [Green Version]
- Pukuma, M.S.; Musa, S.P. Prevalence of urinary schistosomiasis among residents of Waduku in Lamurde Local Government Area of Adamawa State Nigeria. Niger. J. Parasitol. 2007, 28, 65–68. [Google Scholar]
- Uwaezuoke, J.C.; Anosike, J.C.; Nwoke, B.E.B.; Dozie, I.N.S. Urinary schistosomiasis in lhitte Uboma Local Government Area of Imo State, Nigeria. Niger. J. Parasitol. 2007, 28, 90–94. [Google Scholar]
- Sacolo-Gwebu, H.; Kabuyaya, M.; Chimbari, M. Knowledge, attitudes and practices on schistosomiasis and soil-transmitted helminths among caregivers in Ingwavuma area in uMkhanyakude district, South Africa. BMC Infect. Dis. 2019, 19, 734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asaolu, S.O.; Ofoezie, I.E. The role of health education and sanitation in the control of helminth infections. Acta Trop. 2003, 86, 283–294. [Google Scholar] [CrossRef]
- Aagaard-Hansen, J.; Mwanga, J.R.; Bruun, B. Social science perspectives on schistosomiasis control in Africa: Past trends and future directions. Parasitology 2009, 136, 1747–1758. [Google Scholar] [CrossRef]
- Anon. Population Census: Final Report; National Population Commission: Abuja, Nigeria, 1991. [Google Scholar]
- Suresh, K.P.; Chandrashekara, S. Sample size estimation and power analysis for clinical research studies. J. Hum. Reprod. Sci. 2012, 5, 7–13. [Google Scholar] [CrossRef]
- World Health Organization. Expert Committee, Prevention and Control of Schistosomiasis and Soil-Transmitted Heliminthiasis; World Health Organization Technical Report Series: Geneva, Switzerland, 2002. [Google Scholar]


| Variables | N | % |
|---|---|---|
| Gender | ||
| Male | 362 | 58.4 |
| Female | 258 | 41.6 |
| Age | ||
| ≤4 | 17 | 2.7 |
| 9–5 | 156 | 25.2 |
| 14–10 | 270 | 43.5 |
| 15–19 | 168 | 27.1 |
| ≥20 | 9 | 1.5 |
| Father’s Occupation | ||
| Fishing | 15 | 2.4 |
| Farming | 263 | 42.4 |
| Trading | 161 | 26 |
| Salary earner | 132 | 21.3 |
| Wage earner | 49 | 7.9 |
| Mother’s Occupation | ||
| Trading | 291 | 46.9 |
| Farming | 138 | 22.3 |
| Salary earner | 129 | 20.8 |
| Unemployed | 62 | 10 |
| Does Your Father Complete Primary Education | ||
| Yes | 477 | 76.9 |
| No | 143 | 23.1 |
| Does Your Mother Complete Primary Education | ||
| Yes | 432 | 69.7 |
| No | 188 | 30.3 |
| Main Source of Water Supply | ||
| Tap | 71 | 11.5 |
| Well | 309 | 49.8 |
| River | 237 | 38.2 |
| Others | 3 | 0.5 |
| Water Contact Activities | ||
| Playing/Bathing | 137 | 22.1 |
| Washing | 344 | 55.5 |
| Agricultural work | 51 | 8.2 |
| Fishing | 13 | 2.1 |
| No contact | 75 | 12.1 |
| Community | ||
| Ajasse Ipo | 212 | 34.2 |
| Ilie | 203 | 32.7 |
| Bacita | 205 | 33.1 |
| TOTAL | 620 | 100 |
| Variables | No Examined N | No Infected n (%) | Mean (S.D) Intensity of Infection | Recent History of Urinary Schistosomiasis n (%) |
|---|---|---|---|---|
| Sex | ||||
| Male | 362 | 224 (61.9) | 69.29 (68.9) | 193 (53.3) |
