Role of Oral Microbiota in Cancer Development
Abstract
:1. Introduction
2. Potentially Oncogenic Oral Bacteria
3. Mechanisms of Carcinogenic Action of Oral Bacteria
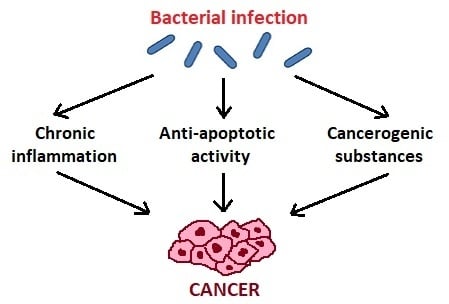
3.1. Chronic Inflammatory Process
3.2. Antiapoptotic Activity
3.3. Cancerogenic Substances
4. Conclusions
Funding
Conflicts of Interest
References
- Cancer. Available online: https://www.who.int/cancer/en/ World Health Organization (accessed on 5 December 2018).
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Oral Cancer. Available online: https://www.who.int/cancer/prevention/diagnosis-screening/oral-cancer/en/ World Health Organization (accessed on 5 December 2018).
- Montero, P.H.; Patel, S.G. Cancer of the oral cavity. Surg. Oncol. Clin. N. Am. 2015, 24, 491–508. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer. Schistosomes, Liver Flukes and Helicobacter pylori. Evaluation of Carcinogenic Risks to Humans; IARC Monograph Evaluating Carcinogenic Risks to Humans; International Agency for Research on Cancer: Lyon, France, 1994; Volume 61, pp. 177–240. [Google Scholar]
- Karpiński, T.M.; Andrzejewska, E.; Eder, P.; Linke, K.; Szkaradkiewicz, A. Evaluation of antimicrobial resistance of Helicobacter pylori in the last 15 years in West Poland. Acta Microbiol. Immunol. Hung. 2015, 62, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.; Yamaura, C.; Ohara-Nemoto, Y.; Tajika, S.; Kodama, Y.; Ohya, T.; Harada, R.; Kimura, S. Streptococcus anginosus infection in oral cancer and its infection route. Oral Dis. 2005, 11, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Mager, D.; Haffajee, A.; Devlin, P.; Norris, C.; Posner, M.; Goodson, J. The salivary microbiota as a diagnostic indicator of oral cancer: A descriptive, nonrandomized study of cancer-free and oral squamous cell carcinoma subjects. J. Transl. Med. 2005, 3, 27. [Google Scholar] [CrossRef] [PubMed]
- Katz, J.; Onate, M.D.; Pauley, K.M.; Bhattacharyya, I.; Cha, S. Presence of Porphyromonas gingivalis in gingival squamous cell carcinoma. Int. J. Oral Sci. 2011, 3, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Pushalkar, S.; Ji, X.; Li, Y.; Estilo, C.; Yegnanarayana, R.; Singh, B.; Li, X.; Saxena, D. Comparison of oral microbiota in tumor and non-tumor tissues of patients with oral squamous cell carcinoma. BMC Microbiol. 2012, 12, 144. [Google Scholar] [CrossRef] [PubMed]
- Atanasova, K.R.; Yilmaz, O. Looking in the Porphyromonas gingivalis cabinet of curiosities: The microbium, the host and cancer association. Mol. Oral Microbiol. 2014, 29, 55–66. [Google Scholar] [CrossRef]
- Galvão-Moreira, L.V.; da Cruz, M.C. Oral microbiome, periodontitis and risk of head and neck cancer. Oral Oncol. 2016, 53, 17–19. [Google Scholar] [CrossRef]
- Lee, W.H.; Chen, H.M.; Yang, S.F.; Liang, C.; Peng, C.Y.; Lin, F.M.; Tsai, L.L.; Wu, B.C.; Hsin, C.H.; Chuang, C.Y.; et al. Bacterial alterations in salivary microbiota and their association in oral cancer. Sci. Rep. 2017, 7, 16540. [Google Scholar] [CrossRef]
- Nagy, K.N.; Sonkodi, I.; Szöke, I.; Nagy, E.; Newman, H.N. The microflora associated with human oral carcinomas. Oral Oncol. 1998, 34, 304–308. [Google Scholar] [CrossRef]
- Castellarin, M.; Warren, R.L.; Freeman, J.D.; Dreolini, L.; Krzywinski, M.; Strauss, J.; Barnes, R.; Watson, P.; Allen-Vercoe, E.; Moore, R.A.; et al. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res. 2012, 22, 299–306. [Google Scholar] [CrossRef]
- Ahn, J.; Sinha, R.; Pei, Z.; Dominianni, C.; Wu, J.; Shi, J.; Goedert, J.J.; Hayes, R.B.; Yang, L. Human gut microbiome and risk for colorectal cancer. J. Natl. Cancer Inst. 2013, 105, 1907–1911. [Google Scholar] [CrossRef]
