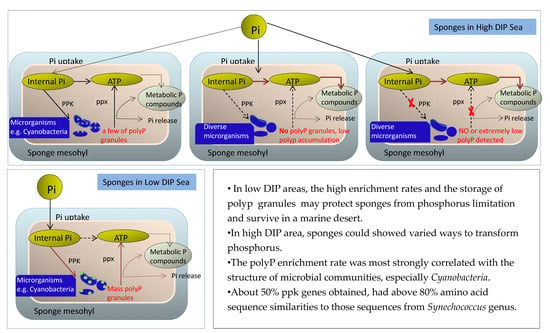
Characteristic Microbiomes Correlate with Polyphosphate Accumulation of Marine Sponges in South China Sea Areas
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Site Description
2.2. Extraction and Measurement of PolyP from Sponge Samples
2.3. Visualization of PolyP Granules by Confocal Microscopy
2.4. DNA Extraction and 16S rDNA PCR Amplification and Sequencing
2.5. Amplicon Sequencing Data Processing and Analysis
2.6. PCR Amplification and Cloning of Polyphosphate Kinase Genes from Sponges
3. Results
3.1. Determination of PolyP Concentrations in Sponges from Different Marine Environments
3.2. PolyP Distribution by Confocal Microscopy
3.3. Sponge Microbiomes
3.4. ppk Gene Identification and Host-Specific Microbial Groups in Relationship to polyP Sequestration
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Colman, A.S. Sponge symbionts and the marine P cycle. Proc. Natl. Acad. Sci. USA 2015, 112, 4191–4192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maldonado, M.; Ribes, M.; van Duyl, F.C. Nutrient fluxes through sponges: Biology, budgets, and ecological implications. Adv. Mar. Biol. 2012, 62, 113–182. [Google Scholar] [PubMed]
- Radwan, M.; Hanora, A.; Zan, J.; Mohamed, N.M.; Abo-Elmatty, D.M.; Abou-El-Ela, S.H.; Hill, R.T. Bacterial community analyses of two Red Sea sponges. Mar. Biotechnol. 2010, 12, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Thomas, T.; Moitinho-Silva, L.; Lurgi, M.; Björk, J.R.; Easson, C.; Astudillo-García, C.; Olson, J.B.; Erwin, P.M.; López-Legentil, S.; Luter, H.; et al. Diversity, structure and convergent evolution of the global sponge microbiome. Nat. Commun. 2016, 7, 11870. [Google Scholar] [CrossRef] [Green Version]
- Enticknap, J.J.; Kelly, M.; Peraud, O.; Hill, R.T. Characterization of a culturable alphaproteobacterial symbiont common to many marine sponges and evidence for vertical transmission via sponge larvae. Appl. Environ. Microbiol. 2006, 72, 3724–3732. [Google Scholar] [CrossRef] [Green Version]
- Taylor, M.W.; Radax, R.; Steger, D.; Wagner, M. Sponge-associated microorganisms: Evolution, ecology, and biotechnological potential. Microbiol. Mol. Biol. Rev. 2007, 71, 295–347. [Google Scholar] [CrossRef] [Green Version]
- Pita, L.; Rix, L.; Slaby, B.M.; Franke, A.; Hentschel, U. The sponge holobiont in a changing ocean: From microbes to ecosystems. Microbiome 2018, 6, 46. [Google Scholar] [CrossRef]
- Hoffmann, F.; Radax, R.; Woebken, D.; Holtappels, M.; Lavik, G.; Rapp, H.T.; Schläppy, M.-L.; Schleper, C.; Kuypers, M.M.M. Complex nitrogen cycling in the sponge Geodia barretti. Environ. Microbiol. 2009, 11, 2228–2243. [Google Scholar] [CrossRef]
- Jensen, S.; Fortunato, S.A.V.; Hoffmann, F.; Hoem, S.; Rapp, H.T.; Øvreås, L.; Torsvik, V.L. The relative abundance and transcriptional activity of marine sponge-associated microorganisms emphasizing groups involved in sulfur cycle. Microb. Ecol. 2017, 73, 668–676. [Google Scholar] [CrossRef]
- Van Mooy, B.A.S.; Fredricks, H.F.; Pedler, B.E.; Dyhrman, S.T.; Karl, D.M.; Koblížek, M.; Lomas, M.W.; Mincer, T.J.; Moore, L.R.; Moutin, T.; et al. Phytoplankton in the ocean use non-phosphorus lipids in response to phosphorus scarcity. Nature 2009, 458, 69–72. [Google Scholar] [CrossRef]
- Delaney, M.L. Phosphorus accumulation in marine sediments and the oceanic phosphorus cycle. Glob. Biogeochem. Cycles 1998, 12, 563–572. [Google Scholar] [CrossRef]
- Fletcher, G.L. Accumulation of yellow phosphorus by several marine invertebrates and seaweed. J. Fish. Res. Board Can. 1971, 28, 793–796. [Google Scholar] [CrossRef]
