Molecular Survey of Dirofilaria and Leishmania Species in Dogs from Central Balkan
Abstract
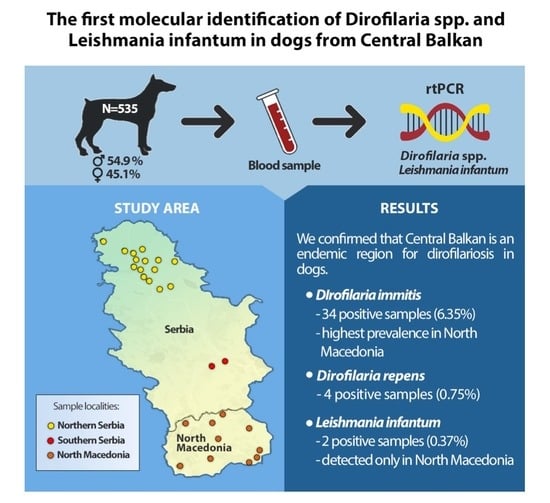
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Dog Population
2.2. Molecular Analyses
2.3. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morchón, R.; Carretón, E.; González Miguel, J.; Mellado Hernández, I. Heartworm Disease (Dirofilaria immitis) and Their Vectors in Europe–New Distribution Trends. Front. Physiol. 2012, 3, 196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otranto, D.; Dantas-Torres, F.; Brianti, E.; Traversa, D.; Petrić, D.; Genchi, C.; Capelli, G. Vector-borne helminths of dogs and humans in Europe. Parasit Vectors 2013, 6, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antoniou, M.; Gramiccia, M.; Molina, R.; Dvorak, V.; Volf, P. The role of indigenous phlebotomine sandflies and mammals in the spreading of leishmaniasis agents in the Mediterranean region. Eurosurveillance 2013, 18, 20540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ready, P.D. Leishmaniasis emergence in Europe. Eurosurveillance 2010, 15, 19505. [Google Scholar] [CrossRef]
- Miró, G.; López-Vélez, R. Clinical management of canine leishmaniosis versus human leishmaniasis due to Leishmania infantum: Putting “One Health” principles into practice. Vet. Parasitol. 2018, 254, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Vaselek, S.; Ayhan, N.; Oguz, G.; Erisoz Kasap, O.; Savić, S.; Di Muccio, T.; Gradoni, L.; Ozbel, Y.; Alten, B.; Petrić, D. Sand fly and Leishmania spp. survey in Vojvodina (Serbia): First detection of Leishmania infantum DNA in sand flies and the first record of Phlebotomus (Transphlebotomus) mascittii Grassi, 1908. Parasites Vectors 2017, 10, 444. [Google Scholar] [CrossRef] [Green Version]
- Gabrielli, S.; Mangano, V.; Furzi, F.; Oliva, A.; Vita, S.; Poscia, R.; Fazii, P. Molecular Identification of New Cases of Human Dirofilariosis (Dirofilaria repens) in Italy. Pathogens 2021, 10, 251. [Google Scholar] [CrossRef]
- El Tai, N.O.; Osman, O.F.; El Fari, M.; Presber, W.; Schönian, G. Genetic heterogeneity of ribosomal internal transcribed spacer in clinical samples of Leishmania donovani spotted on filter paper as revealed by single-strand conformation polymorphisms and sequencing. Trans. R. Soc. Trop. Med. Hyg. 2000, 94, 575–579. [Google Scholar] [CrossRef]
- Schönian, G.; Nasereddin, A.; Dinse, N.; Schweynoch, C.; Schallig, H.D.F.H.; Presber, W.; Jaffe, C.L. PCR diagnosis and characterization of Leishmania in local and imported clinical samples. Diagn. Microbiol. Infect. Dis. 2003, 47, 349–358. [Google Scholar] [CrossRef]
- Fuehrer, H.-P.; Auer, H.; Leschnik, M.; Silbermayr, K.; Duscher, G.; Joachim, A. Dirofilaria in Humans, Dogs, and Vectors in Austria (1978–2014)—From Imported Pathogens to the Endemicity of Dirofilaria repens. PLoS Negl. Trop. Dis. 2016, 10, e0004547. [Google Scholar] [CrossRef]