| Female | 258 | 122 (47.3) | 58.81 (34.6) | 127 (49.2) |
| χ2 | 13.005 | 1.009 | ||
| p value | < 0.001 | 0.116 | 0.315 | |
| Age (Years) | ||||
| ≤4 | 17 | 7 (41.2) | 80.14 (54.2) | 8 (47.1) |
| 5–9 | 156 | 84 (53.8) | 77.77 (97.2) | 80 (51.3) |
| 10–14 | 270 | 178 (65.9) | 67.37 (44.1) | 159 (58.9) |
| 15–19 | 168 | 74 (44.0) | 47.43 (18.6) | 71 (42.3) |
| ≥20 | 9 | 3 (33.3) | 33.67 (11.8) | 2 (22.2) |
| χ2 | 24.191 | 14.867 | ||
| p value | < 0.001 | 0.017 | p = 0.005 | |
| Total | 620 | 346 (55.81) | 65.60 (59.33) | 320 (53.24) |
| Variables | No Examined | No Infected (%) | Mean (S.D) Intensity of Infection | Recent History of Urinary Schistosomiasis (%) |
|---|---|---|---|---|
| Father’s Occupation | ||||
| Fishing | 15 | 9 (60.0) | 109.33 (110.68) | 9 (60.0) |
| Farming | 263 | 174 (66.2) | 64.71 (63.46) | 136 (51.7) |
| Trading | 161 | 86 (53.4) | 61.43 (41.27) | 80 (49.7) |
| Salary earner | 132 | 55 (41.7) | 68.76 (64.42) | 70 (53.0) |
| Wage earner | 49 | 22 (44.9) | 63.09 (38.55) | 25 (51.0) |
| χ2 | 24.975 | 0.775 | ||
| p value | < 0.001 | 0.236 | p = 0.942 | |
| Mother’s Occupation | ||||
| Trading | 291 | 156 (53.6) | 59.33 (38.35) | 144 (49.5) |
| Farming | 138 | 106 (76.8) | 75.67 (81.83) | 77 (55.8) |
| Salary earner | 129 | 50 (38.8) | 65.00 (66.97) | 59 (45.7) |
| Unemployed | 62 | 34 (54.8) | 63.82 (37.56) | 40 (64.5) |
| χ2 | 40.481 | 7.412 | ||
| p value | < 0.001 | 0.185 | p = 0.060 | |
| Does Your Father Complete Primary Education | ||||
| Yes | 477 | 242 (50.7) | 58.87 (41.47) | 234 (49.1) |
| No | 143 | 104 (72.7) | 81.26 (86.09) | 86 (60.1) |
| χ2 | 21.578 | 5.411 | ||
| p value | < 0.001 | < 0.001 | 0.02 | |
| Does Your Mother Complete Primary Education | ||||
| Yes | 432 | 214 (49.5) | 59.88 (43.83) | 209 (48.4) |
| No | 188 | 132 (70.2) | 74.86 (77.50) | 111 (59.0) |
| χ2 | 22.705 | 5.964 | ||
| p value | < 0.001 | = 0.022 | p = 0.015 | |
| Main Source of Water Supply | ||||
| Tap | 71 | 35 (49.3) | 53.37 (24.01) | 38 (53.5) |
| Well | 309 | 134 (43.4) | 63.03 (50.92) | 144 (46.6) |
| River/stream | 237 | 176 (74.3) | 70.07 (69.28) | 137 (57.8) |
| Others | 3 | 1 (33.3) | 51 | 1 (33.3) |
| χ2 | 53.956 | 7.251 | ||
| p value | < 0.001 | 0.425 | 0.064 | |
| Water Contact Activities | ||||
| Playing/Bathing | 137 | 84 (61.3) | 67.69 (63.17) | 62 (45.3) |
| Washing | 344 | 214 (62.2) | 60.70 (47.28) | 187 (54.4) |
| Agricultural work | 51 | 39 (76.5) | 82.82 (93.08) | 29 (56.9) |
| Fishing | 13 | 8 (61.5) | 92.00 (88.63) | 7 (53.8) |
| No contact | 75 | 1 (1.3) | 56 | 35 (46.7) |
| χ2 | 1.066 | 4.581 | ||
| p value | < 0.001 | 0.167 | 0.333 | |
| TOTAL | 620 | 346 (55.8) | 65.60 (59.33) | 320 (53.24) |
| Variables | OR (95% CI) | p-value |
|---|---|---|
| Gender | ||
| Male | 1 | |
| Female | 1.59 (1.07–2.36) | 0.022 |
| Age | ||
| ≤4 | 1 | 0 |
| 5–9 | 0.30 (0.10–0.93) | 0.038 |
| 10–14 | 0.27 (0.09–0.79) | 0.017 |
| 15–19 | 0.80 (0.27–2.39) | 0.693 |
| ≥20 | 1.52 (0.24–9.65) | 0.656 |