- Suzumura, M. Phospholipids in marine environments: A review. Talanta 2005, 66, 1–434. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Blasiak, L.C.; Karolin, J.O.; Powell, R.J.; Geddes, C.D.; Hill, R.T. Phosphorus sequestration in the form of polyphosphate by microbial symbionts in marine sponges. Proc. Natl. Acad. Sci. USA 2015, 112, 4381–4386. [Google Scholar] [CrossRef] [Green Version]
- Powell, N.; Shilton, A.; Chisti, Y.; Pratt, S. Towards a luxury uptake process via microalgae-defining the polyphosphate dynamics. Water Res. 2009, 43, 4207–4213. [Google Scholar] [CrossRef]
- Martin, P.; Dyhrman, S.T.; Lomas, M.W.; Poulton, N.J.; Van Mooy, B.A.S. Accumulation and enhanced cycling of polyphosphate by Sargasso Sea plankton in response to low phosphorus. Proc. Natl. Acad. Sci. USA 2014, 111, 8089–8094. [Google Scholar] [CrossRef] [Green Version]
- Rao, N.N.; Gomez-Garcia, M.R.; Kornberg, A. Inorganic polyphosphate: Essential for growth and survival. Annu. Rev. Biochem. 2009, 78, 605–647. [Google Scholar] [CrossRef]
- Arthur, K. Inorganic polyphosphate: Toward making a forgotten polymer unforgettable. J. Bacteriol. 1995, 177, 491–496. [Google Scholar]
- Holden, D.W. Persisters unmasked. Science 2015, 347, 30–32. [Google Scholar] [CrossRef]
- Azevedo, C.; Saiardi, A. Functions of inorganic polyphosphates in eukaryotic cells: A coat of many colours. Biochem. Soc. Trans. 2014, 42, 98–102. [Google Scholar] [CrossRef]
- Kulaev, I.; Vagabov, V.; Kulakovskaya, T. New aspects of inorganic polyphosphate metabolism and function. J. Biosci. Bioeng. 1999, 88, 111–129. [Google Scholar] [CrossRef]
- Zhou, J.; Jin, H.; Cai, Z.H. A review of the role and function of microbes in coral reef ecosystem. Chin. J. Appl. Ecol. 2014, 25, 919–930. [Google Scholar]
- Martin, P.; Van Mooy, B.A. Fluorometric quantification of polyphosphate in environmental plankton samples: Extraction protocols, matrix effects, and nucleic acid interference. Appl. Environ. Microbiol. 2013, 79, 273–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.; Bruns, M.A.; Tiedje, J.M. DNA recovery from soils of diverse composition. Appl. Environ. Microbiol. 1996, 62, 316–322. [Google Scholar]
- Caporaso, G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Lozupone, C.A.; Turnbaugh, P.J.; Fierer, N.; Knight, R. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. USA 2011, 108, 4516–4522. [Google Scholar] [CrossRef] [Green Version]
- Magoc, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [Green Version]
- Bokulich, N.A.; Subramanian, S.; Faith, J.J.; Gevers, D.; Gordon, J.I.; Knight, R.; Mills, D.A.; Caporaso, J.G. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat. Methods 2013, 10, 57–59. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [Green Version]
- Pruesse, E.; Quast, C.; Knittel, K.; Fuchs, B.M.; Ludwig, W.; Peplies, J.; Glöckner, F.O. SILVA: A comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids. Res. 2007, 35, 7188–7196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dang, H.; Li, J.; Chen, R.; Wang, L.; Guo, L.; Zhang, Z.; Klotz, M.G. Diversity, abundance, and spatial distribution of sediment ammonia-oxidizing Betaproteobacteria in response to environmental gradients and coastal eutrophication in Jiaozhou Bay, China. Appl. Environ. Microbiol. 2010, 76, 4691–4702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minhin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. R-Package Version 2.0–10. 2013. Available online: http://CRAN.R-project.org/package=vegan (accessed on 24 September 2019).
