Clinical Features Associated with Strongyloidiasis in Migrants and the Potential Impact of Immunosuppression: A Case Control Study
Abstract
:1. Introduction
2. Results
2.1. Crude Analysis
2.2. Multivariable Analysis
2.3. Response to Treatment and Outcome
3. Discussion
Limitations of the Study
4. Materials and Methods
4.1. Study Population, Data Collection and Patient Management
4.2. Data Analysis
4.3. Ethical Aspects
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bisoffi, Z.; Buonfrate, D.; Montresor, A.; Requena-Mendez, A.; Munoz, J.; Krolewiecki, A.J.; Gotuzzo, E.; Mena, M.A.; Chiodini, P.L.; Anselmi, M.; et al. Strongyloides stercoralis: A Plea for Action. PLoS Negl. Trop. Dis. 2013, 7, e2214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puthiyakunnon, S.; Boddu, S.; Li, Y.; Zhou, X.; Wang, C.; Li, J.; Chen, X. Strongyloidiasis—An insight into its global prevalence and management. PLoS Negl. Trop. Dis. 2014, 8, e3018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duvignaud, A.; Pistone, T.; Malvy, D. Strongyloidiasis in a young French woman raises concern about possible ongoing autochthonous transmission in Spain. Int. J. Infect. Dis. 2016, 42, 43–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez, P.R.; Guzman, A.P.; Guillen, S.M.; Adell, R.I.; Estruch, A.M.; Gonzalo, I.N.; Olmos, C.R. Endemic strongyloidiasis on the Spanish Mediterranean coast. QJM 2001, 94, 357–363. [Google Scholar] [CrossRef] [Green Version]
- Pacheco-Tenza, M.I.; Ruiz-Maciá, J.A.; Navarro-Cots, M.; Gregori-Colomé, J.; Cepeda-Rodrigo, J.M.; Llenas-García, J. Strongyloides stercoralis infection in a Spanish regional hospital: Not just an imported disease. Enferm. Infecc. Microbiol. Clin. 2016, 36, 24–28. [Google Scholar] [CrossRef]
- Requena-Méndez, A.; Buonfrate, D.; Gomez-Junyent, J.; Zammarchi, L.; Bisoffi, Z.; Muñoz, J. Evidence-Based Guidelines for Screening and Management of Strongyloidiasis in Non-Endemic Countries. Am. J. Trop. Med. Hyg. 2017, 97, 645–652. [Google Scholar] [CrossRef]
- Buonfrate, D.; Angheben, A.; Gobbi, F.; Muñoz, J.; Requena-Mendez, A.; Gotuzzo, E.; Mena, M.A.; Bisoffi, Z. Imported strongyloidiasis: Epidemiology, presentations, and treatment. Curr. Infect. Dis. Rep. 2012, 14, 256–262. [Google Scholar] [CrossRef]
- Schär, F.; Trostdorf, U.; Giardina, F.; Khieu, V.; Muth, S.; Marti, H.; Vounatsou, P.; Odermatt, P. Strongyloides stercoralis: Global Distribution and Risk Factors. PLoS Negl. Trop. Dis. 2013, 7, e2288. [Google Scholar] [CrossRef] [Green Version]
- Requena-Méndez, A.; Salas-Coronas, J.; Salvador, F.; Gomez-Junyent, J.; Villar-Garcia, J.; Santin, M.; Muñoz, C.; Gonzalez-Cordon, A.; Cabezas-Fernandez, M.T.; Sulleiro, E.; et al. High Prevalence of Strongyloidiasis in Spain: A Hospital-Based Study. Pathogens 2020, 9, 107. [Google Scholar] [CrossRef] [Green Version]
- Sequeira-Aymar, E.; Osorio-Lopez, Y.; Gonçalves, A.Q.; Subirà, C.; Requena-Méndez, A. Recommendations for the screening for infectious diseases, mental health, and female genital mutilation in immigrant patients seen in Primary Care. Aten. Primaria 2020, 52, 193–205. [Google Scholar] [CrossRef]