| Father’s Occupation | ||
| Fishing | 1 | 0.874 |
| Farming | 0.90 (0.23–3.46) | 0.879 |
| Trading | 0.72 (0.17–2.89) | 0.643 |
| Salary earner | 0.69 (0.16–2.97) | 0.625 |
| Wage earner | 0.91 (0.20–4.13) | 0.905 |
| Mother’s Occupation | ||
| Trading | 1 | 0.266 |
| Farming | 0.56 (0.29–1.07) | 0.08 |
| Salary earner | 1.10 (0.61–1.96) | 0.748 |
| Unemployed | 0.69 (0.33–1.41) | 0.313 |
| Does Your Father Complete Primary Education | ||
| No | ||
| Does Your Mother Complete Primary Education | 0.88 (0.46–1.65) | 0.69 |
| No | ||
| Main Source of Water Supply | ||
| Tap | 0.96 (0.54–1.70) | 0.897 |
| Well | ||
| River | 1 | 0.003 |
| Others | 1.53 (0.80–2.92) | 0.189 |
| Water Contact Activities | 0.67 (0.33–1.33) | 0.259 |
| Playing/Bathing | 0.74 (0.04–13.24) | 0.841 |
| Washing | ||
| Agricultural work | 1 | 0 |
| Fishing | 0.96 (0.61–1.51) | 0.871 |
| 0.69 (0.30–1.57) | 0.386 | |
| 1.00 (0.24–4.15) | 0.992 |
| Variables | Number Examined | (%) |
|---|---|---|
| Route of Schistosome Infection | ||
| Contact with contaminated natural water | 128 | 20.6 |
| Eating unhygienic food | 40 | 6.5 |
| Playing with soil | 45 | 7.3 |
| I don’t know | 407 | 65.6 |
| Source of Infection | ||
| River water | 120 | 19.4 |
| Playing with infected friends | 62 | 10 |
| Foods | 53 | 8.5 |
| I don’t know | 385 | 62.1 |
| Intermediate Host | ||
| House fly | 282 | 45.5 |
| Water snail | 99 | 16 |
| Fish | 135 | 21.8 |
| House rat | 51 | 8.2 |
| I don’t know | 53 | 8.5 |
| Symptoms of Infection | ||
| Blood in urine | 213 | 34.4 |
| Stomach pain | 231 | 37.3 |
| Waist pain | 89 | 14.4 |
| Blurred vision | 27 | 4.4 |
| I don’t know | 60 | 9.7 |
| Prevention of Infection | ||
| Stop going to river water | 80 | 12.9 |
| Eating good food | 188 | 30.3 |
| Bathing regularly | 63 | 10.2 |
| Treating drinking water | 206 | 33.2 |
| I don’t know | 83 | 13.4 |
| Total | 620 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Awosolu, O.B.; Shariman, Y.Z.; Haziqah M. T., F.; Olusi, T.A. Will Nigerians Win the War Against Urinary Schistosomiasis? Prevalence, Intensity, Risk Factors and Knowledge Assessment among Some Rural Communities in Southwestern Nigeria. Pathogens 2020, 9, 128. https://0-doi-org.brum.beds.ac.uk/10.3390/pathogens9020128
Awosolu OB, Shariman YZ, Haziqah M. T. F, Olusi TA. Will Nigerians Win the War Against Urinary Schistosomiasis? Prevalence, Intensity, Risk Factors and Knowledge Assessment among Some Rural Communities in Southwestern Nigeria. Pathogens. 2020; 9(2):128. https://0-doi-org.brum.beds.ac.uk/10.3390/pathogens9020128
Chicago/Turabian StyleAwosolu, Oluwaseun B., Yahaya Z. Shariman, Farah Haziqah M. T., and Titus A. Olusi. 2020. "Will Nigerians Win the War Against Urinary Schistosomiasis? Prevalence, Intensity, Risk Factors and Knowledge Assessment among Some Rural Communities in Southwestern Nigeria" Pathogens 9, no. 2: 128. https://0-doi-org.brum.beds.ac.uk/10.3390/pathogens9020128