Thermostable Xylanase Production by Geobacillus sp. Strain DUSELR13, and Its Application in Ethanol Production with Lignocellulosic Biomass
Abstract
:Highlights:
- A thermostable xylanase production from a Geobacillus sp. strain DUSELR13 with t1/2 of 38 days at 60 °C.
- Use of the lignocellulosic biomass for the xylanase production, and ethanol production.
- Better xylan hydrolysis by DUSELR13 xylanase than the commercial counterpart at higher temperature.
1. Introduction
2. Materials and Methods
2.1. Microorganism and Enzyme Assay
2.2. Enzyme Assay
2.3. Cell Morphology
2.4. Optimization Studies for Xylanase Production
2.4.1. One Factor at a Time
2.4.2. Response Surface Methodology
2.5. Xylanase Characterization
2.6. Hydrolysis of Birchwood Xylan
2.7. Enzyme Production with Lignocellulosic Biomass
2.8. Co-Culture of Geobacillus sp. strain DUSELR13 and Geobacillus thermoglucosidasius for Ethanol Production
2.9. Material and Energy Balance
3. Results and Discussion
3.1. Microorganism
3.2. Optimization of Endoxylanase Production
3.2.1. One Factor at a Time
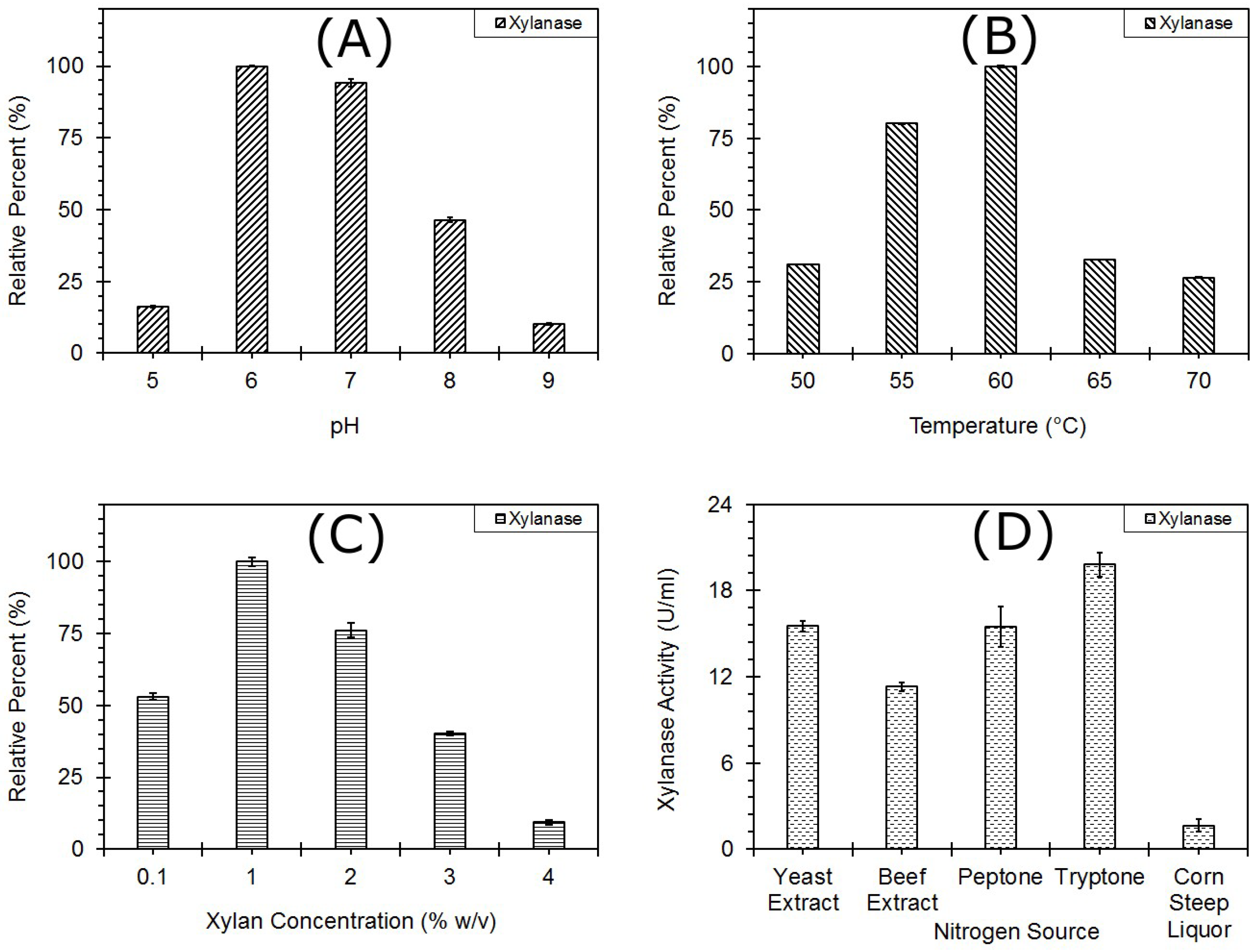
pH
Temperature
Xylan Concentration
Nitrogen Source
3.2.2. Response Surface Methodology
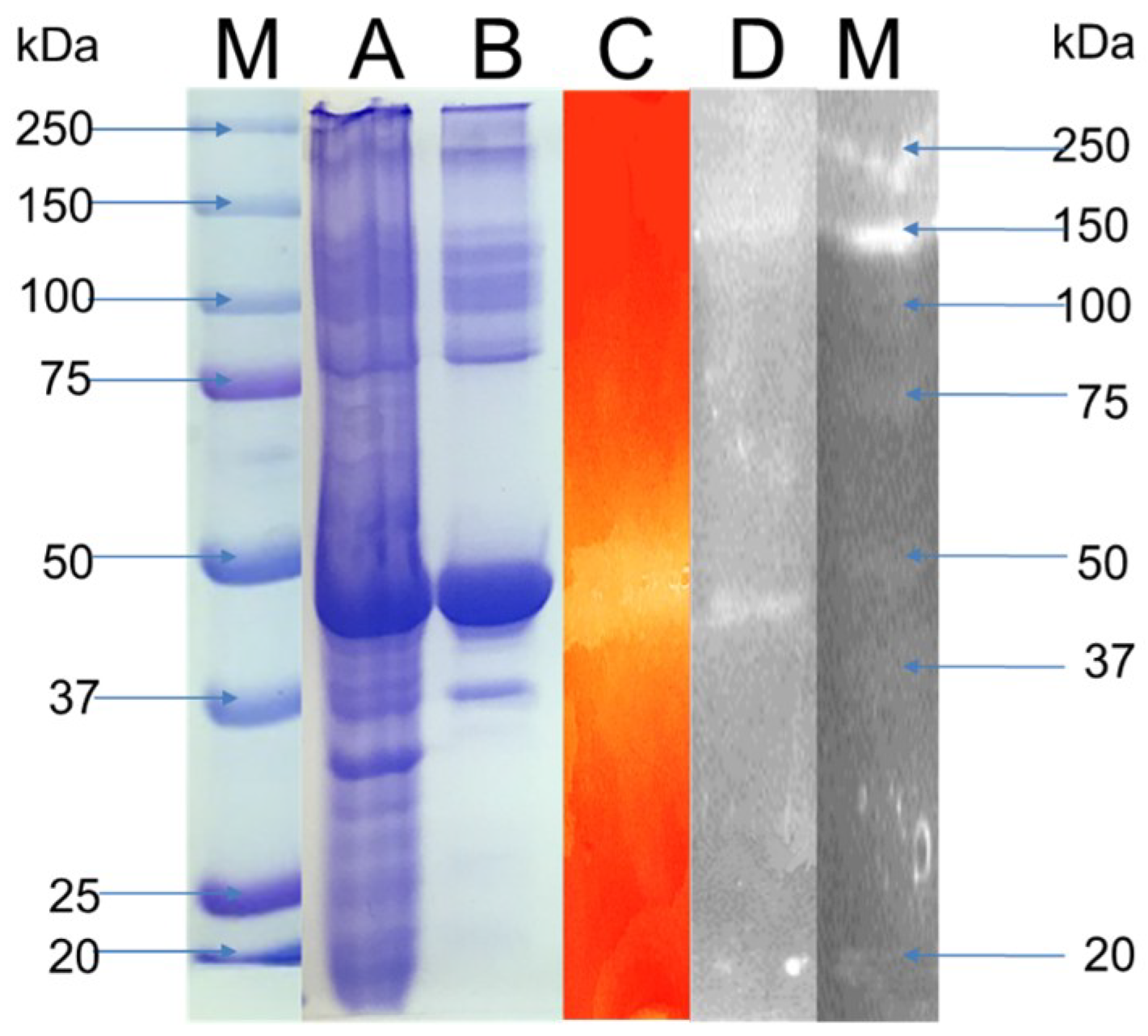
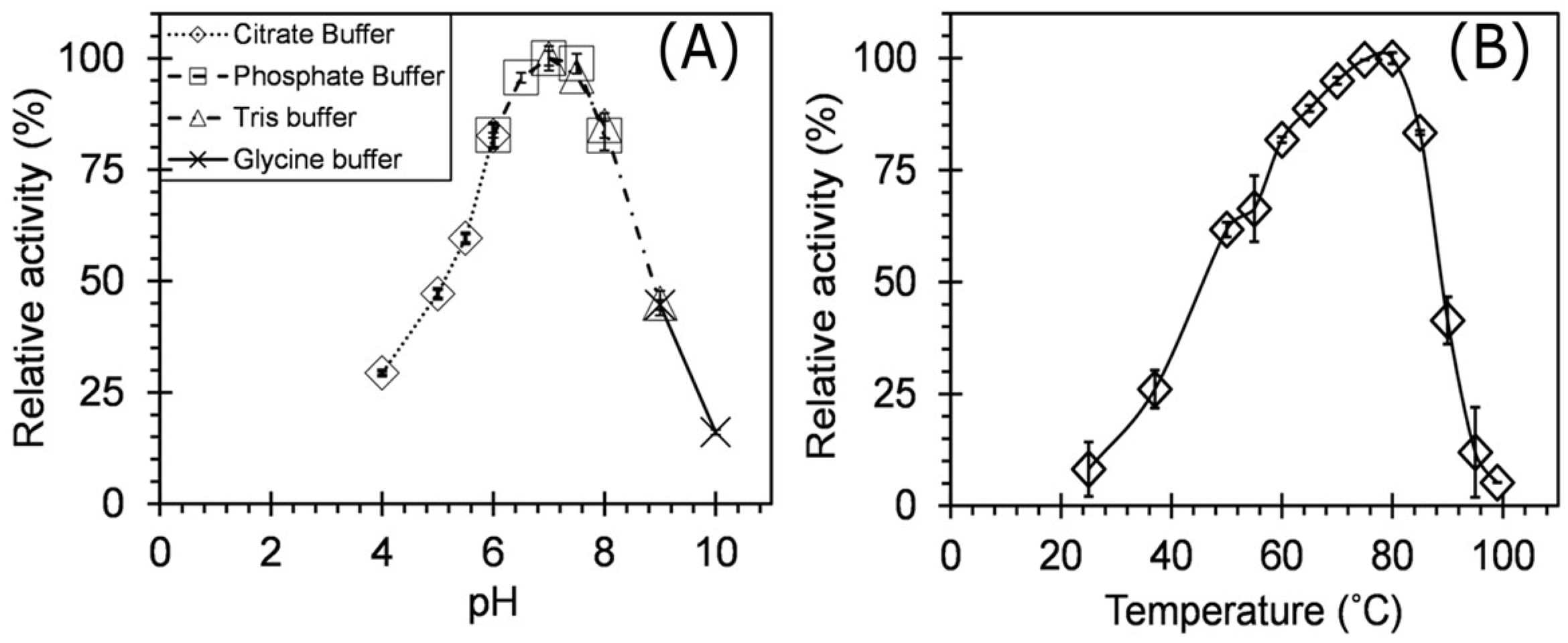
3.3. Enzyme Characterization
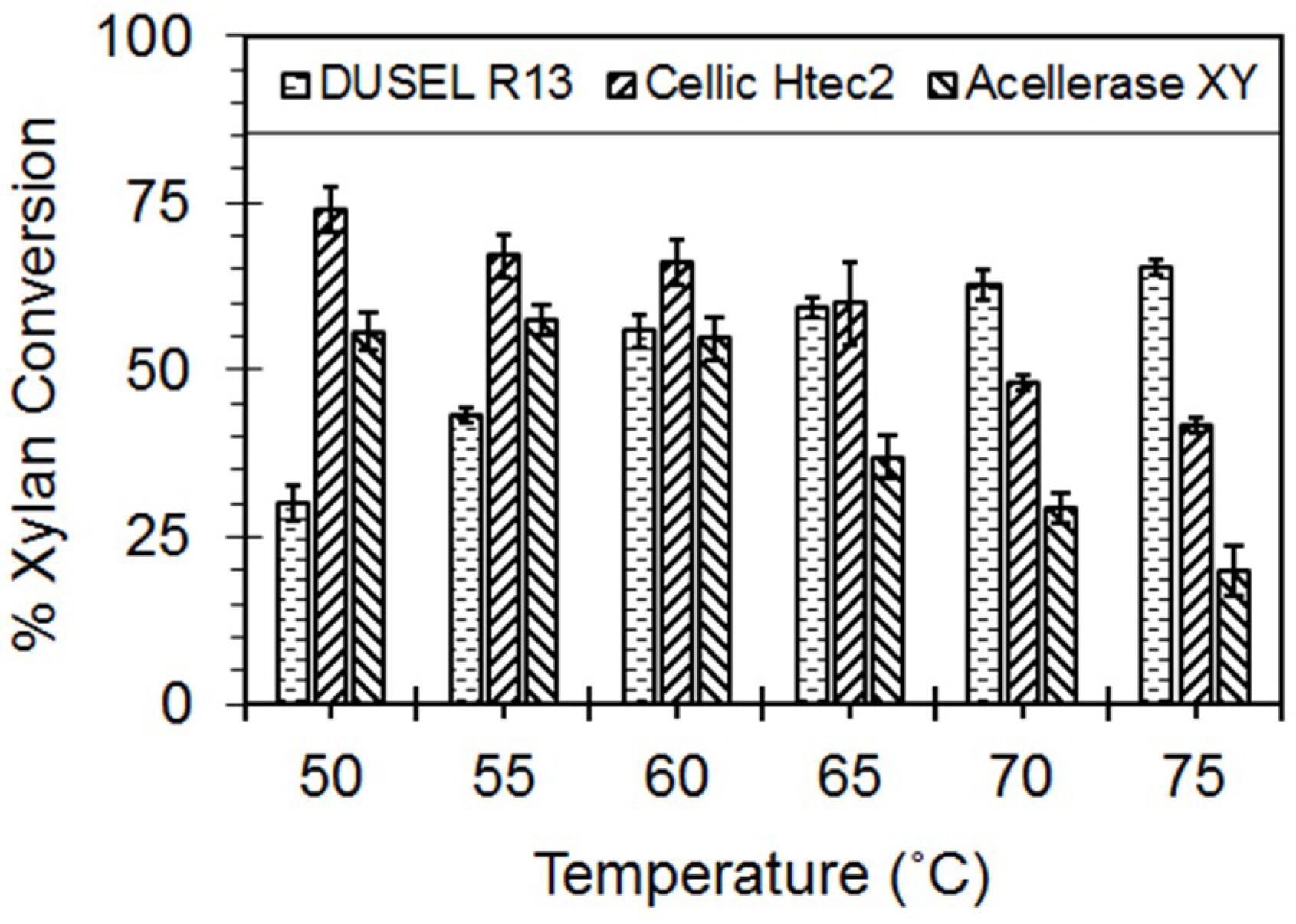
3.4. Hydrolysis of the Beechwood Xylan
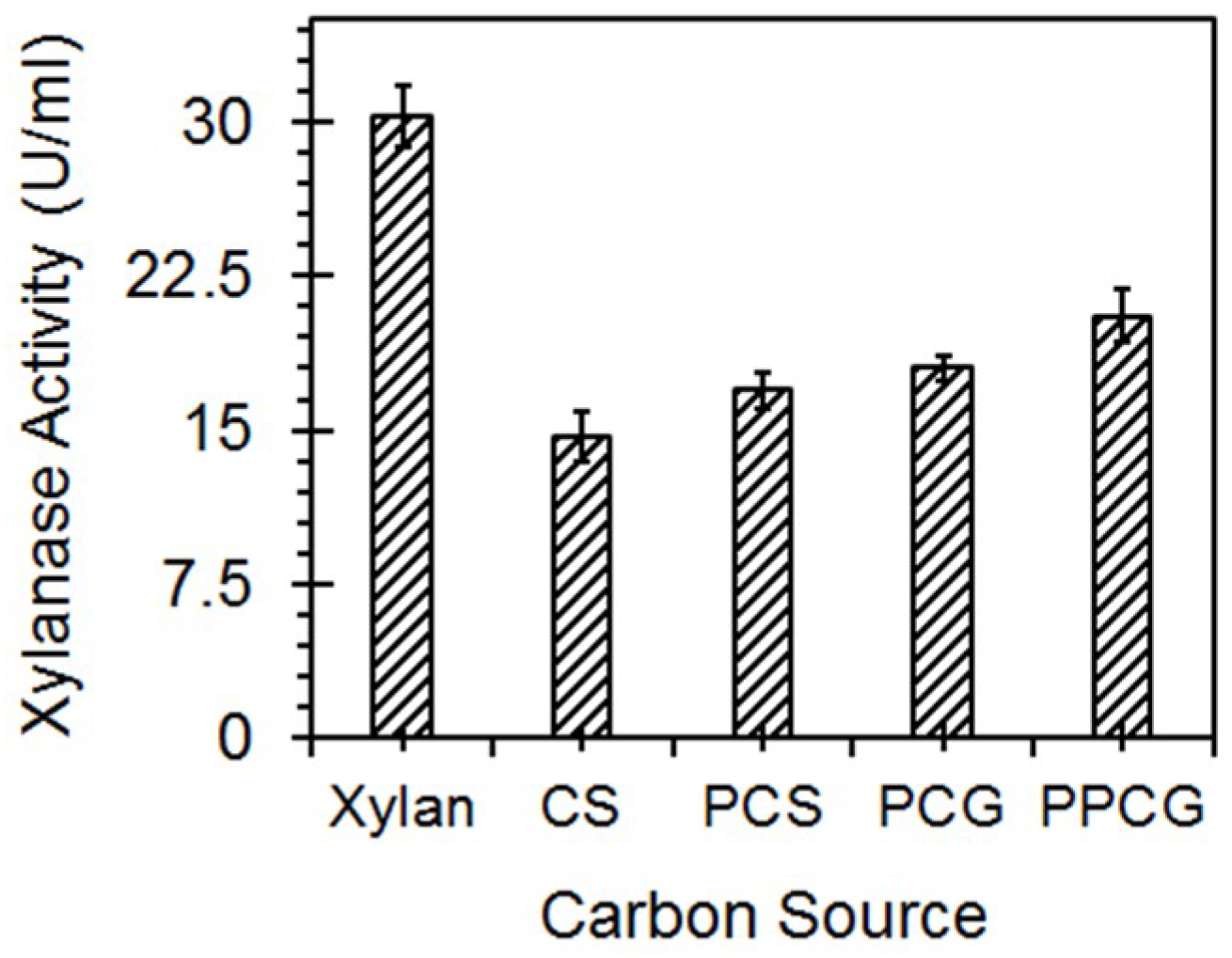
3.5. Enzyme Production with Lignocellulosic Biomass
4. Ethanol Production with Lignocellulosic Biomass
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Maurya, D.P.; Singla, A.; Negi, S. An overview of key pretreatment processes for biological conversion of lignocellulosic biomass to bioethanol. 3 Biotech 2015, 5, 597–609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Bhalla, A.; Bibra, M.; Wang, J.; Morisette, K.; Subramanian, M.R.; Salem, D.; Sani, R.K. Thermophilic biohydrogen production: Challenges at the industrial scale. In Bioenergy: Opportunities and Challenges; Krishnaraj, R.N., Yu, J.S., Eds.; CRC Press, Taylor and Francis: Boca Raton, FL, USA, 2015; p. 382. [Google Scholar]
- Bhalla, A.; Bansal, N.; Kumar, S.; Bischoff, K.M.; Sani, R.K. Improved lignocellulose conversion to biofuels with thermophilic bacteria and thermostable enzymes. Bioresour. Technol. 2013, 128, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Marcolongo, L.; La Cara, F.; Morana, A.; Di Salle, A.; Del Monaco, G.; Paixão, S.M.; Alves, L.; Ionata, E. Properties of an alkali-thermo stable xylanase from Geobacillus thermodenitrificans A333 and applicability in xylooligosaccharides generation. World J. Microbiol. Biotechnol. 2015, 31, 633–648. [Google Scholar] [CrossRef] [PubMed]
- Bhalla, A.; Bischoff, K.M.; Sani, R.K. Highly thermostable xylanase production from A thermophilic Geobacillus sp. Strain WSUCF1 utilizing lignocellulosic biomass. Front. Bioeng. Biotechnol. 2015, 3, 84. [Google Scholar] [CrossRef] [PubMed]