- de Goeij, J.M.; van Oevelen, D.; Vermeij, M.J.A.; Osinga, R.; Middelburg, J.J.; de Goeij, A.F.P.M.; Admiraal1, W. Surviving in a marine desert: The sponge loop retains resources within coral reefs. Science 2013, 342, 108–110. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.R.; Kornberg, A. Inorganic polyphosphate in the origin and survival of species. Proc. Natl. Acad. Sci. USA 2004, 101, 16085–16087. [Google Scholar] [CrossRef] [Green Version]
- Benitez-Nelson, C.R. The biogeochemical cycling of phosphorus in marine systems. Earth Sci. Rev. 2000, 51, 109–135. [Google Scholar] [CrossRef]
- Easson, C.G.; Thanker, R.W. Phylogenetic signal in the community structure of host-specific microbiomes of tropical marine sponge. Front. Microbiolgy 2014, 5, 532–542. [Google Scholar] [CrossRef] [Green Version]
- Paytan, A.; McLaughlin, K. The oceanic phosphorus cycle. Chem. Rev. 2007, 107, 563–576. [Google Scholar] [CrossRef]
- Corno, G.; Modenutti, B.E.; Callieri, C.; Balseiro, E.G.; Bertoni, R.; Caravatia, E. Bacterial diversity and morphology in deep ultraoligotrophic Andean lakes: Role of UVR on vertical distribution. Limnol. Oceanogr. 2009, 54, 1098–1112. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.K.; Lee, J.H.; Lee, H.K. Microbial symbiosis in marine sponges. J. Microbiol. 2001, 39, 254–264. [Google Scholar]
- Grozdanov, L.; Hentschel, U. An environmental genomics perspective on the diversity and function of marine sponge-associated microbiota. Curr. Opin. Microbiol. 2007, 10, 215–220. [Google Scholar] [CrossRef]
- Wilkinson, C.R. Net Primary Productivity in Coral Reef Sponges. Science 1983, 219, 410–412. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, C.; Chowdhury, R.; Ray, K. Phosphorus recycling from an unexplored source by polyphosphate accumulating microalgae and cyanobacteria-A step to phosphorus security in agriculture. Front. Microbiol. 2015, 6, 1421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kromkamp, J. Formation and functional significance of storage products in cyanobacteria. N. Z. J. Mar. Freshw. Res. 1987, 21, 457–465. [Google Scholar] [CrossRef] [Green Version]
- Seki, Y.; Nitta, K.; Kaneko, Y. Observation of polyphosphate bodies and DNA during the cell division cycle of Synechococcus elongatus PCC 7942. Plant Biol. 2014, 16, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Mateo, P.; Douterelo, I.; Berrendero, E.; Perona, E. Physiological differences between two species of Cyanobacteria in relation to phosphorus limitation. J. Phycol. 2006, 42, 61–66. [Google Scholar] [CrossRef]
- Schulz, H.N.; Schulz, H.D. Large sulfur bacteria and the formation of phosphorite. Science 2005, 307, 416–418. [Google Scholar] [CrossRef]
- Aguilar-May, B.; Pilar Sánchez-Saavedra, M. Growth and removal of nitrogen and phosphorus by free-living and chitosan-immobilized cells of the marine cyanobacterium Synechococcus elongatus. J. Appl. Phycol. 2008, 21, 353–360. [Google Scholar] [CrossRef]
- Dvořák, P.; Hindák, F.; Hašler, P.; Hindáková, A.; Poulíčková, A. Morphological and molecular studies of Neosynechococcus sphagnicola, gen. et sp. nov. (Cyanobacteria, Synechococcales). Phytotaxa 2014, 170, 24–34. [Google Scholar]
- Li, X.; Li, R. Limnolyngbya circumcreta gen. & comb. nov. (Synechococcales, Cyanobacteria) with three geographical (provincial) genotypes in China. Phycologia 2016, 55, 478–491. [Google Scholar]