- Monge-Maillo, B.; Navarro, M.; Rodríguez, E.; Rincón, J.R.; Tojeiro, S.C.; Sánchez, S.J.; del Corral, M.C.; López-Vélez, R. Community screening campaign for Strongyloides stercoralis among Latin American immigrants in Spain. Clin. Microbiol. Infect. 2018, 24, 1220–1221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Requena-Méndez, A.; Chiodini, P.; Bisoffi, Z.; Buonfrate, D.; Gotuzzo, E.; Muñoz, J. The laboratory diagnosis and follow up of strongyloidiasis: A systematic review. PLoS Negl. Trop. Dis. 2013, 7, e2002. [Google Scholar] [CrossRef] [PubMed]
- Albonico, M.; Becker, S.; Odermatt, P.; Angheben, A.; Anselmi, M.; Amor, A.; Barda, B.; Buonfrate, D.; Cooper, P.; Gétaz, L.; et al. StrongNet: An International Network to Improve Diagnostics and Access to Treatment for Strongyloidiasis Control. PLoS Negl. Trop. Dis. 2016, 10, e0004898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montes, M.; Sawhney, C.; Barros, N. Strongyloides stercoralis: There but not seen. Curr. Opin. Infect. Dis. 2010, 23, 500–504. [Google Scholar] [CrossRef]
- Belhassen-García, M.; Alonso-Sardón, M.; Martinez-Perez, A.; Soler, C.; Carranza-Rodriguez, C.; Pérez-Arellano, J.L.; Muro, A.; Salvador, F.; Soil-Transmitted Helminths Study Group of the SEMTSI. Surveillance of strongyloidiasis in Spanish in-patients (1998–2014). PLoS ONE 2017, 12, e0189449. [Google Scholar]
- Buonfrate, D.; Requena-Mendez, A.; Angheben, A.; Muñoz, J.; Gobbi, F.; Van Den Ende, J.; Bisoffi, Z. Severe strongyloidiasis: A systematic review of case reports. BMC Infect. Dis. 2013, 13, 78–87. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Perez, A.; Díez, S.R.; Belhasen-Garcia, M.; Torrús-Tendero, D.; Perez-Arellano, J.L.; Cabezas, T.; Soler, C.; Díaz-Menéndez, M.; Navarro, M.; Treviño, B.; et al. Management of severe strongyloidiasis attended at reference centers in Spain. PLoS Negl. Trop. Dis. 2018, 12, e0006272. [Google Scholar] [CrossRef] [Green Version]
- Buonfrate, D.; Sequi, M.; Mejia, R.; Cimino, R.O.; Krolewiecki, A.J.; Albonico, M.; Degani, M.; Tais, S.; Angheben, A.; Requena-Mendez, A.; et al. Accuracy of five serologic tests for the follow up of Strongyloides stercoralis infection. PLoS Negl. Trop. Dis. 2015, 9, e0003491. [Google Scholar] [CrossRef] [Green Version]
- Salvador, F.; Sulleiro, E.; Sánchez-Montalvá, A.; Saugar, J.M.; Rodríguez, E.; Pahissa, A.; Molina, I. Usefulness of Strongyloides stercoralis serology in the management of patients with eosinophilia. Am. J. Trop. Med. Hyg. 2014, 90, 830–834. [Google Scholar] [CrossRef]
- Valerio, L.; Roure, S.; Fernández-Rivas, G.; Basile, L.; Martínez-Cuevas, O.; Ballesteros, Á.L.; Ramos, X.; Sabrià, M.; North Metropolitan Working Group on Imported Diseases. Strongyloides stercoralis, the hidden worm. Epidemiological and clinical characteristics of 70 cases diagnosed in the North Metropolitan Area of Barcelona, Spain, 2003–2012. Trans. R. Soc. Trop. Med. Hyg. 2013, 107, 465–470. [Google Scholar] [CrossRef] [Green Version]
- Instituto Nacional de Estadistica. Avance de la Estadística del Padrón Continuo a 1 de enero de 2019. 2019. Available online: https://www.ine.es/prensa/pad_2019_p.pdf (accessed on 6 June 2020).
