The Influence of the Degradation of Tetracycline by Free Radicals from Riboflavin-5′-Phosphate Photolysis on Microbial Viability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Set-Up of the Photolysis System
2.3. FMN or TC under Blue Light Photoreaction
2.4. Detection of O2•−
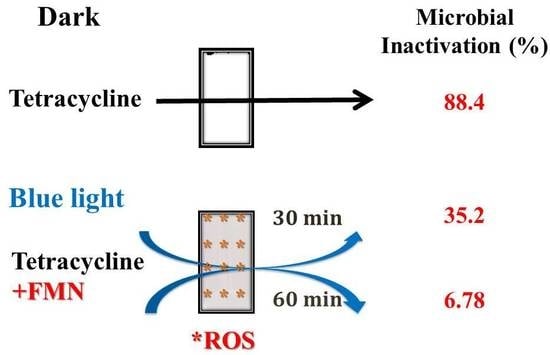
2.5. Influences of TC and Degraded TC on Bacterial Viability
2.6. TC and D-TCF Analysis by LC-MS/MS
2.7. Statistics
3. Results
3.1. Spectral Effects of Blue Light on FMN and TC
3.2. Detection of O2•− via FMN or TC Photolysis
3.3. Effects of TC and D-TCF on E. coli Viability
3.4. Molecular Identification via LC-MS/MS Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chopra, I.; Roberts, M. Tetracycline antibiotics: Mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol. Mol. Biol. Rev. 2001, 65, 232–260. [Google Scholar] [CrossRef]
- Roberts, M.C. Tetracycline resistance determinants: Mechanisms of action, regulation of expression, genetic mobility, and distribution. FEMS. Microbiol. Rev. 1996, 19, 1–24. [Google Scholar] [CrossRef]
- Daghrir, R.; Drogui, P. Tetracycline antibiotics in the environment: A review. Environ. Chem. Lett. 2013, 11, 209–227. [Google Scholar] [CrossRef]
- Chen, Y.; Hu, C.; Qu, J.; Yang, M. Photodegradation of tetracycline and formation of reactive oxygen species in aqueous tetracycline solution under simulated sunlight irradiation. J. Photochem. Photobiol. A Chem. 2008, 197, 81–87. [Google Scholar] [CrossRef]
- Redelsperger, I.M.; Taldone, T.; Riedel, E.R.; Lepherd, M.L.; Lipman, N.S.; Wolf, F.R. Stability of doxycycline in feed and water and minimal effective doses in tetracycline-inducible systems. J. Am. Assoc. Lab. Anim. Sci. 2016, 55, 467–474. [Google Scholar]
- Andreozzi, R.; Raffaele, M.; Nicklas, P. Pharmaceuticals in STP effluents and their solar photodegradation in aquatic environment. Chemosphere 2003, 50, 1319–1330. [Google Scholar] [CrossRef]
- Huang, S.T.; Wu, C.Y.; Lee, N.Y.; Cheng, C.W.; Yang, M.J.; Hung, Y.A.; Wong, T.W.; Liang, J.Y. Effects of 462 nm light-emitting diode on the inactivation of Escherichia coli and a multidrug-resistant by tetracycline photoreaction. J. Clin. Med. 2018, 7, 278. [Google Scholar] [CrossRef]
- Yuann, J.M.P.; Wang, J.S.; Jian, H.L.; Lin, C.C.; Liang, J.Y. Effects of Clinacanthus nutans (Burm. f) Lindau leaf extracts on protection of plasmid DNA from riboflavin photoreaction. MC-Trans. Biotech. 2012, 4, 45–59. [Google Scholar]
- Jian, H.L.; Cheng, C.W.; Chen, L.Y.; Liang, J.Y. The photochemistry of riboflavin. MC-Trans. Biotechnol. 2011, 3, 1–11. [Google Scholar]
- Lin, Y.; Eitenmiller, R.R.; Landen, W.O. Riboflavin. In Vitamin Analysis for the Health and Food Sciences, 2nd ed.; CRC Press: Boca Raton, Florida, USA, 2008; pp. 325–354. [Google Scholar]
- Lu, C.Y.; Wang, W.F.; Lin, W.Z.; Han, Z.H.; Yao, S.D.; Lin, N.Y. Generation and photosensitization properties of the oxidized radical of riboflavin: A laser flash photolysis study. J. Photochem. Photobiol. B Biol. 1999, 52, 111–116. [Google Scholar] [CrossRef]
- Sato, K.; Taguchi, H.; Maeda, T.; Minami, H.; Asada, Y.; Watanabe, Y.; Yoshikawa, K. The primary cytotoxicity in ultraviolet-a-irradiated riboflavin solution is derived from hydrogen peroxide. J. Investig. Dermatol. 1995, 105, 608–612. [Google Scholar] [CrossRef]
