Epidemiology of Non-Hodgkin’s Lymphoma
Abstract
:1. Introduction
2. Epidemiology
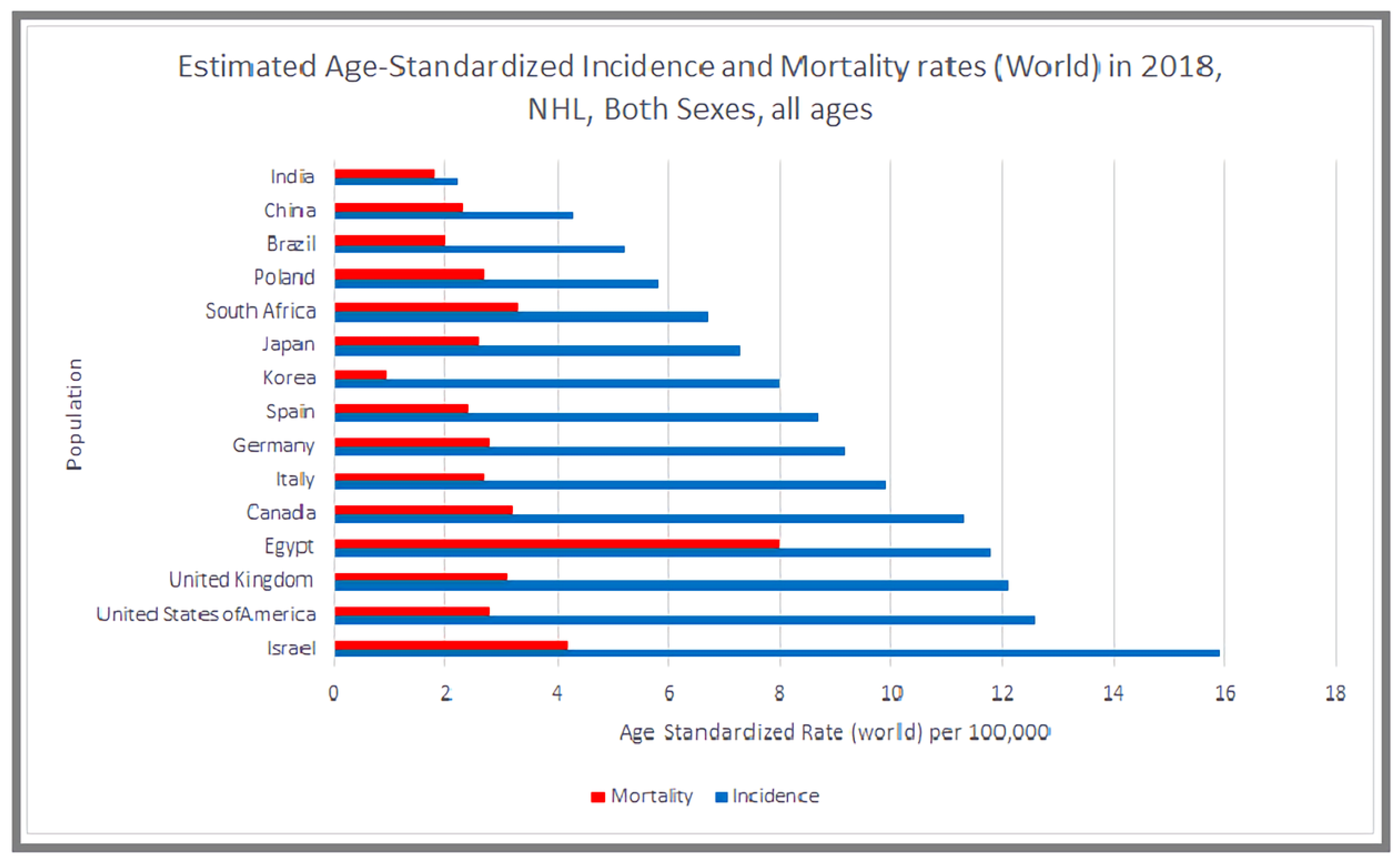
2.1. Incidence
2.2. Mortality
2.3. Survival
3. Non-Modifiable Risk Factors
3.1. Age
3.2. Gender
3.3. Race/Ethnicity
3.4. Family History
3.5. Autoimmune Diseases
3.6. Immunosuppression
4. Modifiable Risk Factors
4.1. Radiation
4.2. Chemical Exposure
4.3. Obesity
4.4. Tobacco Smoking and Alcohol
4.5. Breast Implants
4.6. Vitamin D Deficiency
5. Infections
5.1. Epstein–Barr Virus (EBV)
5.2. Human Immunodeficiency Virus (HIV)
5.3. Human T-Cell Lymphotropic Virus (HTLV-1)
5.4. Human Herpes Virus 8 (HHV-8)
5.5. Hepatitis C
5.6. Helicobacter Pylori
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- De Leval, L.; Jaffe, E.S. Lymphoma classification. Cancer J. 2020, 26, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.; Howell, D.; Crouch, S.; Painter, D.; Blase, J.; Wang, H.-I.; Hewison, A.; Bagguley, T.; Appleton, S.; Kinsey, S.; et al. Cohort Profile: The Haematological Malignancy Research Network (HMRN): A UK population-based patient cohort. Int. J. Epidemiol. 2018, 47, 700–700g. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morton, L.M.; Sampson, J.N.; Cerhan, J.R.; Turner, J.J.; Vajdic, C.M.; Wang, S.S.; Smedby, K.E.; De Sanjosé, S.; Monnereau, A.; Benavente, Y.; et al. Rationale and design of the International Lymphoma Epidemiology Consortium (InterLymph) non-Hodgkin lymphoma subtypes project. J. Natl. Cancer Inst. Monogr. 2014, 2014, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Howlander, N.; Noone, A.M.; Krapcho, M.; Miller, D.; Brest, A.; Yu, M.; Ruhl, J.; Tatalovich, Z.; Mariotto, A.; Lewis, D.R.; et al. SEER Cancer Statistics Review 1975–2016. Natl. Cancer Inst. Available online: https://seer.cancer.gov/csr/1975_2016/ (accessed on 28 December 2020).
- Esau, D. Viral Causes of Lymphoma: The History of Epstein-Barr Virus and Human T-Lymphotropic Virus 1. Virol. Res. Treat. 2017, 8. [Google Scholar] [CrossRef] [Green Version]
- Cerhan, J.R.; Kricker, A.; Paltiel, O.; Flowers, C.R.; Wang, S.S.; Monnereau, A.; Blair, A.; Maso, L.D.; Kane, E.V.; Nieters, A.; et al. Medical history, lifestyle, family history, and occupational risk factors for Diffuse Large B-cell Lymphoma: The interLymph non-Hodgkin lymphoma subtypes project. J. Natl. Cancer Inst. Monogr. 2014, 2014, 15–25. [Google Scholar] [CrossRef] [Green Version]