- Verma, D.; Satyanarayana, T. Cloning, expression and applicability of thermo-alkali-stable xylanase of Geobacillus thermoleovorans in generating xylooligosaccharides from agro-residues. Bioresour. Technol. 2012, 107, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Nagar, S.; Gupta, V.K.; Kumar, D.; Kumar, L.; Kuhad, R.C. Production and optimization of cellulase-free, alkali-stable xylanase by Bacillus pumilus SV-85S in submerged fermentation. J. Ind. Microbiol. Biotechnol. 2010, 37, 71–83. [Google Scholar] [PubMed]
- Ribeiro, L.F.; De Lucas, R.C.; Vitcosque, G.L.; Ribeiro, L.F.; Ward, R.J.; Rubio, M.V.; Damásio, A.R.; Squina, F.M.; Gregory, R.C.; Walton, P.H. A novel thermostable xylanase GH10 from Malbranchea pulchella expressed in Aspergillus nidulans with potential applications in biotechnology. Biotechnol. Biofuels 2014, 7, 115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Sales, A.N.; de Souza, A.C.; Moutta, R.d.O.; Ferreira-Leitão, V.S.; Schwan, R.F.; Dias, D.R. Use of lignocellulose biomass for endoxylanase production by Streptomyces termitum. Prep. Biochem. Biotechnol. 2017, 47, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Zhang, Y.; Li, X.; Huang, Y.; Wang, L.; Wang, Y.; Ding, H.; Wang, F. A novel highly thermostable xylanase stimulated by Ca2+ from Thermotoga thermarum: cloning, expression and characterization. Biotechnol. Biofuels 2013, 6, 26. [Google Scholar] [PubMed]
- Irfan, M.; Asghar, U.; Nadeem, M.; Nelofer, R.; Syed, Q. Optimization of process parameters for xylanase production by Bacillus sp. in submerged fermentation. J. Radiat. Res. Appl. Sci. 2016, 9, 139–147. [Google Scholar] [CrossRef]
- Carlson, C.; Singh, N.K.; Bibra, M.; Sani, R.K.; Venkateswaran, K. Pervasiveness of UVC 254-resistant Geobacillus strains in extreme environments. Appl. Microbiol. Biotechnol. 2018, 102, 1869–1887. [Google Scholar] [CrossRef] [PubMed]
- Cripps, R.; Eley, K.; Leak, D.J.; Rudd, B.; Taylor, M.; Todd, M.; Boakes, S.; Martin, S.; Atkinson, T. Metabolic engineering of Geobacillus thermoglucosidasius for high yield ethanol production. Metab. Eng. 2009, 11, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Raita, M.; Ibenegbu, C.; Champreda, V.; Leak, D.J. Production of ethanol by thermophilic oligosaccharide utilising Geobacillus thermoglucosidasius TM242 using palm kernel cake as a renewable feedstock. Biomass Bioenergy 2016, 95, 45–54. [Google Scholar] [CrossRef]
- Shulami, S.; Shenker, O.; Langut, Y.; Lavid, N.; Gat, O.; Zaide, G.; Zehavi, A.; Sonenshein, A.L.; Shoham, Y. Multiple regulatory mechanisms control the expression of the Geobacillus stearothermophilus gene for extracellular xylanase. J. Biolog. Chem. 2014, 289, 25957–25975. [Google Scholar] [CrossRef] [PubMed]
