Refined versus Extra Virgin Olive Oil High-Fat Diet Impact on Intestinal Microbiota of Mice and Its Relation to Different Physiological Variables
Abstract
:1. Introduction
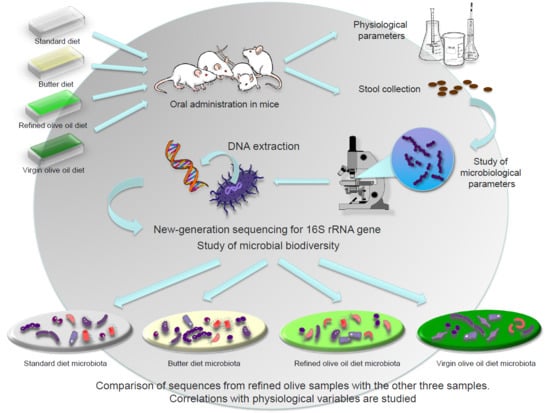
2. Materials and Methods
2.1. Animals
2.2. Bacterial Biodiversity
2.3. Statistical Studies
3. Results
3.1. Physiological Parameters
3.2. Sequencing, Taxa Adscription, Percentage Comparison, and Correlations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Riccardi, G.; Giacco, R.; Rivellese, A.A. Dietary fat, insulin sensitivity and the metabolic syndrome. Clin. Nutr. 2004, 23, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.M.; et al. Enterotypes of the human gut microbiome. Nature 2011, 12, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Albenberg, L.G.; Wu, G.D. Diet and the intestinal microbiome: Associations, functions, and implications for health and disease. Gastroenterology 2014, 146, 1564–1572. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, M.; Wang, S.; Han, R.; Cao, Y.; Hua, W. Interactions between gut microbiota, host genetics and diet relevant to development of metabolic syndromes in mice. ISME J. 2010, 4, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Sunkara, T.; Rawla, P.; Ofosu, A.; Gaduputi, V. Fecal microbiota transplant—A new frontier in inflammatory bowel disease. J. Inflamm. Res. 2018, 11, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Poudyal, H.; Panchal, S.K.; Diwan, V.; Brown, L. Omega-3 fatty acids and metabolic syndrome: Effects and emerging mechanisms of action. Prog. Lipid Res. 2011, 50, 372–387. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.W.; Chien, Y.S.; Chen, Y.J.; Ajuwon, K.M.; Mersmann, H.M.; Ding, S.T. Role of n-3 Polyunsaturated Fatty Acids in Ameliorating the Obesity-Induced Metabolic Syndrome in Animal Models and Humans. Int. J. Mol. Sci. 2016, 17, 1689. [Google Scholar] [CrossRef] [PubMed]
- Fava, F.; Gitau, R.; Griffin, B.A.; Gibson, G.R.; Tuohy, K.M.; Lovegrove, J.A. The type and quantity of dietary fat and carbohydrate alter faecal microbiome and short-chain fatty acid excretion in a metabolic syndrome ‘at-risk’ population. Int. J. Obes. 2013, 37, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Gillingham, L.G.; Harris-Janz, S.; Jones, P.J. Dietary monounsaturated fatty acids are protective against metabolic syndrome and cardiovascular disease risk factors. Lipids 2011, 46, 209–228. [Google Scholar] [CrossRef] [PubMed]
- Martínez-González, M.A.; Martín-Calvo, N. The major European dietary patterns and metabolic syndrome. Rev. Endocr. Metab. Disord. 2013, 14, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, M.; Prieto, I.; Abriouel, H.; Cobo, A.; Benomar, N.; Gálvez, A.; Martínez-Cañamero, M. Effect of virgin and refined olive oil consumption on gut microbiota. Comparison to butter. Food Res. Int. 2014, 64, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Prieto, I.; Hidalgo, M.; Segarra, A.B.; Martínez-Rodríguez, A.M.; Cobo, A.; Ramírez, M.; Abriouel, H.; Gálvez, A.; Martínez-Cañamero, M. Influence of a diet enriched in virgin olive oil or butter on mouse gut microbiota and its correlation to physiological and biochemical parameters related to metabolic syndrome. PLoS ONE 2018, 13, e0190368. [Google Scholar] [CrossRef] [PubMed]