- Vajdic, C.M.; Landgren, O.; McMaster, M.L.; Slager, S.L.; Brooks-Wilson, A.; Smith, A.; Staines, A.; Dogan, A.; Ansell, S.M.; Sampson, J.N.; et al. Medical history, lifestyle, family history, and occupational risk factors for lymphoplasmacytic lymphoma/Waldenström’s macroglobulinemia: The InterLymph non-Hodgkin lymphoma subtypes project. J. Natl. Cancer Inst. Monogr. 2014, 2014, 87–97. [Google Scholar] [CrossRef] [Green Version]
- Linet, M.S.; Vajdic, C.M.; Morton, L.M.; De Roos, A.J.; Skibola, C.F.; Boffetta, P.; Cerhan, J.R.; Flowers, C.R.; De Sanjosé, S.; Monnereau, A.; et al. Medical history, lifestyle, family history, and occupational risk factors for follicular lymphoma: The interlymph non-hodgkin lymphoma subtypes project. J. Natl. Cancer Inst. Monogr. 2014, 2014, 26–40. [Google Scholar] [CrossRef] [Green Version]
- Slager, S.L.; Benavente, Y.; Blair, A.; Vermeulen, R.; Cerhan, J.R.; Costantini, A.S.; Monnereau, A.; Nieters, A.; Clavel, J.; Call, T.G.; et al. Medical history, lifestyle, family history, and occupational risk factors for chronic lymphocytic leukemia/small lymphocytic lymphoma: The InterLymph non-Hodgkin lymphoma subtypes project. J. Natl. Cancer Inst. Monogr. 2014, 2014, 41–51. [Google Scholar] [CrossRef] [Green Version]
- Smedby, K.E.; Sampson, J.N.; Turner, J.J.; Slager, S.L.; Maynadié, M.; Roman, E.; Habermann, T.M.; Flowers, C.R.; Berndt, S.I.; Bracci, P.M.; et al. Medical history, lifestyle, family history, and occupational risk factors for mantle cell lymphoma: The interlymph non-hodgkin lymphoma subtypes project. J. Natl. Cancer Inst. Monogr. 2014, 2014, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Bracci, P.M.; Benavente, Y.; Turner, J.J.; Paltiel, O.; Slager, S.L.; Vajdic, C.M.; Norman, A.D.; Cerhan, J.R.; Chiu, B.C.H.; Becker, N.; et al. Medical history, lifestyle, family history, and occupational risk factors for marginal zone lymphoma: The interLymph non-Hodgkin lymphoma subtypes project. J. Natl. Cancer Inst. Monogr. 2014, 2014, 52–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.S.; Flowers, C.R.; Kadin, M.E.; Chang, E.T.; Hughes, A.M.; Ansell, S.M.; Feldman, A.L.; Lightfoot, T.; Boffetta, P.; Melbye, M.; et al. Medical history, lifestyle, family history, and occupational risk factors for peripheral T-cell lymphomas: The interlymph non-hodgkin lymphoma subtypes project. J. Natl. Cancer Inst. Monogr. 2014, 2014, 66–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mbulaiteye, S.M.; Morton, L.M.; Sampson, J.N.; Chang, E.T.; Costas, L.; de Sanjosé, S.; Lightfoot, T.; Kelly, J.; Friedberg, J.W.; Cozen, W.; et al. Medical history, lifestyle, family history, and occupational risk factors for sporadic Burkitt lymphoma/leukemia: The InterLymph non-Hodgkin lymphoma subtypes project. J. Natl. Cancer Inst. Monogr. 