- Salvador, F.; Treviño, B.; Bosch-Nicolau, P.; Serre-Delcor, N.; Sánchez-Montalvá, A.; Oliveira, I.; Sulleiro, E.; Aznar, M.L.; Pou, D.; Sao-Avilés, A.; et al. Strongyloidiasis screening in migrants living in Spain: Systematic review and meta-analysis. Trop. Med. Int. Health 2020, 25, 281–290. [Google Scholar] [CrossRef] [Green Version]
- Oyebamiji, D.A.; Ebisike, A.N.; Egede, J.O.; Hassan, A.A. Knowledge, attitude and practice with respect to soil contamination by Soil-Transmitted Helminths in Ibadan, Southwestern Nigeria. Parasite Epidemiol. Control 2018, 3, e00075. [Google Scholar] [CrossRef]
- Genta, R.M.; Weesner, R.; Douce, R.W.; Huitger-O’Connor, T.; Walzer, P.D. Strongyloidiasis in US veterans of the Vietnam and other wars. JAMA 1987, 258, 49–52. [Google Scholar] [CrossRef]
- Buonfrate, D.; Salas-Coronas, J.; Muñoz, J.; Maruri, B.T.; Rodari, P.; Castelli, F.; Zammarchi, L.; Bianchi, L.; Gobbi, F.; Cabezas-Fernández, T.; et al. Multiple-dose versus single-dose ivermectin for Strongyloides stercoralis infection (Strong Treat 1 to 4): A multicentre, open-label, phase 3, randomised controlled superiority trial. Lancet Infect. Dis. 2019, 19, 1181–1190. [Google Scholar] [CrossRef]
- Salvador, F.; Treviño, B.; Chamorro-Tojeiro, S.; Sánchez-Montalvá, A.; Herrero-Martínez, J.M.; Rodríguez-Guardado, A.; Serre-Delcor, N.; Torrús, D.; Goikoetxea, J.; Zubero, Z.; et al. Imported strongyloidiasis: Data from 1245 cases registered in the +REDIVI Spanish Collaborative Network (2009–2017). PLoS Negl. Trop. Dis. 2019, 13, e0007399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buonfrate, D.; Requena-Mendez, A.; Angheben, A.; Cinquini, M.; Cruciani, M.; Fittipaldo, A.; Giorli, G.; Gobbi, F.; Piubelli, C.; Bisoffi, Z. Accuracy of molecular biology techniques for the diagnosis of Strongyloides stercoralis infection-A systematic review and meta-analysis. PLoS Negl. Trop. Dis. 2018, 12, e0006229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agbata, E.N.; Morton, R.L.; Bisoffi, Z.; Bottieau, E.; Greenaway, C.; Biggs, B.A.; Montero, N.; Tran, A.; Rowbotham, N.; Arevalo-Rodriguez, I.; et al. Effectiveness of Screening and Treatment Approaches for Schistosomiasis and Strongyloidiasis in Newly-Arrived Migrants from Endemic Countries in the EU/EEA: A Systematic Review. Int. J. Environ. Res. Public Health 2018, 16, 11. [Google Scholar] [CrossRef] [Green Version]
- Asundi, A.; Beliavsky, A.; Liu, X.J.; Akaberi, A.; Schwarzer, G.; Bisoffi, Z.; Requena-Méndez, A.; Shrier, I.; Greenaway, C. Prevalence of strongyloidiasis and schistosomiasis among migrants: A systematic review and meta-analysis. Lancet Glob. Health 2019, 7, e236–e248. [Google Scholar] [CrossRef] [Green Version]
- GeoSentinel. GeoSentinel, the Global Surveillance Network of the ISTM in Partnership with the CDC. Available online: https://www.istm.org/memberexclusivehandouts (accessed on 22 March 2020).