- Mazard, S.; Wilson, W.H.; Scanlan, D.J. Dissecting the physiological response to phosphorus stress in marine Synechococcus isolates (Cyanophyceae). J. Phycol. 2012, 48, 94–105. [Google Scholar] [CrossRef]
- Wu, S.; Ou, H.; Liu, T.; Wang, D.; Zhao, J. Structure and dynamics of microbiomes associated with the marine sponge Tedania sp. during its life cycle. Fems. Microbiol. Ecol. 2018, 94, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yellowlees, D.; Rees, T.A.; Leggat, W. Metabolic interactions between algal symbionts and invertebrate hosts. Plant Cell Environ. 2008, 31, 679–694. [Google Scholar] [CrossRef] [PubMed]
- Arndt-Sullivan, C.; Lechaire, J.P.; Felbeck, H. Extreme tolerance to anoxia in the Lucinoma aequizonata symbiosis. J. Shellfish Res. 2008, 27, 119–127. [Google Scholar] [CrossRef]




| Sample Sources | DIP 1 in Surrounding Sea Water (μM) | ID | Sponge Taxonomy | Abundance (Individuals/20 m2) 3 | polyP/Sponge Dry Weight (mg/g) | Enrichment Rate 2 |
|---|---|---|---|---|---|---|
| Dongshan bay (E) 23.80° N, 117.59° E | ~4.796 | DM | Mycale sp. | 5 | 0.008 ± 0.004 | 53 ± 27 |
| DT | Tedania sp. | 16 | 3.722 ± 0.152 | 24813 ± 1807 | ||
| DC | Callyspongia sp. | 10 | 0.024 ± 0.001 | 157 ± 10 | ||
| Changhua Town (O) 19.25° N, 109.03° E | ~0.040 | CH01 | Haliclona sp. | 12 | 3.729 ± 1.711 | 3107893 ± 247702 |
| CH02 | Cladocroce sp. | 7 | 2.911 ± 0.133 | 2425581 ± 111206 | ||
| Linqiangshi lsland (O) 19.53° N, 109.26° E | ~0.210 | LQ01 | Lissodendoryx sp. | 4 | 0.484 ± 0.079 | 110015 ± 17986 |
| LQ02 | Mycale sp. | 2 | 0.137 ± 0.041 | 31215 ± 9399 | ||
| Qizhou Island (O) 19.55° N, 111.11° E | ~0.081 | QZ01 | Sigmaxinella sp. | 3 | 1.355 ± 0.161 | 541971 ± 64371 |
| QZ02 | Ircinia sp. | 1 | 0.977 ± 0.619 | 390672 ± 247702 | ||
| Meixia Port (O) 20.00° N, 109.35° E | ~0.114 | MX01 | Lissodendoryx sp. | 4 | 1.159 ± 0.443 | 331027 ± 126645 |
| MX02 | Ircinia dendroides | 2 | 0.492 ± 0.194 | 140559 ± 55423 | ||
| MX03 | Callyspongia sp. | 3 | 0.046 ± 0.019 | 13121 ± 5455 |
| ID | Closest ppk Relative and Its Accession Number | Bacteria Group | AA Identities | Taxonomy |
|---|---|---|---|---|
| DM.1 | polyphosphate kinase 1 WP_045783506.1 | Klebsiella michiganensis | 97% | Gammaproteobacteria; Enterobacterales |
| DM.2 | polyphosphate kinase KRO79340.1 | OM182 bacterium BACL3 MAG-120920-bin41 | 96% | Gammaproteobacteria; OMG group |
| DM.3 | polyphosphate kinase 1 WP_049475655.1 | Stenotrophomonas maltophilia | 99% | Gammaproteobacteria; Xanthomonadales |
| DM.4 | polyphosphate kinase 1 WP_071965591.1 | Streptomyces cinnamoneus | 73% | Actinobacteria; Streptomycetales |
| DM.5 | polyphosphate kinase KPK47330.1 | Thiotrichales bacterium SG8_50 | 72% | Gammaproteobacteria; Thiotrichales |
| DM.6 | polyphosphate kinase 1 OYV98205.1 | Acidobacteria bacterium 21-70-11 | 72% | Acidobacteria |
| DM.7 | polyphosphate kinase 1 WP_019874751.1 | Sporichthya polymorpha | 77% | Actinobacteria; Frankiales |
| DM.8 | polyphosphate kinase KRO79340.1 | OM182 bacterium BACL3 MAG-120920-bin41 | 96% | Gammaproteobacteria; OMG group |
| DM.9 | polyphosphate kinase 1 PWL23716.1 | Synechococcus sp. XM-24 | 86% | Cyanobacteria; Synechococcales |