2014, 2014, 106–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chihara, D.; Nastoupil, L.J.; Williams, J.N.; Lee, P.; Koff, J.L.; Flowers, C.R. New insights into the epidemiology of non-Hodgkin lymphoma and implications for therapy. Expert Rev. Anticancer Ther. 2015, 15, 531–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mbulaiteye, S.M.; Clarke, C.A.; Morton, L.M.; Gibson, T.M.; Pawlish, K.; Weisenburger, D.D.; Lynch, C.F.; Goodman, M.T.; Engels, E.A. Burkitt lymphoma risk in U.S. solid organ transplant recipients. Am. J. Hematol. 2013, 88, 245–250. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.J.; Freedman, D.M.; Curtis, R.E.; De Gonzalez, A.B.; Morton, L.M. Risk of non-Hodgkin lymphoma after radiotherapy for solid cancers. Leuk. Lymphoma 2013, 54, 1691–1697. [Google Scholar] [CrossRef] [Green Version]
- Krishnan, B.; Morgan, G.J. Non-Hodgkin lymphoma secondary to cancer chemotherapy. In Cancer Epidemiology Biomarkers and Prevention; Timothy R. Rebbeck: Philadelphia, PA, USA, 2007; Volume 16, pp. 377–380. [Google Scholar]
- Aschebrook-Kilfoy, B.; Cocco, P.; La Vecchia, C.; Chang, E.T.; Vajdic, C.M.; Kadin, M.E.; Spinelli, J.J.; Morton, L.M.; Kane, E.V.; Sampson, J.N.; et al. Medical history, lifestyle, family history, and occupational risk factors for mycosis fungoides and sézary syndrome: The InterLymph non-Hodgkin lymphoma subtypes project. J. Natl. Cancer Inst. Monogr. 2014, 2014, 98–105. [Google Scholar] [CrossRef] [Green Version]
- McGlynn, K.A.; Abnet, C.C.; Zhang, M.; Sun, X.D.; Fan, J.H.; O’Brien, T.R.; Wei, W.Q.; Ortiz-Conde, B.A.; Dawsey, S.M.; Weber, J.P.; et al. Serum concentrations of 1,1,1-Trichloro-2,2-bis(p-chlorophenyl)ethane (DDT) and 1,1-Dichloro-2,2-bis(p-chlorophenyl)ethylene (DDE) and risk of primary liver cancer. J. Natl. Cancer Inst. 2006, 98, 1005–1010. [Google Scholar] [CrossRef]
- Geyer, S.M.; Morton, L.M.; Habermann, T.M.; Allmer, C.; Davis, S.; Cozen, W.; Severson, R.K.; Lynch, C.F.; Wang, S.S.; Maurer, M.J.; et al. Smoking, alcohol use, obesity, and overall survival from non-Hodgkin lymphoma: A population-based study. Cancer 2010, 116, 2993–3000. [Google Scholar] [CrossRef]
- Kricheldorff, J.; Fallenberg, E.M.; Solbach, C.; Gerber-Schäfer, C.; Rancsó, C.; Von Fritschen, U. Breast implant-associated lymphoma—The diagnosis and treatment of a new disease entity. Dtsch. Arztebl. Int. 2018, 115, 628. [Google Scholar] [PubMed]