- Medina, E.; de Castro, A.; Romero, C.; Brenes, M. Comparison of the concentrations of phenolic compounds in olive oils and other plant oils: Correlation with antimicrobial activity. J. Agric. Food Chem. 2006, 54, 4954–4961. [Google Scholar] [CrossRef] [PubMed]
- Grossi, C.; Rigacci, S.; Ambrosini, S.; Dami, T.E.; Luccarini, I.; Traini, C.; Failli, P.; Berti, A.; Casamenti, F.; Stefani, M. The polyphenol oleuropein aglycone protects TgCRND8 mice against Aß plaque pathology. PLoS ONE 2013, 8, e71702. [Google Scholar] [CrossRef] [PubMed]
- Anhê, F.F.; Roy, D.; Pilon, G.; Dudonné, S.; Matamoros, S.; Varin, T.V.; Garofalo, C.; Moine, Q.; Desjardins, Y.; Levy, E.; et al. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut 2015, 64, 872–883. [Google Scholar] [CrossRef] [PubMed]
- Roopchand, D.E.; Carmody, R.N.; Kuhn, P.; Moskal, K.; Rojas-Silva, P.; Turnbaugh, P.J.; Raskin, I. Dietary Polyphenols Promote Growth of the Gut Bacterium Akkermansia muciniphila and Attenuate High-Fat Diet-Induced Metabolic Syndrome. Diabetes 2015, 64, 2847–2858. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Tortosa, C.; López-Pedrosa, J.M.; Suarez, A.; Ros, E.; Mataix, J.; Gil, A. Olive oil- and fish oil-enriched diets modify plasma lipids and susceptibility of LDL to oxidative modification in free-living male patients with peripheral vascular disease: The Spanish Nutrition Study. Br. J. Nutr. 1999, 82, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Gorzynik-Debicka, M.; Przychodzen, P.; Cappello, F.; Kuban-Jankowska, A.; Marino Gammazza, A.; Knap, N.; Wozniak, M.; Gorska-Ponikowska, M. Potential health benefits of olive oil and plant polyphenols. Int. J. Mol. Sci. 2018, 19, 686. [Google Scholar] [CrossRef] [PubMed]
- Ministerio de Agricultura y Pesca, Alimentación y Medio Ambiente. Informe del Consumo de Alimentación en España 2016; Servicio de Publicaciones del MAPAMA: Madrid, Spain, 2017.
- Vázquez-Araújo, L.; Adhikari, K.; Chambers, E.; Chambers, D.H.; Carbonell-Barrachina, A.A. Cross-cultural perception of six commercial olive oils: A study with Spanish and US consumers. Food Sci. Technol. Int. 2015, 21, 454–466. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, Y.M.; López, S.; Bermúdez, B.; Abia, R.; Muriana, F.J. Extra-virgin vs. refined olive oil on postprandial hemostatic markers in healthy subjects. J. Thromb. Haemost. 