- Daas, M.J.; Martínez, P.M.; van de Weijer, A.H.; van der Oost, J.; de Vos, W.M.; Kabel, M.A.; van Kranenburg, R. Biochemical characterization of the xylan hydrolysis profile of the extracellular endo-xylanase from Geobacillus thermodenitrificans T12. BMC Biotechnol. 2017, 17, 44. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, U.; Ertunga, N.S. Gene cloning, expression, immobilization and characterization of endo-xylanase from Geobacillus sp. TF16 and investigation of its industrial applications. J. Mol. Catal. B Enzym. 2017, 133, 288–298. [Google Scholar] [CrossRef]
- Irfan, M.; Gonzalez, C.F.; Raza, S.; Rafiq, M.; Hasan, F.; Khan, S.; Shah, A.A. Improvement in thermostability of xylanase from Geobacillus thermodenitrificans C5 by site directed mutagenesis. Enzyme Microb. Technol. 2018, 111, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Bhalla, A.; Bischoff, K.M.; Uppugundla, N.; Balan, V.; Sani, R.K. Novel thermostable endo-xylanase cloned and expressed from bacterium Geobacillus sp. WSUCF1. Bioresour. Technol. 2014, 165, 314–318. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-G.; Yi, Z.-L.; Pei, X.-Q.; Wu, Z.-L. Improving the thermostability of Geobacillus stearothermophilus xylanase XT6 by directed evolution and site-directed mutagenesis. Bioresour. Technol. 2010, 101, 9272–9278. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Yan, Y.; Zhang, C.; Dalby, P.A. Two strategies to engineer flexible loops for improved enzyme thermostability. Sci. Rep. 2017, 7, 41212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.-F.; Yang, G.-Y.; Zhang, Y.; Xie, Y.; Withers, S.G.; Feng, Y. A general and efficient strategy for generating the stable enzymes. Sci. Rep. 2016, 6, 33797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Haq, I.; Prakash, J.; Singh, S.K.; Mishra, S.; Raj, A. Purification, characterization and thermostability improvement of xylanase from Bacillus amyloliquefaciens and its application in pre-bleaching of kraft pulp. 3 Biotech 2017, 7, 20. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, G.; Bhalla, A.; Adhikari, A.; Bischoff, K.M.; Hughes, S.R.; Christopher, L.P.; Sani, R.K. Characterization of thermostable cellulases produced by Bacillus and Geobacillus strains. Bioresour. Technol. 2010, 101, 8798–8806. [Google Scholar] [CrossRef] [PubMed]
- Bibra, M.; Kumar, S.; Wang, J.; Bhalla, A.; Salem, D.R.; Sani, R.K. Single Pot Bioconversion of Prairie Cordgrass into Biohydrogen by Thermophiles. Bioresour. Technol. 2018, 266, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Bailey, M.J.; Biely, P.; Poutanen, K. Interlaboratory testing of methods for assay of xylanase activity. J. Biotechnol. 1992, 23, 257–270. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Bibra, M.; Venkateswaran, K.; Salem, D.R.; Rathinam, N.K.; Gadhamshetty, V.; Sani, R.K. Biohydrogen production from space crew’s waste simulants using thermophilic consolidated bioprocessing. Bioresour. Technol. 2018, 255, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Purohit, A.; Rai, S.K.; Chownk, M.; Sangwan, R.S.; Yadav, S.K. Xylanase from Acinetobacter pittii MASK 25 and developed magnetic cross-linked xylanase aggregate produce predominantly xylopentose and xylohexose from agro biomass. Bioresour. Technol. 2017, 244, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Bibi, Z.; Ansari, A.; Zohra, R.R.; Aman, A.; Ul Qader, S.A. Production of xylan degrading endo-1,4-β-xylanase from thermophilic Geobacillus stearothermophilus KIBGE-IB29. J. Radiat. Res. Appl. Sci. 2014, 7, 478–485. [Google Scholar] [CrossRef]