- Tripathi, A.K.; Dwivedi, A.; Pal, M.K.; Rastogi, N.; Gupta, P.; Ali, S.; Prabhu, M.B.; Kushwaha, H.N.; Ray, R.S.; Singh, S.K.; et al. Attenuated neuroprotective effect of riboflavin under UV-B irradiation via miR-203/c-Jun signaling pathway in vivo and in vitro. J. Biomed. Sci. 2014, 21, 39. [Google Scholar] [CrossRef]
- Liang, J.Y.; Yuann, J.M.; Cheng, C.W.; Jian, H.L.; Lin, C.C.; Chen, L.Y. Blue light induced free radicals from riboflavin on E. coli DNA damage. J. Photochem. Photobiol. B Biol. 2013, 119, 60–64. [Google Scholar] [CrossRef]
- Liang, J.Y.; Cheng, C.W.; Yu, C.H.; Chen, L.Y. Investigations of blue light-induced reactive oxygen species from flavin mononucleotide on inactivation of E. coli. J. Photochem. Photobiol. B Biol. 2015, 143, 82–88. [Google Scholar] [CrossRef]
- Ottaway, P.B. Stability of vitamins in food. In The Technology of Vitamins in Food; Chapman and Hall: London, UK, 1993; pp. 233–244. [Google Scholar]
- Yang, M.Y.; Chang, C.J.; Chen, L.Y. Blue light induced reactive oxygen species from flavin mononucleotide and flavin adenine dinucleotide on lethality of HeLa cells. J. Photochem. Photobiol. B Biol. 2017, 173, 325–332. [Google Scholar] [CrossRef]
- Liang, J.Y.; Yuann, J.P.; Hsie, Z.J.; Huang, S.T.; Chen, C.C. Blue light induced free radicals from riboflavin in degradation of crystal violet by microbial viability evaluation. J. Photochem. Photobiol. B: Biol. 2017, 174, 355–363. [Google Scholar] [CrossRef]
- Bouafıa-Cherguı, S.; Zemmourı, H.; Chabanı, M.; Bensmaılı, A. TiO2-photocatalyzed degradation of tetracycline: Kinetic study, adsorption isotherms, mineralization and toxicity reduction. Desalin. Water Treat. 2016, 57, 16670–16677. [Google Scholar] [CrossRef]
- Reyes, C.; Fernandez, J.; Freer, J.; Mondaca, M.; Zaror, C.; Malato, S.; Mansilla, H. Degradation and inactivation of tetracycline by TiO2 photocatalysis. J. Photochem. Photobiol. A Chem. 2006, 184, 141–146. [Google Scholar] [CrossRef]
- Cai, F.; Tang, Y.; Chen, F.; Yan, Y.; Shi, W. Enhanced visible-light-driven photocatalytic degradation of tetracycline by Cr3+ doping SrTiO3 cubic nanoparticles. RSC Adv. 2015, 5, 21290–21296. [Google Scholar] [CrossRef]
- Wang, H.; Yao, H.; Pei, J.; Liu, F.; Li, D. Photodegradation of tetracycline antibiotics in aqueous solution by UV/ZnO. Desalin. Water Treat. 2016, 57, 19981–19987. [Google Scholar] [CrossRef]
- Saghi, M.; Mahanpoor, K. Photocatalytic degradation of tetracycline aqueous solutions by nanospherical α-Fe2O3 supported on 12-tungstosilicic acid as catalyst: Using full factorial experimental design. Int. J. Ind. Chem. 2017, 8, 297–313. [Google Scholar] [CrossRef]
- Yamal-Turbay, E.; Jaén, E.; Graells, M.; Pérez-Moya, M. Enhanced photo-Fenton process for tetracycline degradation using efficient hydrogen peroxide dosage. J. Photochem. Photobiol. A Chem. 2013, 267, 11–16. [Google Scholar] [CrossRef]
- Castillo, C.; Criado, S.; Díaz, M.; García, N.A. Riboflavin as a sensitiser in the photodegradation of tetracyclines. Kinetics, mechanism and microbiological implications. Dyes Pigm. 2007, 72, 178–184. [Google Scholar] [CrossRef]
- Jiao, S.; Zheng, S.; Yin, D.; Wang, L.; Chen, L. Aqueous photolysis of tetracycline and toxicity of photolytic products to luminescent bacteria. Chemosphere 2008, 73, 377–382. [Google Scholar] [CrossRef]
- Wong, T.W.; Cheng, C.W.; Hsieh, Z.J.; Liang, J.Y. Effects of blue or violet light on the inactivation of Staphylococcus aureus by riboflavin-5′-phosphate photolysis. J. Photochem. Photobiol. B Biol. 2017, 173, 672–680. [Google Scholar] [CrossRef]