- UNICEF and WHO. Progress on Household Drinking Water, Sanitation and Hygiene 2000–2017. Special Focus on Inequalities; United Nations Children’s Fund (UNICEF) and World Health Organization (WHO): New York, NY, USA, 2019; Available online: https://www.who.int/water_sanitation_health/publications/jmp-2019-full-report.pdf (accessed on 22 March 2020).

| Variable Name | Cases n = 158 | Controls n = 294 | Discrepancies within Matched Pairs | Overall p-Value |
|---|---|---|---|---|
| Age * | 39.88 (SD 12) | 44.6 (SD 60.5) | −4.8 (61.4) | 0.18 |
| Female gender | 70 (44%) | 124 (42%) | 61/129 (47%) | 1.00 |
| Pregnancy | 2 (3%) | 8 (6%) | 1/2 (50%) | 0.18 |
| Geographic area: | 0.001 | |||
| South America | 100 (63%) | 137 (47%) | 71/181 (39%) 5 | |
| Central America | 4 (3%) | 12(4%) | 5/8 (62%) 5 | |
| Caribbean | 4 (3%) | 9 (3%) | 7/8 (88%) | |
| Sub-Saharan Africa | 41 (26%) | 102 (35%) | 18/81 (22%) | |
| North Africa | 4 (3%) | 15 (5%) | 2/8 (25%) | |
| South-Central Asia | 0 (1%) | 12 (4%) | 0/0 (%) | |
| North-East Asia | 3 (2%) | 4 (1%) | 5/5 (100%) | |
| South East Asia | 0 (0%) | 0 (0%) | 0/0 (0%) | |
| Europe | 2 (1%) | 2 (1%) | 3/3 (100%) | |
| Unknown | 0 (0%) | 1 (0%) | 0/0 (%) | |
| Open Defecation | ||||
| Low | 132 (84%) | 236 (80%) | 24/230 (10%) | 0.45 |
| Medium | 16 (10%) | 39 (13%) | 19/30 (63%) | |
| Unknown | 10 (6%) | 19 (6%) | 15/15 (100%) | |
| Years in Spain * | 10 (6.2) | 9.8 (7.4) | −0.03 (9.6) | 0.9 |
| Variable Name | Cases n = 158 | Controls n = 293 | Odds Ratio (95% CI) | p-Value |
|---|---|---|---|---|
| Pathological Background | ||||
| Immunosuppressed patient | 29/158 (18.4%) | 67/293 (22.9%) | 0.8 (0.5, 1.2) | 0.26 |