- Adhyaru, D.N.; Bhatt, N.S.; Modi, H.A. Enhanced production of cellulase-free, thermo-alkali-solvent-stable xylanase from Bacillus altitudinis DHN8, its characterization and application in sorghum straw saccharification. Biocatal. Agric. Biotechnol. 2014, 3, 182–190. [Google Scholar] [CrossRef]
- Ellis, J.T.; Magnuson, T.S. Thermostable and alkalistable xylanases produced by the thermophilic bacterium Anoxybacillus flavithermus TWXYL3. ISRN Microbiol. 2012, 2012, 517524. [Google Scholar] [CrossRef] [PubMed]
- Forster, B.M.; Marquis, H. Protein transport across the cell wall of monoderm Gram-positive bacteria. Mol. Microbiol. 2012, 84, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Satyanarayana, T. Production of endoxylanase with enhanced thermostability by a novel polyextremophilic Bacillus halodurans TSEV1 and its applicability in waste paper deinking. Process Biochem. 2014, 49, 386–394. [Google Scholar] [CrossRef]
- Sharma, A.; Adhikari, S.; Satyanarayana, T. Alkali-thermostable and cellulase-free xylanase production by an extreme thermophile Geobacillus thermoleovorans. World J. Microbiol. Biotechnol. 2007, 23, 483–490. [Google Scholar] [CrossRef]
- Adıgüzel, A.O.; Tunçer, M. Production and Characterization of Partially Purified Thermostable Endoxylanase and Endoglucanase from Novel Actinomadura geliboluensis and the Biotechnological Applications in the Saccharification of Lignocellulosic Biomass. BioResources 2017, 12, 2528–2547. [Google Scholar] [CrossRef]
- Khusro, A.; Kaliyan, B.K.; Al-Dhabi, N.A.; Arasu, M.V.; Agastian, P. Statistical optimization of thermo-alkali stable xylanase production from Bacillus tequilensis strain ARMATI. Electron. J. Biotechnol. 2016, 22, 16–25. [Google Scholar] [CrossRef]
- Kumar, V.; Chhabra, D.; Shukla, P. Xylanase production from Thermomyces lanuginosus VAPS-24 using low cost agro-industrial residues via hybrid optimization tools and its potential use for saccharification. Bioresour. Technol. 2017, 243, 1009–1019. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Shi, P.; Huang, H.; Zhang, X.; Luo, H.; Wang, Y.; Yao, B. Characterization of three novel thermophilic xylanases from Humicola insolens Y1 with application potentials in the brewing industry. Bioresour. Technol. 2013, 130, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Hempelmann, E.; Krafts, K. The mechanism of silver staining of proteins separated by SDS polyacrylamide gel electrophoresis. Biotech. Histochem. 2017, 92, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Bin, L.; Zhang, N.N.; Zhao, C.; Lin, B.X.; Xie, L.H.; Huang, Y.F. haracterization of a Recombinant Thermostable Xylanase from Hot Spring Thermophilic Geobacillus sp. TC-W7. J. Microbiol. Biotechnol. 2012, 22, 1388–1394. [Google Scholar]