- Tasić, A.; Rossi, L.; Tasić, S.; Miladinović-Tasić, N.; Ilić, T.; Dimitrijević, S. Survey of canine dirofilariasis in Vojvodina, Serbia. Parasitol. Res. 2008, 103, 1297–1302. [Google Scholar] [CrossRef] [PubMed]
- Tasić, A.; Tasić-Otašević, S.; Gabrielli, S.; Miladinović-Tasić, N.; Ignjatović, A.; Dorđević, J.; Dimitrijević, S.; Cancrini, G. Canine Dirofilaria infections in two uninvestigated areas of Serbia: Epidemiological and genetic aspects. Vector Borne Zoonotic Dis. 2012, 12, 1031–1035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tasić, A.; Tasić, S.; Miladinović-Tasić, N.; Zdravković, D.; Djordjević, J. Prevalence of Dirofilaria repens-cause of zoonosis in dogs. Acta Fac. Med. Naiss. 2007, 24, 71–74. [Google Scholar]
- Penezić, A.; Selaković, S.; Pavlović, I.; Ćirović, D. First findings and prevalence of adult heartworms (Dirofilaria immitis) in wild carnivores from Serbia. Parasitol. Res. 2014, 113, 3281–3285. [Google Scholar] [CrossRef]
- Gavrilović, P.; Blitva-Robertson, G.; Özvegy, J.; Kiskároly, F.; Becskei, Z. Case Report of dirofilariasis in grey wolf in Serbia. Acta Parasitol. 2014, 60, 175–178. [Google Scholar] [CrossRef]
- Potkonjak, A.; Rojas, A.; Gutiérrez, R.; Nachum-Biala, Y.; Kleinerman, G.; Savić, S.; Polaček, V.; Pušić, I.; Harrus, S.; Baneth, G. Molecular survey of Dirofilaria species in stray dogs, red foxes and golden jackals from Vojvodina, Serbia. Comp. Immunol. Microbiol. Infect. Dis. 2020, 68, 101409. [Google Scholar] [CrossRef]
- Jurhar, M.; Cvetković, D.; Duma, H.; Kochevski, Z.; Jankoska, G.; Petrovska, M. Dirofilariosis in R. Macedonia, an overview of literature data and our experience. In Proceedings of the 46th Days of Preventive Medicine, Niš, Serbia, 25–29 August 2012. [Google Scholar]
- Ćirović, D.; Penezić, A.; Pavlović, I.; Kulišić, Z.; Ćosić, N.; Burazerović, J.; Maletić, V. First records of Dirofilaria repens in wild canids from the region of Central Balkan. Acta Vet. Hung. 2014, 62, 481–488. [Google Scholar] [CrossRef] [Green Version]
- Dvorak, V.; Kasap, O.E.; Ivovic, V.; Mikov, O.; Stefanovska, J.; Martinkovic, F.; Omeragic, J.; Pajovic, I.; Baymak, D.; Oguz, G.; et al. Sand flies (Diptera: Psychodidae) in eight Balkan countries: Historical review and region-wide entomological survey. Parasites Vectors 2020, 13, 573. [Google Scholar] [CrossRef]
- Dumitrache, M.O.; Nachum-Biala, Y.; Gilad, M.; Mircean, V.; Cazan, C.D.; Mihalca, A.D.; Baneth, G. The quest for canine leishmaniasis in Romania: The presence of an autochthonous focus with subclinical infections in an area where disease occurred. Parasites Vectors 2016, 9, 297. [Google Scholar] [CrossRef] [Green Version]
- Pavel, G.; Timofte, D.; Mocanu, D.; Malancus, R.; Solcan, C. 2017. Imported leishmaniasis in a dog in a sandfly-populated area in northeastern Romania. J. Vet. Diagn. Investig. 2017, 29, 683–685. [Google Scholar] [CrossRef]