| HIV | 28/139 (20%) | 63/235 (27%) | 0.7 (0.2, 2.2) | 0.82 |
| >500 CD4 | 16 (10%) | 37 (12.6%) | - | - |
| 500–200 CD4 | 11 (7%) | 21 (7%) | 1.1 (0.3, 3.4) | 0.93 |
| <200 CD4 | 1 (1%) | 5 (2%) | 0.53 (0.05, 5.9) | 0.60 |
| Last viral load (copies/mL) | 1606 (6352.8) | 8302.1 (28174) | * | |
| HTLV-1 positive serology | 2/68 (3%) | 0/1 (0%) | * | - |
| Transplanted | 3/158 (1.9%) | 7/294 (2.3%) | * | - |
| Neoplasia | 6/137 (4%) | 8/206 (4%) | 1.4 (0.4, 5.1) | 0.6 |
| Other pathogenic parasites in stool | 14/144 (10%) | 15/200 (8%) | 1.76 (0.76, 4.1) | 0.19 |
| Clinical Manifestations and Blood Test Results | ||||
| Diffuse abdominal pain | 33/157 (21%) | 39/291 (13%) | 1.90 (1.1, 3.2) | 0.02 |
| Epigastralgia | 29/144 (20%) | 18/269 (7%) | 3.6 (1.8, 7.1) | <0.001 |
| Urticaria/Pruritus | 25/158 (16%) | 21/291 (7%) | 2.5 (1.3, 4.6) | 0.005 |
| Maximum eosinophil count cells/µL | 494.8 (598.1) | 220.5 (213.6) | 1.58 (1.4, 1.8) | <0.001 |
| (n = 156) | (n = 276) | |||
| Maximum leukocyte count cells/µL (n = 153) | 6942.3 (2542.3) | 6495.4 (2591.1) | 1.1 (1, 1.2) | 0.1 |
| (n = 157) | (n = 287) | |||
| Total IgE (IU/L) (n = 153) | 354 (593.1) | 157.9 (237.6) | 1.5 (1.2, 1.8) | <0.001 |
| (n = 122) | (n = 106) | |||
| HTLV-1 serology | 2/57 (4%) | - | - | - |
| Main Variables n = 117 | Cured = 111 | Uncured n = 6 | p-Value |
|---|---|---|---|
| Female gender | 48 (43.2%) | 3 (50%) | 0.7 |
| Age | 38.3 (SD 13.1) | 33.9 (SD 10.5) | 0.2 |
| Geographic area n = 117 | n = 111 | n = 6 | 0.06 |
| South America | 71 (64%) | 2 (33.3%) | |
| Central America and Caribbean | 6 (5.4%) | 1 (16.7%) | |
| North Africa | 4 (3.6%) | 0 | |
| Sub-Saharan Africa | 28 (25.3%) | 2 (33.3%) | |
| South-East Asia | 1(0.9%) | 1 (16.7%) | |