- Verma, D.; Anand, A.; Satyanarayana, T. Thermostable and alkalistable endoxylanase of the extremely thermophilic bacterium Geobacillus thermodenitrificans TSAA1: cloning, expression, characteristics and its applicability in generating xylooligosaccharides and fermentable sugars. Appl. Biochem. Biotechnol. 2013, 170, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Liu, J.; Qi, Y.; Yang, K.; Xu, Y.; Feng, L. Synergistic hydrolysis of xylan using novel xylanases, β-xylosidases, and an α-l-arabinofuranosidase from Geobacillus thermodenitrificans NG80-2. Appl. Biochem. Biotechnol. 2017, 101, 6023–6037. [Google Scholar] [CrossRef] [PubMed]
- Guan, G.-Q.; Zhao, P.-X.; Zhao, J.; Wang, M.-J.; Huo, S.-H.; Cui, F.-J.; Jiang, J.-X. Production and partial characterization of an alkaline xylanase from a novel fungus Cladosporium oxysporum. BioMed Res. Int. 2016, 2016, 4575024. [Google Scholar]
- Lehninger, A. Role of metal ions in enzyme systems. Physiol. Rev. 1950, 30, 393–429. [Google Scholar] [CrossRef] [PubMed]
- Amel, B.-D.; Nawel, B.; Khelifa, B.; Mohammed, G.; Manon, J.; Salima, K.-G.; Farida, N.; Hocine, H.; Bernard, O.; Jean-Luc, C. Characterization of a purified thermostable xylanase from Caldicoprobacter algeriensis sp. nov. strain TH7C1T. Carbohydr. Res. 2016, 419, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Novozymes, F.E.A. CELLIC Ctec2 and Htec2—Enzymes for Hydrolysis of Lignocellulosic Materials. Novozymes A/S, Luna, p 01. Available online: http://www.shinshu-u.ac.jp/faculty/engineering/chair/chem010/manual/Ctec2.pdf (accessed on 28 August 2018).
- DuPont Accellerase XY. Available online: accellerase.dupont.com/fileadmin/.../accellerase/.../DUP-00413_ProdSheet_XY_web.page (accessed on 22 July 2018).
- Peng, X.; Qiao, W.; Mi, S.; Jia, X.; Su, H.; Han, Y. Characterization of hemicellulase and cellulase from the extremely thermophilic bacterium Caldicellulosiruptor owensensis and their potential application for bioconversion of lignocellulosic biomass without pretreatment. Biotechnol. Biofuels 2015, 8, 131. [Google Scholar] [CrossRef] [PubMed]
- Balan, V. Current challenges in commercially producing biofuels from lignocellulosic biomass. ISRN Biotechnol. 2014, 2014, 463074. [Google Scholar] [CrossRef] [PubMed]
- Bibra, M.; Wang, J.; Squillace, P.; Pinkelman, R.; Papendick, S.; Schneiderman, S.; Wood, V.; Amar, V.; Kumar, S.; Salem, D. Biofuels and value-added products from extremophiles. In Advances in Biotechnology; Nawani, N.N., Khetmalas, M., Razdan, P.N., Pandey, A., Eds.; I K International Publishing House Pvt. Ltd.: New Delhi, India, 2014; p. 268. [Google Scholar]