- Kostic, A.D.; Chun, E.; Robertson, L.; Glickman, J.N.; Gallini, C.A.; Michaud, M.; Clancy, T.E.; Chung, D.C.; Lochhead, P.; Hold, G.L.; et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe 2013, 14, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Michaud, D.S.; Izard, J.; Wilhelm-Benartzi, C.S.; You, D.H.; Grote, V.A.; Tjønneland, A.; Dahm, C.C.; Overvad, K.; Jenab, M.; Fedirko, V.; et al. Plasma antibodies to oral bacteria and risk of pancreatic cancer in a large European prospective cohort study. Gut 2013, 62, 1764–1770. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, L.; Schmid, J.; Ebert, M.; Soucek, P.; Kunicka, T.; Liska, V.; Bruha, J.; Neary, P.; Dezeeuw, N.; Tommasino, M.; et al. Fusobacterium nucleatum associates with stages of colorectal neoplasia development, colorectal cancer and disease outcome. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 1381–1390. [Google Scholar] [CrossRef] [PubMed]
- Mitsuhashi, K.; Nosho, K.; Sukawa, Y.; Matsunaga, Y.; Ito, M.; Kurihara, H.; Kanno, S.; Igarashi, H.; Naito, T.; Adachi, Y.; et al. Association of Fusobacterium species in pancreatic cancer tissues with molecular features and prognosis. Oncotarget 2015, 6, 7209–7220. [Google Scholar] [CrossRef]
- Fan, X.; Alekseyenko, A.V.; Wu, J.; Peters, B.A.; Jacobs, E.J.; Gapstur, S.M.; Purdue, M.P.; Abnet, C.C.; Stolzenberg-Solomon, R.; Miller, G.; et al. Human oral microbiome and prospective risk for pancreatic cancer: A population-based nested case-control study. Gut 2018, 67, 120–127. [Google Scholar] [CrossRef]
- Shiga, K.; Tateda, M.; Saijo, S.; Hori, T.; Sato, I.; Tateno, H.; Matsuura, K.; Takasaka, T.; Miyagi, T. Presence of Streptococcus infection in extra-oropharyngeal head and neck squamous cell carcinoma and its implication in carcinogenesis. Oncol Rep. 2001, 8, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Narikiyo, M.; Tanabe, C.; Yamada, Y.; Igaki, H.; Tachimori, Y.; Kato, H.; Muto, M.; Montesano, R.; Sakamoto, H.; Nakajima, Y.; et al. Frequent and preferential infection of Treponema denticola, Streptococcus mitis, and Streptococcus anginosus in esophageal cancers. Cancer Sci. 2004, 95, 569–574. [Google Scholar] [CrossRef]
- Sakamoto, H.; Naito, H.; Ohta, Y.; Tanakna, R.; Maeda, N.; Sasaki, J.; Nord, C.E. Isolation of bacteria from cervical lymph nodes in patients with oral cancer. Arch. Oral Biol. 1999, 44, 789–793. [Google Scholar] [CrossRef]
- Kostic, A.D.; Gevers, D.; Pedamallu, C.S.; Michaud, M.; Duke, F.; Earl, A.M.; Ojesina, A.I.; Jung, J.; Bass, A.J.; Tabernero, J.; et al. Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res. 2012, 22, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Tahara, T.; Yamamoto, E.; Suzuki, H.; Maruyama, R.; Chung, W.; Garriga, J.; Jelinek, J.; Yamano, H.O.; Sugai, T.; An, B.; et al. Fusobacterium in colonic flora and molecular features of colorectal carcinoma. Cancer Res. 2014, 74, 1311–1318. [Google Scholar] [CrossRef] [PubMed]
- Mima, K.; Cao, Y.; Chan, A.T.; Qian, Z.R.; Nowak, J.A.; Masugi, Y.; Shi, Y.; Song, M.; da Silva, A.; Gu, M.; et al. Fusobacterium nucleatum in colorectal carcinoma tissue according to tumor location. Clin. Transl. Gastroenterol. 2016, 7, e200. [Google Scholar] [CrossRef]
- Farrell, J.J.; Zhang, L.; Zhou, H.; Chia, D.; Elashoff, D.; Akin, D.; Paster, B.J.; Joshipura, K.; Wong, D.T. Variations of oral microbiota are associated with pancreatic diseases including pancreatic cancer. Gut 2012, 61, 582–588. [Google Scholar] [CrossRef]