| DT.1 | polyphosphate kinase 1 WP_045783506.1 | Klebsiella michiganensis | 98% | Gammaproteobacteria; Enterobacterales |
| DT.2 | polyphosphate kinase 1 WP_010309567.1 | Synechococcus sp. CB0101 | 84% | Cyanobacteria; Synechococcales |
| DT.3 | polyphosphate kinase 1 WP_049475655.1 | Stenotrophomonas maltophilia | 98% | Gammaproteobacteria; Xanthomonadales |
| DT.4 | polyphosphate kinase 1 PWL23716.1 | Synechococcus sp. XM-24 | 89% | Cyanobacteria; Synechococcales |
| DT.5 | polyphosphate kinase 1 OYV98205.1 | Acidobacteria bacterium 21-70-11 | 71% | Acidobacteria. |
| DT.6 | polyphosphate kinase 1 WP_094558932.1 | Synechococcus sp. 8F6 | 86% | Cyanobacteria; Synechococcales |
| CH01.1 | polyphosphate kinase 1 WP_010309567.1 | Synechococcus sp. CB0101 | 86% | Cyanobacteria; Synechococcales |
| CH01.2 | polyphosphate kinase 1 WP_007099397.1 | Synechococcus sp. RS9916 | 97% | Cyanobacteria; Synechococcales |
| CH01.3 | polyphosphate kinase 1 WP_010317776.1 | Synechococcus sp. CB0205 | 85% | Cyanobacteria; Synechococcales |
| CH01.4 | polyphosphate kinase 1 PWL23716.1 | Synechococcus sp. XM-24 | 84% | Cyanobacteria; Synechococcales |
| CH02.1 | polyphosphate kinase 1 WP_007099397.1 | Synechococcus sp. RS9916 | 97% | Cyanobacteria; Synechococcales |
| CH02.2 | polyphosphate kinase 1 WP_041025907.1 | Alcanivorax pacificus | 84% | Gammaproteobacteria; Oceanospirillales |
| LQ01.1 | polyphosphate kinase 1 WP_010309567.1 | Synechococcus sp. CB0101 | 87% | Cyanobacteria; Synechococcales |
| LQ01.2 | polyphosphate kinase 1 PWL23716.1 | Synechococcus sp. XM-24 | 88% | Cyanobacteria; Synechococcales |
| LQ01.3 | polyphosphate kinase 1 WP_010309567.1 | Synechococcus sp. CB0101 | 89% | Cyanobacteria; Synechococcales |
| LQ02.1 | polyphosphate kinase 1 WP_010309567.1 | Synechococcus sp. CB0101 | 89% | Cyanobacteria; Synechococcales |
| QZ01.1 | polyphosphate kinase 1 WP_007099397.1 | Synechococcus sp. RS9916 | 97% | Cyanobacteria; Synechococcales |
| QZ01.2 | polyphosphate kinase 1 PWL23716.1 | Synechococcus sp. XM-24 | 87% | Cyanobacteria; Synechococcales |
| QZ02.1 | polyphosphate kinase PPR24019.1 | Alphaproteobacteria bacterium MarineAlpha10_Bin2 | 74% | Alphaproteobacteria |
| QZ02.2 | polyphosphate kinase 1 WP_090634088.1 | Nitrosomonas marina | 74% | Betaproteobacteria; Nitrosomonadales |
| QZ02.3 | polyphosphate kinase 1 WP_096458022.1 | Sulfurifustis variabilis | 65% | Gammaproteobacteria; Acidiferrobacterales |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ou, H.; Li, M.; Wu, S.; Jia, L.; Hill, R.T.; Zhao, J. Characteristic Microbiomes Correlate with Polyphosphate Accumulation of Marine Sponges in South China Sea Areas. Microorganisms 2020, 8, 63. https://0-doi-org.brum.beds.ac.uk/10.3390/microorganisms8010063
Ou H, Li M, Wu S, Jia L, Hill RT, Zhao J. Characteristic Microbiomes Correlate with Polyphosphate Accumulation of Marine Sponges in South China Sea Areas. Microorganisms. 2020; 8(1):63. https://0-doi-org.brum.beds.ac.uk/10.3390/microorganisms8010063
Chicago/Turabian StyleOu, Huilong, Mingyu Li, Shufei Wu, Linli Jia, Russell T. Hill, and Jing Zhao. 2020. "Characteristic Microbiomes Correlate with Polyphosphate Accumulation of Marine Sponges in South China Sea Areas" Microorganisms 8, no. 1: 63. https://0-doi-org.brum.beds.ac.uk/10.3390/microorganisms8010063