- Park, E.Y.; Naruse, K.; Kato, T. One-pot bioethanol production from cellulose by co-culture of Acremonium cellulolyticus and Saccharomyces cerevisiae. Biotechnol. Bbiofuels 2012, 5, 64. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, K.; Irbis, C.; Takada, J.; Matsuura, A. An ability of isolated strains to efficiently cooperate in ethanolic fermentation of agricultural plant refuse under initially aerobic thermophilic conditions: Oxygen deletion process appended to consolidated bioprocessing (CBP). Bioresour. Technol. 2008, 99, 1768–1775. [Google Scholar] [CrossRef] [PubMed]







| Variables | Code | Range and Levels | ||||
|---|---|---|---|---|---|---|
| −1 | −0.5 | 0 | +0.5 | +1 | ||
| Temperature (°C) | A | 55 | 57.5 | 60 | 62.5 | 65 |
| pH | B | 6 | 6.25 | 6.5 | 6.75 | 7 |
| Xylan (g/L) | C | 5 | 7.5 | 10 | 12.5 | 15 |
| Tryptone (g/L) | D | 1 | 3.25 | 5.5 | 7.75 | 10 |
| Run | A | B | C | D | Xylanase Activity (IU/mL) | |
|---|---|---|---|---|---|---|
| Experimental | Predicted | |||||
| 1 | 1.000 | 1.000 | −1.000 | 1.000 | 13.60 | 15.06 |
| 2 | 0.500 | 0.000 | 0.000 | 0.000 | 26.40 | 24.92 |
| 3 | −1.000 | −1.000 | 1.000 | 1.000 | 17.60 | 17.51 |
| 4 | 1.000 | 1.000 | −1.000 | −1.000 | 12.00 | 11.75 |
| 5 | 0.000 | 0.000 | 0.000 | 0.000 | 24.70 | 24.93 |
| 6 | 0.500 | 0.000 | 0.000 | 0.000 | 16.30 | 15.80 |
| 7 | −1.000 | −1.000 | 1.000 | −1.000 | 16.10 | 15.06 |
| 8 | −1.000 | 1.000 | 1.000 | −1.000 | 23.40 | 23.24 |
| 9 | −1.000 | 1.000 | 1.000 | −1.000 | 23.10 | 24.23 |
| 10 | −0.500 | 0.000 | 0.000 | 0.000 | 26.20 | 24.23 |
| 11 | 1.000 | 1.000 | 1.000 | 1.000 | 18.70 | 19.83 |
| 12 | 1.000 | −1.000 | −1.000 | 1.000 | 27.50 | 26.72 |
| 13 | 0.000 | 0.000 | 0.000 | 0.000 | 20.00 | 22.51 |
| 14 | 1.000 | −1.000 | −1.000 | −1.000 | 18.30 | 17.51 |
| 15 | 1.000 | −1.000 | 1.000 | −1.000 | 26.20 | 24.92 |
| 16 | 0.000 | 0.000 | 0.000 | −0.500 | 15.70 | 15.80 |
| 17 | 1.000 | −1.000 | 1.000 | 1.000 | 15.60 | 15.16 |
| 18 | 0.000 | 0.000 | 0.500 | 0.000 | 22.40 | 24.23 |
| 19 | −1.000 | 1.000 | −1.000 | −1.000 | 12.30 | 12.21 |
| 20 | 0.000 | 0.500 | 0.000 | 0.000 | 19.30 | 19.48 |
| 21 | 0.000 | 0.500 | 0.000 | 0.000 | 18.40 | 19.08 |
| 22 | 0.000 | 0.000 | 0.000 | −0.500 | 16.30 | 16.51 |
| 23 | 0.000 | 0.000 | 0.000 | 0.500 | 22.60 | 21.35 |
| 24 | 1.000 | 1.000 | 1.000 | 1.000 | 23.20 | 25.89 |
| 25 | 0.000 | −0.500 | 0.000 | 0.000 | 18.40 | 19.83 |
| 26 | −1.000 | 1.000 | −1.000 | 1.000 | 18.60 | 19.48 |
| 27 | 1.000 | −1.000 | 1.000 | 1.000 | 23.80 | 23.24 |
| 28 | 1.000 | 1.000 | 1.000 | −1.000 | 13.30 | 12.52 |
| 29 | 0.000 | 0.000 | 0.000 | 0.000 | 15.30 | 15.06 |
| 30 | 0.000 | 0.000 | 0.000 | 0.000 | 27.00 | 24.92 |
| 31 | 0.000 | 0.000 | −0.500 | 0.000 | 17.30 | 16.57 |
| 32 | −1.000 | −1.000 | 1.000 | −1.000 | 21.20 | 19.83 |
| 33 | −1.000 | 1.000 | 1.000 | 1.000 | 23.10 | 23.97 |
| 34 | 0.500 | 0.000 | 0.000 | 0.000 | 23.70 | 25.89 |
| 35 | 1.000 | 1.000 | 1.000 | −1.000 | 22.50 | 23.40 |
| 36 | −1.000 | 1.000 | 1.000 | 1.000 | 21.30 | 23.24 |
| 37 | 1.000 | 1.000 | −1.000 | 1.000 | 16.80 | 17.85 |
| 38 | −1.000 | 1.000 | −1.000 | −1.000 | 18.30 | 17.85 |
| 39 | −1.000 | −1.000 | −1.000 | −1.000 | 15.30 | 15.16 |