- Yan, X.; Xinmin, Y.; Yang, M.; Liu, J.; Gao, R.; Hu, J.; Li, J.; Zhang, L.; Shi, Y.; Guo, H.; et al. Discovery and validation of potential bacterial biomarkers for lung cancer. Am. J. Cancer Res. 2015, 5, 3111–3122. [Google Scholar]
- Guerrero-Preston, R.F.; Godoy-Vitorino, F.; Jedlicka, A.; Rodríguez-Hilario, A.; González, H.; Bondy, J.; Lawson, F.; Folawiyo, O.; Michailidi, C.; Dziedzic, A.; et al. 16S rRNA amplicon sequencing identifies microbiota associated with oral cancer, human papilloma virus infection and surgical treatment. Oncotarget 2016, 7, 51320–51334. [Google Scholar] [CrossRef] [Green Version]
- Ahn, J.; Segers, S.; Hayes, R.B. Periodontal disease, Porphyromonas gingivalis serum antibody levels and orodigestive cancer mortality. Carcinogenesis 2012, 33, 1055–1058. [Google Scholar] [CrossRef]
- Peters, B.A.; Wu, J.; Pei, Z.; Yang, L.; Purdue, M.P.; Freedman, N.D.; Jacobs, E.J.; Gapstur, S.M.; Hayes, R.B.; Ahn, J. Oral microbiome composition reflects prospective risk for esophageal cancers. Cancer Res. 2017, 77, 6777–6787. [Google Scholar] [CrossRef]
- Gao, Z.; Guo, B.; Gao, R.; Zhu, Q.; Qin, H. Microbiota disbiosis is associated with colorectal cancer. Front. Microbiol. 2015, 6, 20. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Li, H.; Ni, C.; Du, Z.; Yan, F. Human oral microbiota and its modulation for oral health. Biomed. Pharmacother. 2018, 99, 883–893. [Google Scholar] [CrossRef] [PubMed]
- Szkaradkiewicz, A.K.; Karpiński, T.M. Microbiology of chronic periodontitis. J. Biol. Earth Sci. 2013, 3, M14–M20. [Google Scholar]
- Hou, L.T.; Liu, C.M.; Liu, B.Y.; Lin, S.J.; Liao, C.S.; Rossomando, E.F. Interleukin-1β, clinical parameters and matched cellular-histopathologic changes of biopsied gingival tissue from periodontitis patients. J. Period. Res. 2003, 38, 247–254. [Google Scholar] [CrossRef]
- Konopka, Ł.; Brzezińska-Błaszczyk, E. Cytokines in gingival crevicular fluid as potential diagnostic and prognostic markers of periodontitis. Dent. Med. Probl. 2010, 47, 206–213. [Google Scholar]
- Carmi, Y.; Dotan, S.; Rider, P.; Kaplanov, I.; White, M.R.; Baron, R.; Abutbul, S.; Huszar, M.; Dinarello, C.A.; Apte, R.N.; et al. The role of IL-1β in the early tumor cell-induced angiogenic response. J. Immunol. 2013, 190, 3500–3509. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Yuan, R.Q.; Fuchs, A.; Yao, Y.; Joseph, A.; Schwall, R.; Schnitt, S.J.; Guida, A.; Hastings, H.M.; Andres, J.; et al. Expression of interleukin-1beta in human breast carcinoma. Cancer 1997, 80, 421–434. [Google Scholar] [CrossRef]
- Voronov, E.; Shouval, D.S.; Krelin, Y.; Cagnano, E.; Benharroch, D.; Iwakura, Y.; Dinarello, C.A.; Apte, R.N. IL-1 is required for tumor invasiveness and angiogenesis. Proc. Natl. Acad. Sci. USA 2003, 100, 2645–2650. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.M.; Liu, H.Q.; Liu, S.R.; Tang, S.P.; Yang, L.; Feng, G.S. SHP-2 promoting migration and metastasis of MCF-7 with loss of E-cadherin, dephosphorylation of FAK and secretion of MMP-9 induced by IL-1beta in vivo and in vitro. Breast Cancer Res. Treat. 2005, 89, 5–14. [Google Scholar] [CrossRef]
- Pannone, G.; Santoro, A.; Feola, A.; Bufo, P.; Papagerakis, P.; Lo Muzio, L.; Staibano, S.; Ionna, F.; Longo, F.; Franco, R.; et al. The role of E-cadherin down-regulation in oral cancer: CDH1 gene expression and epigenetic blockage. Curr. Cancer Drug Targets 2014, 14, 115–127. [Google Scholar] [CrossRef]
- Wong, S.H.M.; Fang, C.M.; Chuah, L.H.; Leong, C.O.; Ngai, S.C. E-cadherin: Its dysregulation in carcinogenesis and clinical implications. Crit. Rev. Oncol. Hematol. 2018, 121, 11–22. [Google Scholar] [CrossRef]