2006, 4, 1421–1422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banegas, I.; Prieto, I.; Vives, F.; Alba, F.; de Gasparo, M.; Duran, R.; de Dios Luna, J.; Segarra, A.B.; Hermoso, F.; Ramírez, M. Asymmetrical response of aminopeptidase A and nitric oxide in plasma of normotensive and hypertensive rats with experimental hemiparkinsonism. Neuropharmacology 2009, 56, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Segarra, A.B.; Ramirez, M.; Banegas, I.; Alba, F.; Vives, F.; de Gasparo, M.; Ortega, E.; Ruiz, E.; Prieto, I. Dietary fat influences testosterone, cholesterol, aminopeptidase A, and blood pressure in male rats. Horm. Metab. Res. 2008, 40, 289–291. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.Y.; Xydakis, A.M.; Hoogeveen, R.C.; Jones, P.H.; Smith, E.B.; Nelson, K.W.; Ballantyne, C.M. Multiplexed analysis of biomarkers related to obesity and the metabolic syndrome in human plasma, using the Luminex-100 system. Clin. Chem. 2005, 51, 1102–1109. [Google Scholar] [CrossRef] [PubMed]
- Nocella, C.; Cammisotto, V.; Fianchini, L.; D’Amico, A.; Novo, M.; Castellani, V.; Stefanini, L.; Violi, F.; Carnevale, R. Extra virgin olive oil and cardiovascular diseases: Benefits for human health. Endocr. Metab. Immune Disord. Drug Targets 2018, 18, 4–13. [Google Scholar] [PubMed]
- Hidalgo, M.; Prieto, I.; Abrioue, H.; Villarejo, A.B.; Ramírez, M.; Cobo, A.; Benomar, N.; Gálvez, A.; Martínez-Cañamero, M. Changes in Gut Microbiota Linked to a Reduction in Systolic Blood Pressure in Spontaneously Hypertensive Rats Fed an Extra Virgin Olive Oil-Enriched Diet. Plant Foods Hum. Nutr. 2018, 73, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Faith, J.J.; McNulty, N.P.; Rey, F.E.; Gordon, J.L. Predicting a human gut microbiota’s response to diet in gnotobiotic mice. Science 2011, 333, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Rey, F.E.; Gonzalez, M.D.; Cheng, J.; Wu, M.; Ahern, P.P.; Gordon, J.I. Metabolic niche of a prominent sulfate-reducing human gut bacterium. Proc. Natl. Acad. Sci. USA 2013, 110, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Devkota, S.; Chang, E.B. Interactions between Diet, Bile Acid Metabolism, Gut Microbiota, and Inflammatory Bowel Diseases. J. Dig. Dis. 2015, 33, 351–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freeman, B.A.; Sissenstein, R.; McManus, T.T.; Woodward, J.E.; Lee, I.M.; Mudd, J.B. Lipid composition and lipid metabolism of Spiroplasma citri. J. Bacteriol. 1976, 125, 946–954. [Google Scholar] [PubMed]
- Shirley, H.Y.; Hung, T.A.; Chen, R.F.; Whitcomb, J.G.; Tully, Y.X. Spiroplasma culicicola sp. nov. from the Salt Marsh Mosquito Aedes sollicitanst. Int. J. Syst. Evol. Microbiol. 1987, 37, 365–370. [Google Scholar]
- Musetti, R.; Favali, M.A.; Pressacco, L. Histopathology and polyphenol content in plants infected by phytoplasmas. Cytobios 2000, 102, 133–147. [Google Scholar] [PubMed]