- Doren, E.L.; Miranda, R.N.; Selber, J.C.; Garvey, P.B.; Liu, J.; Medeiros, L.J.; Butler, C.E.; Clemens, M.W.U.S. epidemiology of breast implant-associated anaplastic large cell lymphoma. Plast. Reconstr. Surg. 2017, 139, 1042–1050. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.L.; Drake, M.T.; Fredericksen, Z.S.; Asmann, Y.W.; Liebow, M.; Shanafelt, T.D.; Feldman, A.L.; Ansell, S.M.; Macon, W.R.; Herr, M.M.; et al. Early life sun exposure, vitamin D-related gene variants, and risk of non-Hodgkin lymphoma. Cancer Causes Control 2012, 23, 1017–1029. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Chen, J.; Jin, J. Vitamin D status and risk of non-Hodgkin lymphoma: A meta-analysis. Cancer Causes Control 2014, 25, 1553–1563. [Google Scholar] [CrossRef] [PubMed]
- Tretli, S.; Schwartz, G.G.; Torjesen, P.A.; Robsahm, T.E. Serum levels of 25-hydroxyvitamin D and survival in Norwegian patients with cancer of breast, colon, lung, and lymphoma: A population-based study. Cancer Causes Control 2012, 23, 363–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robsahm, T.E.; Tretli, S.; Torjesen, P.A.; Babigumira, R.; Schwartz, G.G. Serum 25-hydroxyvitamin D levels predict cancer survival: A prospective cohort with measurements prior to and at the time of cancer diagnosis. Clin. Epidemiol. 2019, 11, 695. [Google Scholar] [CrossRef] [Green Version]
- Shanafelt, T.D.; Drake, M.T.; Maurer, M.J.; Allmer, C.; Rabe, K.G.; Slager, S.L.; Weiner, G.J.; Call, T.G.; Link, B.K.; Zent, C.S.; et al. Vitamin D insufficiency and prognosis in chronic lymphocytic leukemia. Blood 2011, 117, 1492–1498. [Google Scholar] [CrossRef]
- Drake, M.T.; Maurer, M.J.; Link, B.K.; Habermann, T.M.; Ansell, S.M.; Micallef, I.N.; Kelly, J.L.; Macon, W.R.; Nowakowski, G.S.; Inwards, D.J.; et al. Vitamin D insufficiency and prognosis in non-Hodgkin’s lymphoma. J. Clin. Oncol. 2010, 28, 4191. [Google Scholar] [CrossRef] [Green Version]
- Brady, G.; MacArthur, G.J.; Farrell, P.J. Epstein-Barr virus and Burkitt lymphoma. Postgrad. Med. J. 2008, 84, 372–377. [Google Scholar] [CrossRef]
- Bohlius, J.; Schmidlin, K.; Costagliola, D.; Fätkenheuer, G.; May, M.; Caro-Murillo, A.M.; Mocroft, A.; Bonnet, F.; Clifford, G.; Karafoulidou, A.; et al. Incidence and risk factors of HIV-related non- Hodgkin’s lymphoma in the era of combination antiretroviral therapy: A European multicohort study. Antivir. Ther. 2009, 14, 1065. [Google Scholar]