- Ishimi, Y.; Miyaura, C.; Jin, C.H.; Akatsu, T.; Abe, E.; Nakamura, Y.; Yamaguchi, A.; Yoshiki, S.; Matsuda, T.; Hirano, T.; et al. IL-6 is produced by osteoblasts and induces bone resorption. J. Immunol. 1990, 145, 3297–3303. [Google Scholar] [PubMed]
- Gabay, C. Interleukin-6 and chronic inflammation. Arthritis Res. Ther. 2006, 8, S3. [Google Scholar] [CrossRef] [PubMed]
- Mathy-Hartert, M.; Hogge, L.; Sanchez, C.; Deby-Dupont, G.; Crielaard, J.M.; Henrotin, Y. Interleukin-1beta and interleukin-6 disturb the antioxidant enzyme system in bovine chondrocytes: A possible explanation for oxidative stress generation. Osteoarthr.Cartil. 2008, 16, 756–763. [Google Scholar] [CrossRef] [PubMed]
- Murata, M.; Thanan, R.; Ma, N.; Kawanishi, S. Role of nitrative and oxidative DNA damage in inflammation-related carcinogenesis. J. Biomed. Biotechnol. 2012, 2012, 623019. [Google Scholar] [CrossRef] [PubMed]
- Kossakowska, A.E.; Edwards, D.R.; Prusinkiewitcz, C.; Zhang, M.C.; Guo, D.; Urbanski, S.J.; Grogan, T.; Marquez, L.A.; Janowska-Wieczorek, A. Interleukin-6 regulation of matrix metalloproteinase (MMP-2 and MMP-9) and tissue inhibitor of metalloproteinase (TIMP-1) expression in malignant non-Hodgkin’s lymphomas. Blood 1999, 94, 2080–2089. [Google Scholar] [PubMed]
- Natali, P.; Nicotra, M.R.; Cavaliere, R.; Bigotti, A.; Romano, G.; Temponi, M.; Ferrone, S. Differential expression of intercellular adhesion molecule 1 in primary and metastatic melanoma lesions. Cancer Res. 1990, 50, 1271–1278. [Google Scholar]
- Haura, E.B.; Turkson, J.; Jove, R. Mechanisms of disease: Insights into the emerging role of signal transducers and activators of transcription in cancer. Nat. Rev. Clin. Oncol. 2005, 2, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Bradley, J.R. TNF-mediated inflammatory disease. J. Pathol. 2008, 214, 149–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurtiş, B.; Tüter, G.; Serdar, M.; Akdemir, P.; Uygur, C.; Firatli, E.; Bal, B. Gingival crevicular fluid levels of monocyte chemoattractant protein-1 and tumor necrosis factor-α in patients with chronic and aggressive periodontitis. J. Periodontol. 2005, 76, 1849–1855. [Google Scholar] [CrossRef] [PubMed]
- Szlosarek, P.; Charles, K.A.; Balkwill, F.R. Tumour necrosis factor-alpha as a tumour promoter. Eur. J. Cancer 2006, 42, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Rivas, M.A.; Carnevale, R.P.; Proietti, C.J.; Rosemblit, C.; Beguelin, W.; Salatino, M.; Charreau, E.H.; Frahm, I.; Sapia, S.; Brouckaert, P.; et al. TNF alpha acting on TNFR1 promotes breast cancer growth via p42/P44 MAPK, JNK, Akt and NF-kappa B-dependent pathways. Exp. Cell Res. 2008, 314, 509–629. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Wang, H.; Rabbani, Z.N.; Zhao, Y.; Li, W.; Yuan, Y.; Li, F.; Dewhirst, M.W.; Li, C.Y. Tumor necrosis factor-alpha is a potent endogenous mutagen that promotes cellular transformation. Cancer Res. 2006, 66, 11565–11570. [Google Scholar] [CrossRef] [PubMed]
- Leber, T.M.; Balkwill, F.R. Regulation of monocyte MMP-9 production by TNF-alpha and a tumour-derived soluble factor (MMPSF). Br. J. Cancer 1998, 78, 724–732. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Ono, M.; Shono, T.; Izumi, H.; Ishibashi, T.; Suzuki, H.; Kuwano, M. Involvement of interleukin-8, vascular endothelial growth factor, and basic fibroblast growth factor in tumor necrosis factor alpha-dependent angiogenesis. Mol. Cell Biol. 1997, 17, 4015–4023. [Google Scholar] [CrossRef] [PubMed]