- Furneri, P.M.; Piperno, A.; Sajia, A.; Bisignano, G. Antimycoplasmal activity of hydroxytyrosol. Anticancer Agents Med. Chem. 2004, 48, 4892–4894. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Xu, S.; Dewhirst, F.E.; Paster, B.J.; Pena, J.A.; Modlin, I.M.; Kidd, M.; Fox, J.G. A novel enterohepatic Helicobacter species ‘Helicobacter mastomyrinus’ isolated from the liver and intestine of rodents. Helicobacter 2005, 10, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, M.A.; Hoffmann, C.; Sherrill–Mix, S.A.; Keilbaugh, S.A.; Hamady, M.; Chen, Y.Y.; Knight, R.; Ahima, R.S.; Bushman, F.; Wu, G.D. High-fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterology 2009, 137, 1716–1724. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef] [PubMed]




| Diet. | SD | EVOO | ROO | BT | ||||
|---|---|---|---|---|---|---|---|---|
| Composition | g/100 g | % energy | g/100 g | % energy | g/100 g | % energy | g/100 g | % energy |
| Protein | 16.5 | 20 | 16.5 | 14 | 16.5 | 14 | 16.5 | 14 |
| Carbohydrates | 60 | 72 | 55 | 48 | 55 | 48 | 55 | 48 |
| Fat | 3 | 8 | 20 | 35 | 20 | 35 | 20 | 35 |
| Total Energy (kJ/g) | 14.2 | 19.6 | 19.6 | 19.6 | ||||
| Diet | SD | EVOO | BT | ROO | p |
|---|---|---|---|---|---|
| Food Intake (g/day) | 3.70 ± 0.65 | 3.74 ± 0.39 | 2.76 ± 0.38 | 3.83 ± 0.41 | n.s. |
| Water intake (mL/day) | 8.71 ± 1.61 | 11.28 ± 1.60 | 6.67 ± 1.95 | 7.14 ± 1.10 | n.s. |
| Diuresis (mL/day) | 2.44 ± 0.70 | 2.68 ± 0.65 | 1.65 ± 0.63 | 2.41 ± 0.50 | n.s. |
| Body Weight (g) | 39.09 ± 1.17 | 38.62 ± 0.71 | 42.15 ± 0.61 | 38.09 ± 0.86 | A * |
| SBP (mmHg) | 161.71 ± 11.83 | 148.11 ± 5.94 | 190.50 ± 8.53 | 156.14 ± 19.54 | A ** |
| Plasma Leptin (pg/mL) | 1929.63 ± 437.86 | 949.89 ± 230.36 | 1433.23 ± 226.95 | 897.33 ± 259.13 | n.s. |
| Plasma Ghrelin (pg/mL) | 55.0 ± 17.56 | 94.3 ± 62.36 | 78.02 ± 42.82 | 89.33 ± 14.97 | n.s. |
| Plasma Insulin (mg/100 mL) | 1253.28 ± 201.32 | 685.71 ± 139.57 | 1518.44 ± 329.97 | 875.97 ± 132.60 | B * |
| Plasma Glucose (mg/100 mL) | 194.13 ± 17.31 | 175.33 ± 18.95 | 192.0 ± 17.2 | 259.63 ± 20.50 | C * |
| Plasma Triglycerides (mg/100 mL) | 39.11 ± 11.88 | 26.01 ± 3.77 | 48.94 ± 4.89 | 47.44 ± 8.89 | n.s. |
| Plasma Total Cholesterol (mg/100mL) | 49.28 ± 10.53 | 84.57 ± 9.98 | 98.67 ± 9.98 | 115.4 ± 11.39 | D ** |
| Plasma HDL/LDL Ratio | 0.20 ± 0.02 | 0.38 ± 0.08 | 0.32 ± 0.03 | 0.28 ± 0.02 | E * |
| Variable | Diuresis (0.23/0.0086) | Leptin (0.79/0.0000) | Insulin * (0.12/0.0456) | Total Cholesterol (0.23/0.0185) | Triglycerides (0.62/0.0000) |
|---|---|---|---|---|---|
| Prevotellaceae | n.s. | n.s. | n.s. | −2.73 ± 1.18 (0.0276) | n.s. |
| Desulfovibrionaceae | n.s. | −309.07 ± 78.84 (0.0006) | n.s. | n.s. | n.s. |
| Marinilabiliaceae | n.s. | −2112.02 ± 1004.75 (0.0462) | n.s. | n.s. | n.s. |
| Erysipelotrichaceae | 1.55 ± 0.54 (0.0086) | n.s. | −0.461 ± 0.221 (0.0456) | n.s. | n.s. |
| Sutterellaceae | n.s. | −1026.14 ± 189.08 (0.0000) | n.s. | n.s. | n.s. |
| Spiroplasmataceae | n.s. | −5033.70 ± 2006.53 (0.0000) | n.s. | 60.63 ± 28.27 (0.0399) | n.s. |
| Staphylococcaceae | n.s. | −5033.70 ± 2006.53 (0.0193) | n.s. | n.s. | 110.39 ± 19.70 (0.0000) |
| Microbacteriaceae | n.s. | n.s. | n.s. | n.s. | 22.73 ± 8.61 (0.0130) |