| Eastern Europe | 1 (0.9%) | 0 | |
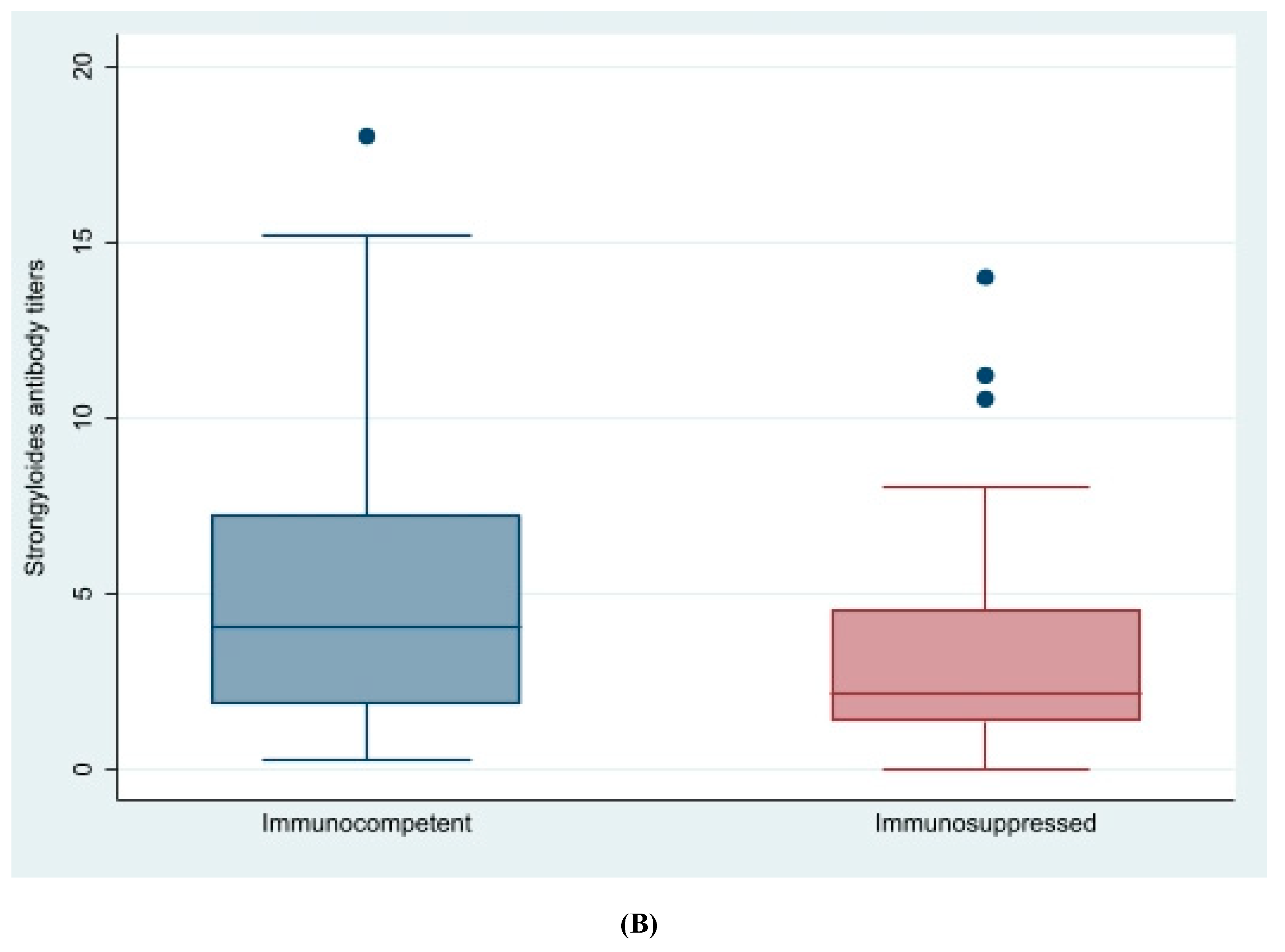
| Immunosuppressed | 37 (30.6%) | 2 (28.6%) | 0.91 |
| Baseline serology titers | 4.3 (SD 3.9) | 5. (SD 3.1) | 0.15 |
| Other parasitic infections | 34 (29.1%) | 2 (28.6%) | 0.98 |
| Treatment provided included ivermectin (n = 124) | 117 (98.3%) | 7 (100%) | 0.64 |
| Time to visit in days | 210 (SD 82.9) | 232.5 (SD 107.1) | 0.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinez-Pérez, A.; Soriano-Pérez, M.J.; Salvador, F.; Gomez-Junyent, J.; Villar-Garcia, J.; Santin, M.; Muñoz, C.; González-Cordón, A.; Salas-Coronas, J.; Sulleiro, E.; et al. Clinical Features Associated with Strongyloidiasis in Migrants and the Potential Impact of Immunosuppression: A Case Control Study. Pathogens 2020, 9, 507. https://0-doi-org.brum.beds.ac.uk/10.3390/pathogens9060507
Martinez-Pérez A, Soriano-Pérez MJ, Salvador F, Gomez-Junyent J, Villar-Garcia J, Santin M, Muñoz C, González-Cordón A, Salas-Coronas J, Sulleiro E, et al. Clinical Features Associated with Strongyloidiasis in Migrants and the Potential Impact of Immunosuppression: A Case Control Study. Pathogens. 2020; 9(6):507. https://0-doi-org.brum.beds.ac.uk/10.3390/pathogens9060507
Chicago/Turabian StyleMartinez-Pérez, Angela, Manuel Jesús Soriano-Pérez, Fernando Salvador, Joan Gomez-Junyent, Judith Villar-Garcia, Miguel Santin, Carme Muñoz, Ana González-Cordón, Joaquín Salas-Coronas, Elena Sulleiro, and et al. 2020. "Clinical Features Associated with Strongyloidiasis in Migrants and the Potential Impact of Immunosuppression: A Case Control Study" Pathogens 9, no. 6: 507. https://0-doi-org.brum.beds.ac.uk/10.3390/pathogens9060507