- Gholizadeh, P.; Eslami, H.; Yousefi, M.; Asgharzadeh, M.; Aghazadeh, M.; Kafil, H.S. Role of oral microbiome on oral cancers, a review. Biomed. Pharmacother. 2016, 84, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, Ö.; Jungas, T.; Verbeke, P.; Ojcius, D.M. Activation of the phosphatidylinositol 3-kinase/Akt pathway contributes to survival of primary epithelial cells infected with the periodontal pathogen Porphyromonas gingivalis. Infect. Immun. 2004, 72, 3743–3751. [Google Scholar] [CrossRef] [PubMed]
- Mao, S.; Park, Y.; Hasegawa, Y.; Tribble, G.D.; James, C.E.; Handfield, M.; Stavropoulos, M.F.; Yilmaz, Ö.; Lamont, R.J. Intrinsic apoptotic pathways of gingival epithelial cells modulated by Porphyromonas gingivalis. Cell. Microbiol. 2007, 9, 1997–2007. [Google Scholar] [CrossRef]
- Whitmore, S.E.; Lamont, R.J. Oral bacteria and cancer. PLoS Pathog. 2014, 10, e1003933. [Google Scholar] [CrossRef]
- Yao, L.; Jermanus, C.; Barbetta, B.; Choi, C.; Verbeke, P.; Ojcius, D.; Yilmaz, Ö. Porphyromonas gingivalis infection sequesters pro-apoptotic Bad through Akt in primary gingival epithelial cells. Mol. Oral Microbiol. 2010, 25, 89–101. [Google Scholar] [CrossRef]
- Nakhjiri, S.F.; Park, Y.; Yilmaz, O.; Chung, W.O.; Watanabe, K.; El-Sabaeny, A.; Park, K.; Lamont, R.J. Inhibition of epithelial cell apoptosis by Porphyromonas gingivalis. FEMS Microbiol. Lett. 2001, 200, 145–149. [Google Scholar] [CrossRef]
- Yilmaz, Ö.; Yao, L.; Maeda, K.; Rose, T.M.; Lewis, E.L.; Duman, M.; Lamont, R.J.; Ojcius, D.M. ATP scavenging by the intracellular pathogen Porphyromonas gingivalis inhibits P2X7-mediated host-cell apoptosis. Cell Microbiol. 2008, 10, 863–875. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.H.; Spooner, R.; DeGuzman, J.; Koutouzis, T.; Ojcius, D.M.; Yilmaz, O. Porphyromonas gingivalis-nucleoside-diphosphate-kinase inhibits ATP-induced reactive-oxygen-species via P2X7 receptor/NADPH-oxidase signalling and contributes to persistence. Cell Microbiol. 2013, 15, 961–976. [Google Scholar] [CrossRef] [PubMed]
- Spooner, R.; Yilmaz, O. The role of reactive-oxygen-species in microbial persistence and inflammation. Int. J. Mol. Sci. 2011, 12, 334–352. [Google Scholar] [CrossRef] [PubMed]
- Inaba, H.; Sugita, H.; Kuboniwa, M.; Iwai, S.; Hamada, M.; Noda, T.; Morisaki, I.; Lamont, R.J.; Amano, A. Porphyromonas gingivalis promotes invasion of oral squamous cell carcinoma through induction of proMMP9 and its activation. Cell Microbiol. 2014, 16, 131–145. [Google Scholar] [CrossRef] [PubMed]
- Baqui, A.; Meiller, T.F.; Chon, J.J.; Turng, B.-F.; Falkler, W.A. Granulocyte- macrophage colony-stimulating factor amplification of interleukin-1b and tumor necrosis factor alpha production in THP-1 human monocytic cells stimulated with lipopolysaccharide of oral microorganisms. Clin. Diagn. Lab. Immunol. 1998, 5, 341–347. [Google Scholar]
- Fischman, S.; Revach, B.; Bulvik, R.; Maliutina, A.; Rubinstein, A.; Nussbaum, G.; Elkin, M. Periodontal pathogens Porphyromonas gingivalis and Fusobacterium nucleatum promote tumor progression in an oral-specific chemical carcinogenesis model. Oncotarget 2015, 6, 22613–22623. [Google Scholar]
- Rubinstein, M.R.; Wang, X.; Liu, W.; Hao, Y.; Cai, G.; Han, Y.W. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/b-catenin signaling via its FadA adhesin. Cell Host Microbe 2013, 14, 195–206. [Google Scholar] [CrossRef]
- Park, H.E.; Kim, J.H.; Cho, N.Y.; Lee, H.S.; Kang, G.H. Intratumoral Fusobacterium nucleatum abundance correlates with macrophage infiltration and CDKN2A methylation in microsatellite-unstable colorectal carcinoma. Virchows Arch. 2017, 471, 329–336. [Google Scholar] [CrossRef]