| 40 | 1.000 | −1.000 | −1.000 | 1.000 | 14.80 | 15.16 |
| 41 | −0.500 | 0.000 | 0.000 | 0.000 | 19.60 | 19.48 |
| 42 | 0.000 | 0.000 | 0.000 | 0.500 | 14.50 | 13.17 |
| 43 | −1.000 | −1.000 | −1.000 | −1.000 | 13.10 | 12.52 |
| 44 | 0.000 | 0.000 | 0.000 | −0.500 | 16.50 | 16.57 |
| 45 | 1.000 | −1.000 | −1.000 | 1.000 | 15.80 | 16.51 |
| 46 | 1.000 | 1.000 | −1.000 | −1.000 | 23.40 | 22.51 |
| 47 | 1.000 | −1.000 | −1.000 | −1.000 | 26.10 | 24.92 |
| 48 | 1.000 | −1.000 | −1.000 | −1.000 | 17.10 | 16.57 |
| 49 | 0.000 | 0.000 | 0.000 | 0.000 | 12.10 | 12.52 |
| 50 | −1.000 | −1.000 | −1.000 | 1.000 | 12.30 | 12.21 |
| 51 | 1.000 | 1.000 | 1.000 | 1.000 | 26.20 | 24.93 |
| 52 | 0.000 | 0.000 | 0.500 | 0.000 | 22.00 | 23.40 |
| 53 | −1.000 | −1.000 | 1.000 | −1.000 | 24.50 | 25.89 |
| 54 | 1.000 | 1.000 | −1.000 | −1.000 | 11.20 | 11.75 |
| 55 | 0.000 | 0.000 | 0.000 | 0.500 | 28.40 | 26.72 |
| 56 | −1.000 | −1.000 | −1.000 | −1.000 | 13.00 | 12.44 |
| 57 | −1.000 | −1.000 | −1.000 | 1.000 | 13.30 | 13.17 |
| 58 | 0.000 | 0.500 | 0.000 | 0.000 | 24.50 | 23.97 |
| 59 | 0.000 | 0.000 | −0.500 | 0.000 | 19.00 | 19.08 |
| 60 | −0.500 | 0.000 | 0.000 | 0.000 | 16.00 | 16.51 |
| 61 | −1.000 | −1.000 | −1.000 | 1.000 | 17.40 | 17.85 |
| 62 | −1.000 | 1.000 | −1.000 | −1.000 | 16.80 | 17.51 |
| 63 | −1.000 | 1.000 | 1.000 | −1.000 | 20.90 | 21.35 |
| 64 | 0.000 | 0.000 | 0.000 | 0.000 | 26.80 | 24.92 |
| 65 | 0.000 | 0.000 | −0.500 | 0.000 | 11.20 | 11.75 |
| 66 | −1.000 | 1.000 | 1.000 | 1.000 | 14.80 | 15.80 |
| 67 | −1.000 | −1.000 | 1.000 | 1.000 | 12.40 | 13.17 |
| 68 | 1.000 | −1.000 | 1.000 | −1.000 | 21.30 | 22.51 |
| 69 | −1.000 | −1.000 | 1.000 | 1.000 | 25.40 | 24.92 |
| 70 | −1.000 | 1.000 | −1.000 | 1.000 | 21.80 | 21.35 |
| 71 | 0.000 | −0.500 | 0.000 | 0.000 | 24.20 | 23.97 |
| 72 | 0.000 | −0.500 | 0.000 | 0.000 | 13.10 | 12.44 |
| 73 | 1.000 | −1.000 | 1.000 | −1.000 | 24.50 | 24.93 |
| 74 | 0.000 | 0.000 | 0.500 | 0.000 | 12.80 | 12.21 |
| 75 | −1.000 | 1.000 | −1.000 | 1.000 | 28.30 | 26.72 |
| 76 | 1.000 | 1.000 | 1.000 | −1.000 | 23.60 | 23.40 |
| 77 | 1.000 | −1.000 | 1.000 | 1.000 | 20.40 | 19.08 |
| 78 | 1.000 | 1.000 | −1.000 | 1.000 | 12.20 | 12.44 |
| Source | Sum of Squares | df | Mean Square | F Value | p-Vale | Prob > F |
|---|---|---|---|---|---|---|
| Model | 0.0734 | 14 | 0.0052 | 146.69 | <0.0001 | significant |
| A-Temperature | 0.0177 | 1 | 0.0177 | 496.16 | <0.0001 | |
| B-pH | 0.0024 | 1 | 0.0024 | 68.47 | <0.0001 | |
| C-Xylan | 0.0010 | 1 | 0.0010 | 27.96 | <0.0001 | |
| D-Tryptone | 0.0023 | 1 | 0.0023 | 63.44 | <0.0001 | |
| AB | 0.0003 | 1 | 0.0003 | 7.64 | 0.0075 | |
| AC | 0.0000 | 1 | 0.0000 | 0.5490 | 0.4615 | |
| AD | 0.0017 | 1 | 0.0017 | 48.19 | <0.0001 | |
| BC | 0.0001 | 1 | 0.0001 | 3.89 | 0.0529 | |
| BD | 0.0003 | 1 | 0.0003 | 8.63 | 0.0046 | |
| CD | 0.0001 | 1 | 0.0001 | 3.37 | 0.0711 | |
| A² | 0.0003 | 1 | 0.0003 | 8.57 | 0.0047 | |
| B² | 0.0003 | 1 | 0.0003 | 7.45 | 0.0082 | |