- Yang, M.J.; Hung, Y.A.; Wong, T.W.; Lee, N.Y.; Yuann, J.M.; Huang, S.T.; Wu, C.Y.; Chen, I.Z.; Liang, J.Y. Effects of blue-light-induced free radical formation from catechin hydrate on the inactivation of Acinetobacter baumannii, Including a carbapenem-resistant strain. Molecules 2018, 23, 1631. [Google Scholar] [CrossRef]
- Cheng, C.W.; Chen, L.Y.; Chou, C.W.; Liang, J.Y. Investigations of riboflavin photolysis via coloured light in the nitro blue tetrazolium assay for superoxide dismutase activity. J. Photochem. Photobiol. B Biol. 2015, 148, 262–267. [Google Scholar] [CrossRef]
- Russell, L.V.; Vanderslice, J.T. Comprehensive review of vitamin B2 analytical methodology. J. Micronutr. Anal. 1990, 8, 257–310. [Google Scholar]
- Barua, M.G.; Escalada, J.P.; Bregliani, M.; Pajares, A.; Criado, S. Antioxidant capacity of (+)-catechin visible-light photoirradiated in the presence of vitamin B2. Redox Rep. 2017, 22, 282–289. [Google Scholar] [CrossRef]
- Huvaere, K.; Sinnaeve, B.; Van Bocxlaer, J.; Skibsted, L.H. Flavonoid deactivation of excited state flavins: Reaction monitoring by mass spectrometry. J. Agric. Food Chem. 2012, 60, 9261–9272. [Google Scholar] [CrossRef]
- Massad, W.A.; Bertolotti, S.; Garcia, N.A. Kinetics and mechanism of the vitamin B2-sensitized photooxidation of isoproterenol. Photochem. Photobiol. 2004, 79, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.J.; Wang, R.L.; Wang, D.; Liu, L.; Cheng, L. Visible-light-mediated oxidative demethylation of N 6-methyl adenines. J. Chem. Soc. Chem. Commun. 2017, 53, 10734–10737. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Shi, Y.; Dong, W.; Wen, X.; Jiang, M.; Lu, J. Thermo-activated persulfate oxidation system for tetracycline antibiotics degradation in aqueous solution. Chem. Eng. J. 2016, 298, 225–233. [Google Scholar] [CrossRef] [Green Version]
- Huvaere, K.; Cardoso, D.R.; Homem-de-Mello, P.; Westermann, S.; Skibsted, L.H. Light-induced oxidation of unsaturated lipids as sensitized by flavins. J. Phys. Chem. B 2010, 114, 5583–5593. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Huang, Y.Y.; Xi, L.; Gelfand, J.A.; Hamblin, M.R. Tetracyclines function as dual-action light-activated antibiotics. PLoS ONE 2018, 13, e0196485. [Google Scholar] [CrossRef]










© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.-T.; Lee, S.-Y.; Wang, S.-H.; Wu, C.-Y.; Yuann, J.-M.P.; He, S.; Cheng, C.-W.; Liang, J.-Y. The Influence of the Degradation of Tetracycline by Free Radicals from Riboflavin-5′-Phosphate Photolysis on Microbial Viability. Microorganisms 2019, 7, 500. https://0-doi-org.brum.beds.ac.uk/10.3390/microorganisms7110500
Huang S-T, Lee S-Y, Wang S-H, Wu C-Y, Yuann J-MP, He S, Cheng C-W, Liang J-Y. The Influence of the Degradation of Tetracycline by Free Radicals from Riboflavin-5′-Phosphate Photolysis on Microbial Viability. Microorganisms. 2019; 7(11):500. https://0-doi-org.brum.beds.ac.uk/10.3390/microorganisms7110500
Chicago/Turabian StyleHuang, Shiuh-Tsuen, Shwu-Yuan Lee, Song-Hua Wang, Chun-Yi Wu, Jeu-Ming P. Yuann, Sin He, Chien-Wei Cheng, and Ji-Yuan Liang. 2019. "The Influence of the Degradation of Tetracycline by Free Radicals from Riboflavin-5′-Phosphate Photolysis on Microbial Viability" Microorganisms 7, no. 11: 500. https://0-doi-org.brum.beds.ac.uk/10.3390/microorganisms7110500