- Chen, Y.; Peng, Y.; Yu, J.; Chen, T.; Wu, Y.; Shi, L.; Li, Q.; Wu, J.; Fu, X. Invasive Fusobacterium nucleatum activates beta-catenin signaling in colorectal cancer via a TLR4/P-PAK1 cascade. Oncotarget 2017, 8, 31802–31814. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, J.; Chen, T.; Li, Q.; Peng, W.; Li, H.; Tang, X.; Fu, X. Fusobacterium nucleatum potentiates intestinal tumorigenesis in mice via a Toll-like receptor 4/p21-activated kinase 1 cascade. Dig. Dis. Sci. 2018, 63, 1210–1218. [Google Scholar] [CrossRef]
- Uitto, V.J.; Baillie, D.; Wu, Q.; Gendron, R.; Grenier, D.; Putnins, E.E.; Kanervo, A.; Firth, J.D. Fusobacterium nucleatum increases collagenase 3 production and migration of epithelial cells. Infect. Immun. 2005, 73, 1171–1179. [Google Scholar] [CrossRef] [PubMed]
- Landskron, G.; De la Fuente, M.; Thuwajit, P.; Thuwajit, C.; Hermoso, M.A. Chronic inflammation and cytokines in the tumor microenvironment. J. Immunol. Res. 2014, 2014, 149185. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [PubMed]
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837, 837a–837d. [Google Scholar] [CrossRef] [PubMed]
- Abranches, J.; Zeng, L.; Kajfasz, J.K.; Palmer, S.R.; Chakraborty, B.; Wen, Z.T.; Richards, V.P.; Brady, L.J.; Lemos, J.A. Biology of oral streptococci. Microbiol. Spectr. 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Brauncajs, M.; Sakowska, D.; Krzemiński, Z. Production of hydrogen peroxide by lactobacilli colonising the human oral cavity. Med. Dośw. Mikrobiol. 2001, 53, 331–336. [Google Scholar]
- Hussain, S.P.; Hofseth, L.J.; Harris, C.C. Radical causes of cancer. Nat. Rev. Cancer 2003, 3, 276–285. [Google Scholar] [CrossRef]
- Piao, J.Y.; Lee, H.G.; Kim, S.J.; Kim, D.H.; Han, H.J.; Ngo, H.K.; Park, S.A.; Woo, J.H.; Lee, J.S.; Na, H.K.; et al. Helicobacter pylori activates IL-6-STAT3 signaling in human gastric cancer cells: Potential roles for reactive oxygen species. Helicobacter 2016, 21, 405–416. [Google Scholar] [CrossRef]
- Koczorowski, R.; Karpiński, T.M. Halitosis—Problem społeczny. Halitosis—A social problem. Now. Lek. 2001, 70, 657–664. [Google Scholar]
- Koczorowski, R.; Karpiński, T.M.; Hofman, J. Badanie zależności między halitosis a chorobami przyzębia. A study of the relationship between halitosis and periodontal diseases. Dent. Forum 2004, 30, 51–56. [Google Scholar]
- Milella, L. The negative effects of volatile sulphur compounds. J. Vet. Dent. 2015, 32, 99–102. [Google Scholar] [CrossRef]
- Attene-Ramos, M.S.; Wagner, E.D.; Plewa, M.J.; Gaskins, H.R. Evidence that hydrogen sulfide is a genotoxic agent. Mol. Cancer Res. 2006, 4, 9–14. [Google Scholar] [CrossRef]
- Hellmich, M.R.; Szabo, C. Hydrogen sulfide and cancer. Handb. Exp. Pharmacol. 2015, 230, 233–241. [Google Scholar]
- Singh, S.B.; Lin, H.C. Hydrogen sulfide in physiology and diseases of the digestive tract. Microorganisms 2015, 3, 866–889. [Google Scholar] [CrossRef] [PubMed]
- Karpiński, T.M.; Szkaradkiewicz, A.K. Characteristic of bacteriocines and their application. Pol. J. Microbiol. 2013, 62, 223–235. [Google Scholar] [PubMed]
- Hooper, S.J.; Crean, S.J.; Fardy, M.J.; Lewis, M.A.; Spratt, D.A.; Wade, W.G.; Wilson, M.J. A molecular analysis of the bacteria present within oral squamous cell carcinoma. J. Med. Microbiol. 2007, 56 Pt 12, 1651–1659. [Google Scholar] [CrossRef] [Green Version]
- Senneby, A.; Davies, J.R.; Svensäter, G.; Neilands, J. Acid tolerance properties of dental biofilms in vivo. BMC Microbiol. 2017, 17, 165. [Google Scholar] [CrossRef] [PubMed]
- Downes, J.; Wade, W.G. Peptostreptococcus stomatis sp. nov., isolated from the human oral cavity. Int. J. Syst. Evol. Microb. 2006, 56, 751–754. [Google Scholar] [CrossRef] [PubMed]