- Huebel, K.; Re, A.; Boumendil, A.; Finel, H.; Hentrich, M.; Michieli, M.; Kanfer, E.; Diez-Martin, J.L.; Balsalobre, P.; Vincent, L.; et al. Autologous Stem Cell Transplantation (autoSCT) for HIV-Associated Lymphoma in the Era of Combination Antiretroviral Therapy (cART): A Retrospective Analysis of the EBMT Lymphoma Working Party. Blood 2019, 54, 1625–1631. [Google Scholar] [CrossRef]
- Gessain, A.; Mahieux, R. Tropical spastic paraparesis and HTLV-1 associated myelopathy: Clinical, epidemiological, virological and therapeutic aspects. Rev. Neurol. (Paris) 2012, 168, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Edlich, R.F.; Arnette, J.A.; Williams, F.M. Global epidemic of human T-cell lymphotropic virus type-I (HTLV-I). J. Emerg. Med. 2000, 18, 109–119. [Google Scholar] [CrossRef]
- Foster, W.R.; Bischin, A.; Dorer, R.; Aboulafia, D.M. Human Herpesvirus Type 8-associated Large B-cell Lymphoma: A Nonserous Extracavitary Variant of Primary Effusion Lymphoma in an HIV-infected Man: A Case Report and Review of the Literature. Clin. Lymphoma Myeloma Leuk. 2016, 16, 311–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biological agents. Volume 100 B. A review of human carcinogens. IARC Monogr. Eval. Carcinog Risks Hum. 2012, 100, 1–441. [Google Scholar]


| Non-Modifiable Risk Factors | Modifiable Risk Factors |
|---|---|
| Age | Radiation |
| Gender | Chemical Exposure |
| Race/Ethnicity | Obesity |
| Family History | Tobacco smoking and alcohol |
| Autoimmune Diseases | Breast Implants |
| Immunosuppression | Vitamin Deficiency |
| Infection | Sub Types of NHL |
|---|---|
| EBV | Burkitt’s lymphoma |
| HIV | DLBCL, Primary brain lymphoma |
| HTLV-1 | DLBCL |
| HHV-8 | Primary effusion lymphoma |
| Hepatitis C | DLBCL |
| Helicobacter Pylori | MALT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thandra, K.C.; Barsouk, A.; Saginala, K.; Padala, S.A.; Barsouk, A.; Rawla, P. Epidemiology of Non-Hodgkin’s Lymphoma. Med. Sci. 2021, 9, 5. https://0-doi-org.brum.beds.ac.uk/10.3390/medsci9010005
Thandra KC, Barsouk A, Saginala K, Padala SA, Barsouk A, Rawla P. Epidemiology of Non-Hodgkin’s Lymphoma. Medical Sciences. 2021; 9(1):5. https://0-doi-org.brum.beds.ac.uk/10.3390/medsci9010005
Chicago/Turabian StyleThandra, Krishna C., Adam Barsouk, Kalyan Saginala, Sandeep Anand Padala, Alexander Barsouk, and Prashanth Rawla. 2021. "Epidemiology of Non-Hodgkin’s Lymphoma" Medical Sciences 9, no. 1: 5. https://0-doi-org.brum.beds.ac.uk/10.3390/medsci9010005