- Harizanov, R.; Rainova, I.; Tzvetkova, N.; Kaftandjiev, I.; Bikov, I.; Mikov, O. Geographical distribution and epidemiological characteristics of visceral leishmaniasis in Bulgaria, 1988 to 2012. Eurosurveillance 2013, 18, 20531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mihalca, A.D.; Cazan, C.D.; Sulesco, T.; Dumitrache, M.O. A historical review on vector distribution and epidemiology of human and animal leishmanioses in Eastern Europe. Res. Vet. Sci 2019, 123, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Tsatchev, I.; Kyriazis, I.D.; Boutsini, S.; Karagouni, E.; Dotsika, E. First report of canine visceral leishmaniasis in Bulgaria. Turkish J. Vet. Anim. Sci. 2010, 34, 465–469. [Google Scholar] [CrossRef]
- Myrseli, T.; Mersini, K.; Nachum-Biala, Y.; Bino, S.; Baneth, G. A Survey of canine leishmaniosis in Albania. In Proceedings of the 3rd Conference on Neglected Vectors and Vector-Borne Diseases, Zaragoza, Spain, 24–26 May 2016. [Google Scholar]
- Hamel, D.; Shukullari, E.; Rapti, D.; Silaghi, C.; Pfister, K.; Rehbein, S. Parasites and vector-borne pathogens in client-owned dogs in Albania. Blood pathogens and seroprevalences of parasitic and other infectious agents. Parasitol. Res. 2016, 115, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Živičnjak, T.; Martinković, F.; Marinculić, A.; Mrljak, V.; Kučer, N.; Matijatko, V.; Mihaljević, Ž.; Barić-Rafaj, R. A seroepidemiologic survey of canine visceral leishmaniosis among apparently healthy dogs in Croatia. Vet. Parasitol. 2005, 131, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Šiško-Kraljević, K.; Jerončić, A.; Mohar, B.; Punda-Polić, V. Asymptomatic Leishmania infantum infections in humans living in endemic and non-endemic areas of Croatia, 2007 to 2009. Eurosurveillance 2013, 18, 20533. [Google Scholar] [CrossRef] [Green Version]
- Medenica, S.; Jovanovic, S.; Dozic, I.; Milicic, B.; Lakicevic, N.; Rakocevic, B. Epidemiological surveillance of Leishmaniasis in Montenegro, 1992–2013. Srp. Arh. Celok. Lek. 2015, 143, 707–711. [Google Scholar] [CrossRef]
- Colella, V.; Hodžić, A.; Iatta, R.; Baneth, G.; Alić, A.; Otranto, D. Zoonotic Leishmaniasis, Bosnia and Herzegovina. Emerg. Infect. Dis. 2019, 25, 385–386. [Google Scholar] [CrossRef]
- Ntais, P.; Sifaki-Pistola, D.; Christodoulou, V.; Messaritakis, I.; Pratlong, F.; Poupalos, G.; Antoniou, M. Leishmaniases in Greece. Am. J. Trop. Med. Hyg. 2013, 89, 906–915. [Google Scholar] [CrossRef] [Green Version]
- Gkolfinopoulou, K.; Bitsolas, N.; Patrinos, S.; Veneti, L.; Marka, A.; Dougas, G.; Pervanidou, D.; Detsis, M.; Triantafillou, E.; Georgakopoulou, T.; et al. Epidemiology of human leishmaniasis in Greece, 1981–2011. Eurosurveillance 2013, 18, 20532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatzis, M.K.; Andreadou, M.; Leontides, L.; Kasabalis, D.; Mylonakis, M.; Koutinas, A.F. Cytological and molecular detection of Leishmania infantum in different tissues of clinically normal and sick cats. Vet. Parasitol. 