- Lunt, S.J.; Chaudary, N.; Hill, R.P. The tumor microenvironment and metastatic disease. Clin. Exp. Metastasis 2009, 26, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Mazzio, E.; Smith, B.; Soliman, K. Evaluation of endogenous acidic metabolic products associated with carbohydrate metabolism in tumor cells. Cell Biol. Toxicol. 2010, 26, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Pavlova, S.I.; Jin, L.; Gasparovich, S.R.; Tao, L. Multiple alcohol dehydrogenases but no functional acetaldehyde dehydrogenase causing excessive acetaldehyde production from ethanol by oral streptococci. Microbiology 2013, 159Pt 7, 1437–1446. [Google Scholar] [CrossRef]
- Marttila, E.; Bowyer, P.; Sanglard, D.; Uittamo, J.; Kaihovaara, P.; Salaspuro, M.; Richardson, M.; Rautemaa, R. Fermentative 2-carbon metabolism produces carcinogenic levels of acetaldehyde in Candida albicans. Mol. Oral Microbiol. 2013, 28, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Salaspuro, M. Microbial metabolism of ethanol and acetaldehyde and clinical consequences. Addict. Biol. 1997, 2, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Meurman, J.H.; Uittamo, J. Oral micro-organisms in the etiology of cancer. Acta Odontol. Scand. 2008, 66, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Muto, M.; Hitomi, Y.; Ohtsu, A.; Shimada, H.; Kashiwase, Y.; Sasaki, H.; Yoshida, S.; Esumi, H. Acetaldehyde production by non-pathogenic Neisseria in human oral microflora: Implications for carcinogenesis in upper aerodigestive tract. Int. J. Cancer 2000, 88, 342–350. [Google Scholar] [CrossRef]



| Cancer Localization | Oral Bacteria as Biomarkers | Main Findings | Reference |
|---|---|---|---|
| Oral squamous cell carcinoma (OSCC) | Streptococcus anginosus | S. anginosus infection is more common in OSCC | [7] |
| Oral squamous cell carcinoma (OSCC) | Capnocytophaga gingivalis, Prevotella melaninogenica, Streptococcus mitis | Levels of mentioned bacteria were elevated in the saliva of patients with OSCC | [8] |
| Oral squamous cell carcinoma (OSCC) | Bacillus, Enterococcus, Parvimonas, Peptostreptococcus, Slackia | Significant differences between epithelial precursor lesion and cancer patients in presented five bacterial genera | [13] |
| Oral squamous cell carcinoma (OSCC) | Streptococcus sp. 058, S. salivarius, S. gordonii, S. parasanguinis, Peptostreptococcus stomatis, Gemella haemolysans, G. morbillorum, Johnsonella ignava | Presented bacteria were highly associated with OSCC tumor sites | [10] |
| Oral squamous cell carcinoma (OSCC) | Capnocytophaga gingivalis, Prevotella melaninogenica, Streptococcus mitis, Porphyromonas gingivalis | The high salivary counts of studied bacteria may be diagnostic indicators of oral squamous cell carcinoma | [12] |
| Gingival squamous cell carcinoma | Porphyromonas gingivalis | P. gingivalis was abundantly present in malignant oral epithelium | [9] |
| Oral mucosal cancer | Streptococcus intermedius, S. constellatus, S. oralis, S. mitis, S. sanguis, S. salivarius, Peptostreptococcus sp. | Bacteria were the most common isolates from cervical lymph nodes in patients with oral cancer | [24] |
| Head and neck squamous cell carcinoma (HNSCC) | Streptococcus sp. and Lactobacillus sp. | HNSCC saliva samples were associated with increased amounts of Streptococcus and Lactobacillus and a decrease in Haemophilus, Neisseria, Gemella, and Aggregatibacter | [30] |
| Head and neck squamous cell carcinoma (HNSCC) | Streptococcus anginosus | S. anginosus infection is implicated in the carcinogenesis of HNSCC | [22] |