| Helicobacteraceae | n.s. | −40363.4 ± 8870.42 (0.0001) | n.s. | n.s. | n.s. |
| Genera | FI 0.34/0.0299 | WI 0.64/0.0001 | Diuresis 0.40/0.0027 | BW 0.13/0.0414 | SBP 0.21/0.0093 | Leptin 0.45/0.0002 | Insulin * 0.14/0.0296 | Triglycerides * 0.14/0.0323 | T-CHO 0.39/0.0018 | HDL/LDL* 0.50/0.0009 |
|---|---|---|---|---|---|---|---|---|---|---|
| Desulfovibrio | 1.81 ± 0.57 (0.0034) | 4.49 ± 1.35 (0.0028) | 1.36 ± 0.46 (0.0075) | n.s. | n.s. | n.s. | n.s. | n.s. | 48.34 ± 13.62 (0.0013) | n.s. |
| Ruminiclostridium | n.s. | −1.41 ± 0.48 (0.0074) | n.s. | n.s. | 8.43 ± 3.02 (0.0093) | n.s. | n.s. | n.s. | n.s. | 0.13 ± 0.06 (0.0295) |
| Fusicatenibacter | n.s. | 13.80 ± 4.05 (0.0023) | 4.78 ± 1.63 (0.0074) | −6.34 ± 2.98 (0.0414) | n.s. | n.s. | n.s. | n.s. | −142.93 ± 37.67 (0.0007) | −1.84 ± 0.45 (0.0004) |
| Parasutterella | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | −0.94 ± 0.41 (0.0296) | n.s. | n.s. | −1.01 ± 0.29 (0.0020) |
| Olivibacter | −6.32 ± 2.78 (0.0310) | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | 2.80 ± 1.25 (0.0324) | n.s. | n.s. |
| Marispirillum | n.s. | n.s. | n.s. | n.s. | n.s. | −3.21 ± 0.89 (0.0012) | n.s. | n.s. | n.s. | n.s. |
| Spiroplasma | −2.94 ± 1.18 (0.0189) | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | −0.85 ± 0.39 (0.0394) |
| Marinilabilia | 16.90 ± 7.08 (0.0239) | 90.74 ± 17.52 (0.0000) | 18.61 ± 8.01 (0.0293) | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| Desulfotomaculum | −34.26 ± 14.03 (0.0212) | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | −764.08 ± 346.92 (0.0355) | n.s. |
| Helicobacter | n.s. | 200.22 ± 68.19 (0.0072) | 81.99 ± 27.92 (0.0074) | n.s. | n.s. | −28.18 ± 12.44 (0.0314) | n.s. | n.s. | n.s. | n.s. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez, N.; Prieto, I.; Hidalgo, M.; Segarra, A.B.; Martínez-Rodríguez, A.M.; Cobo, A.; Ramírez, M.; Gálvez, A.; Martínez-Cañamero, M. Refined versus Extra Virgin Olive Oil High-Fat Diet Impact on Intestinal Microbiota of Mice and Its Relation to Different Physiological Variables. Microorganisms 2019, 7, 61. https://0-doi-org.brum.beds.ac.uk/10.3390/microorganisms7020061
Martínez N, Prieto I, Hidalgo M, Segarra AB, Martínez-Rodríguez AM, Cobo A, Ramírez M, Gálvez A, Martínez-Cañamero M. Refined versus Extra Virgin Olive Oil High-Fat Diet Impact on Intestinal Microbiota of Mice and Its Relation to Different Physiological Variables. Microorganisms. 2019; 7(2):61. https://0-doi-org.brum.beds.ac.uk/10.3390/microorganisms7020061
Chicago/Turabian StyleMartínez, Nieves, Isabel Prieto, Marina Hidalgo, Ana Belén Segarra, Ana M. Martínez-Rodríguez, Antonio Cobo, Manuel Ramírez, Antonio Gálvez, and Magdalena Martínez-Cañamero. 2019. "Refined versus Extra Virgin Olive Oil High-Fat Diet Impact on Intestinal Microbiota of Mice and Its Relation to Different Physiological Variables" Microorganisms 7, no. 2: 61. https://0-doi-org.brum.beds.ac.uk/10.3390/microorganisms7020061