2014, 202, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Diakou, A.; Di Cesare, A.; Accettura, P.M.; Barros, L.; Iorio, R.; Paoletti, B.; Frangipane di Regalbono, A.; Halos, L.; Beugnet, F.; Traversa, D. Intestinal parasites and vector-borne pathogens in stray and free-roaming cats living in continental and insular Greece. PLoS Negl. Trop. Dis. 2017, 11, e0005335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ćirović, D.; Chochlakis, D.; Tomanović, S.; Sukara, R.; Penezić, A.; Tselentis, Y. Presence of Leishmania and Brucella Species in the Golden Jackal Canis aureus in Serbia. BioMed Res. Int. 2014, 2014, 728516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savić, S.; Vidić, B.; Grgić, Ž.; Gioia, B.; Gradoni, L. Serological and clinical evidence of leishmaniosis in a dog population living in a farm in Northern Serbia. In Proceedings of the International SCIVAC congress; Canine leishmaniosis and other vector-borne diseases: Our current state of knowledge, Pisa, Italy, 8–10 March 2013. [Google Scholar]
- Savić, S.; Vidić, B.; Fenjac, I.; Bekvalac, R.; Radičević, M.; Lolić, Z. Canine leishmaniosis in Serbia exists or not? In Proceedings of the Symposium of small animal practicioners SIVEMAP, Belgrade, Serbia, 22–24 October 2010. [Google Scholar]
- Cimpan, A.A.; Baneth, G.; Nachum-Biala, Y.; Miron, L.; Rojas, A. Dirofilaria repens predominates in shelter dogs from South Romania. Comp. Immunol. Microbiol. Infect. Dis. 2022, 84, 101793. [Google Scholar] [CrossRef] [PubMed]
- Dakic, Z.D.; Pelemis, M.R.; Stevanovic, G.D.; Poluga, J.L.; Lavadinovic, L.S.; Milosevic, I.S. Epidemiology and diagnostics of visceral leishmaniasis in Serbia. Clin. Microbiol. Infect. 2009, 15, 1173–1176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Francino, O.; Altet, L.; Sánchez-Robert, E.; Rodriguez, A.; Solano-Gallego, L.; Alberola, J.; Ferrer, L.; Sánchez, A.; Roura, X. Advantages of real-time PCR assay for diagnosis and monitoring of canine leishmaniosis. Vet. Parasitol. 2006, 137, 214–221. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tasić-Otašević, S.; Savić, S.; Jurhar-Pavlova, M.; Stefanovska, J.; Stalević, M.; Ignjatović, A.; Ranđelović, M.; Gajić, B.; Cvetkovikj, A.; Gabrielli, S. Molecular Survey of Dirofilaria and Leishmania Species in Dogs from Central Balkan. Animals 2022, 12, 911. https://0-doi-org.brum.beds.ac.uk/10.3390/ani12070911
Tasić-Otašević S, Savić S, Jurhar-Pavlova M, Stefanovska J, Stalević M, Ignjatović A, Ranđelović M, Gajić B, Cvetkovikj A, Gabrielli S. Molecular Survey of Dirofilaria and Leishmania Species in Dogs from Central Balkan. Animals. 2022; 12(7):911. https://0-doi-org.brum.beds.ac.uk/10.3390/ani12070911
Chicago/Turabian StyleTasić-Otašević, Suzana, Sara Savić, Maja Jurhar-Pavlova, Jovana Stefanovska, Marko Stalević, Aleksandra Ignjatović, Marina Ranđelović, Bojan Gajić, Aleksandar Cvetkovikj, and Simona Gabrielli. 2022. "Molecular Survey of Dirofilaria and Leishmania Species in Dogs from Central Balkan" Animals 12, no. 7: 911. https://0-doi-org.brum.beds.ac.uk/10.3390/ani12070911