Holistic Assessment of Carbon Capture and Utilization Value Chains
Abstract
:1. Introduction
1.1. Carbon Capture as a Mitigation Option
1.2. Carbon, Capture and Utilization
2. Technical Characteristics of a CCU Value Chain
2.1. Carbon Source Characterization
2.2. Carbon Capture
- Post-combustion carbon capture, when separation takes place after combustion. This process involves CO2 capture from the exhaust gases after the combustion of fossil fuels. The most common separation technique in this category is separation by chemical absorption.
- Pre-combustion carbon capture, when separation takes place before combustion. This process involves CO2 capture from streams containing H2 and CO2 that usually result from natural gas reforming and the produced syngas reacts with H2 through a water gas shift reaction to produce the mixture of H2 and CO2. The most common separation technique in this category would be separation by physical sorbents.
- Oxy-fuel combustion carbon capture, when the combustion is performed while using pure oxygen. This is essentially the same with post combustion carbon capture, but in this case, a lot of impurities can be avoided due to oxygen being pure (21% of air is oxygen), therefore the product would be a high purity CO2 stream.
2.3. Carbon Purification
2.4. Carbon Transportation
2.5. Carbon Utilization
2.5.1. Direct Use
2.5.2. Mineral Carbonation
2.5.3. Fuels Production
2.5.4. Chemicals Production
3. Economic Characteristics of a CCU Value Chain
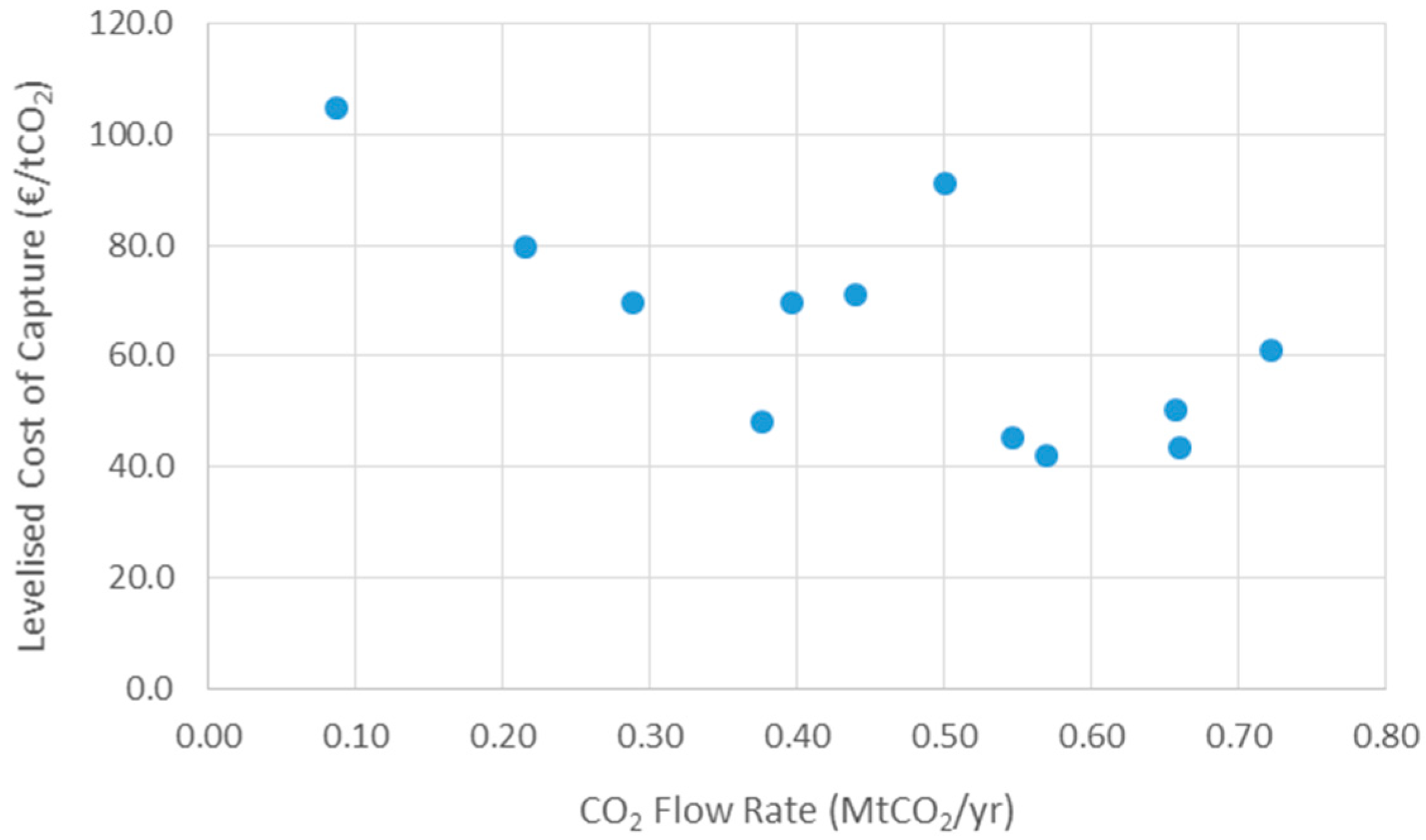
3.1. Carbon Capture
3.2. Carbon Transportation
4. Existing Optimization Models and Algorithms
5. Conclusions and Suggestions for Future Work
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- NASA. Available online: https://climate.nasa.gov/climate_resources/24/ (accessed on 31 May 2018).
- European Commission. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions, A Roadmap for Moving to a Competitive Low Carbon Economy in 2050. 2011. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:52011DC0112&from=GA (accessed on 31 May 2018).