| Keratinizing squamous cell carcinoma | Veillonella sp., Fusobacterium sp., Prevotella sp., Porphyromonas sp., Actinomyces sp., Clostridium sp., Haemophilus sp., Streptococcus sp., and Enterobacteriaceae | Higher numbers of presented bacteria in keratinizing squamous cell carcinoma | [14] |
| Orodigestive cancer | Porphyromonas gingivalis | P. gingivalis is a biomarker for microbe-associated risk of death due to orodigestive cancer | [31] |
| Esophageal cancer | Streptococcus anginosus, S. mitis, Treponema denticola | Studied bacteria could have a significant role in the carcinogenic process by causing inflammation and by promoting the carcinogenesis | [23] |
| Esophageal adenocarcinoma and esophageal squamous cell carcinoma | Porphyromonas gingivalis, Tannerella forsythia | The abundance of P. gingivalis is trended with higher risk of esophageal squamous cell carcinoma, and T. forsythia is associated with higher risk of esophageal adenocarcinoma | [32] |
| Colorectal cancer (CRC) | Fusobacterium sp., Porphyromonas sp. | Increased carriage of presented bacteria was found in patients with CRC; lower abundance of Clostridium sp. was simultaneously observed | [16] |
| Colorectal cancer (CRC) | Fusobacterium sp. | Fusobacterium enrichment is associated with specific molecular subsets of colorectal cancers | [26] |
| Colorectal cancer (CRC) | Fusobacterium sp. | Fusobacterium sp. are enriched in human colonic adenomas. F. nucleatum increases tumor multiplicity and can promote tumor progression | [17] |
| Colorectal cancer (CRC) | Fusobacterium nucleatum | Patients with low F. nucleatum levels had a significantly longer overall survival time than patients with moderate and high levels of the bacterium | [19] |
| Colorectal cancer (CRC) | Fusobacterium sp. | Overabundance of Fusobacterium in tumor has positive association with lymph node metastasis | [15] |
| Colorectal cancer (CRC) | Fusobacterium sp. | Fusobacterium sequences were enriched in CRC | [25] |
| Colorectal cancer (CRC) | Fusobacterium sp., Lactococcus sp. | Presented bacteria exhibited a higher abundance in cancerous tissues, while Pseudomonas and Escherichia-Shigella were reduced | [33] |
| Pancreatic cancer | Porphyromonas gingivalis | Individuals with high levels of antibodies against P. gingivalis had a higher risk of pancreatic cancer | [18] |
| Pancreatic cancer | Porphyromonas gingivalis, Aggregatibacter actinomycetemcomitans | Carriage of both pathogens was associated with higher risk of pancreatic cancer | [21] |
| Pancreatic cancer | Fusobacterium sp. | Level of Fusobacterium species in the tumor is associated with a worse prognosis of pancreatic cancer | [20] |
| Pancreatic cancer | Streptococcus mitis, Neisseria elongata | Bacteria can be used as biomarkers for distinguishing patients with pancreatic cancer from healthy subjects | [28] |
| Lung cancer | Capnocytophaga sp., Veillonella sp. | Levels of presented bacteria were significantly higher in the saliva from lung cancer patients | [29] |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karpiński, T.M. Role of Oral Microbiota in Cancer Development. Microorganisms 2019, 7, 20. https://0-doi-org.brum.beds.ac.uk/10.3390/microorganisms7010020
Karpiński TM. Role of Oral Microbiota in Cancer Development. Microorganisms. 2019; 7(1):20. https://0-doi-org.brum.beds.ac.uk/10.3390/microorganisms7010020
Chicago/Turabian StyleKarpiński, Tomasz M. 2019. "Role of Oral Microbiota in Cancer Development" Microorganisms 7, no. 1: 20. https://0-doi-org.brum.beds.ac.uk/10.3390/microorganisms7010020