| C² | 0.0003 | 1 | 0.0003 | 7.51 | 0.0080 | |
| D² | 0.0002 | 1 | 0.0002 | 4.55 | 0.0368 | |
| Residual | 0.0023 | 63 | 0.0000 | |||
| Lack of Fit | 0.0005 | 10 | 0.0001 | 1.61 | 0.1297 | Not significant |
| Pure Error | 0.0017 | 53 | 0.0000 | |||
| Cor Total | 0.0756 | 77 |
| Organism | Type | Enzyme Activity (U/mL) | M.Wt. # (kDa) | Topt (°C) | pHopt | Thermostability | Reference |
|---|---|---|---|---|---|---|---|
| Geobacillus sp. strain DUSELR13 | Wild type | 31.0 | ~45 | 75 | 7.0 | t1/2 = 13 days at 70 °C | This Study |
| Geobacillus sp. strain WSUCF1 | Wild type | 23.8 | 37 | 70 | 6.5 | t1/2 = 12 days at 70 °C | [5] |
| Geobacillus thermolevorans | Wild type | 26.52 | ~45 | 80 | 8.5 | t1/2 = 50–55 min at 80 °C | [6] |
| Geobacillus thermodinitrificans strain A333 | Wild type | 0.02 | 44 | 70 | 7.5 | t1/2 = 60 min at 70 °C | [4] |
| Geobacillus thermodinitrificans strain NG80-2 XynA1 | Recombinant | 40.4 | N.A.* | 70 | 7.6 | t1/2 = 28 h at 65 °C | [43] |
| Geobacillus thermodinitrificans strain NG80-2 XynA2 | Recombinant | 36.8 | N.A. | 70 | 6.5 | t1/2 = 26 h at 65 °C | [43] |
| Geobacillus sp. strain TF16 | Recombinant | 7.92 | 38.9 | 55 | 8.5 | t1/2 = 10 min at 70 °C | [17] |
| Bacillus amyloliquefaciens | Wild type | 48.5 | ~50 | 50 | 9.0 | t1/2 = 45 min at 70 °C | [23] |
| Bacillus pumilus SV-85S | Wild type | 2995 | N.A. | 50 | 6.0 | t1/2 = 25 min at 70 °C | [7] |
| Cladosporium oxysporum GQ-3 | Wild type | 55.92 | N.A. | 50 | 8.0 | t1/2 = 30 min at 70 °C | [44] |
| Malbranchea pulchela | Wild type | 3.0 | 49 | 80 | 5.5 | t1/2 = 260 min at 70 °C | [8] |
| Substrate/Metabolite | Mass Balance | |||
|---|---|---|---|---|
| PCG | % | CS | % | |
| Mass | Mass * | |||
| Substrate Utilized | 13.8 | 100 | 16.7 # | 100 |
| Biomass | - | - | - | - |
| Acetate | 1.46 | 10.57971 | 1.68 | 10.05988 |
| Lactate | 4.12 | 29.85507 | 5.66 | 33.89222 |
| Propionate | 0.37 | 2.681159 | 0.64 | 3.832335 |
| Ethanol | 3.35 | 24.27536 | 3.72 | 22.27545 |
| CO2 | 3.2 | 23.18841 | 3.6 | 21.55689 |
| Total | 12.5 | 11.7 | ||
| Recovery | 90.57971 | 91.61677 | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bibra, M.; Kunreddy, V.R.; Sani, R.K. Thermostable Xylanase Production by Geobacillus sp. Strain DUSELR13, and Its Application in Ethanol Production with Lignocellulosic Biomass. Microorganisms 2018, 6, 93. https://0-doi-org.brum.beds.ac.uk/10.3390/microorganisms6030093
Bibra M, Kunreddy VR, Sani RK. Thermostable Xylanase Production by Geobacillus sp. Strain DUSELR13, and Its Application in Ethanol Production with Lignocellulosic Biomass. Microorganisms. 2018; 6(3):93. https://0-doi-org.brum.beds.ac.uk/10.3390/microorganisms6030093
Chicago/Turabian StyleBibra, Mohit, Venkat Reddy Kunreddy, and Rajesh K. Sani. 2018. "Thermostable Xylanase Production by Geobacillus sp. Strain DUSELR13, and Its Application in Ethanol Production with Lignocellulosic Biomass" Microorganisms 6, no. 3: 93. https://0-doi-org.brum.beds.ac.uk/10.3390/microorganisms6030093