- European Commission. 2050 Energy Strategy. Available online: https://ec.europa.eu/energy/en/topics/energy-strategy-and-energy-union/2050-energy-strategy (accessed on 13 September 2018).
- European Commission. Directive 2009/28/EC—Promoting the Use of Energy from Renewable Sources. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=LEGISSUM:en0009 (accessed on 13 September 2018).
- European Commission. 2020 Climate & Energy Package. Available online: https://ec.europa.eu/clima/policies/strategies/2020_en#tab-0-0 (accessed on 13 September 2018).
- European Commission. Energy Efficiency Directive. Available online: https://ec.europa.eu/energy/en/topics/energy-efficiency/energy-efficiency-directive (accessed on 13 September 2018).
- European Commission. Energy Efficiency. Available online: https://ec.europa.eu/energy/en/topics/energy-efficiency (accessed on 13 September 2018).
- Global CCS Institute. Understanding CCS—Storage. Available online: https://www.globalccsinstitute.com/understanding-ccs/how-ccs-works-storage (accessed on 13 September 2018).
- Cuellar-Franca, R.M.; Azapagic, A. Carbon capture, storage and utilization technologies: A critical analysis and comparison of their life cycle environmental impacts. J. CO2 Util. 2015, 9, 82–102. [Google Scholar] [CrossRef]
- IPCC. IPCC Special Report on Carbon Dioxide Capture and Storage. In Prepared by Working Group III of the Intergovernmental Panel on Climate Change; Metz, B., Davidson, O., de Coninck, H.C., Loos, M., Meyer, L.A., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2005. [Google Scholar]
- Patricio, J.; Angelis Dimakis, A.; Castillo-Castillo, A.; Kalmykova, Y.; Rosado, L. Method to identify opportunities for CCU at a regional level—Matching sources and receivers. J. CO2 Util. 2017, 22, 330–345. [Google Scholar] [CrossRef]
- Zakkour, P.; Cook, G. CCS Roadmap for Industry: High-Purity CO2 Sources; Carbon counts: London, UK, 2010. [Google Scholar]
- Fennell, P.S.; Florin, N.; Napp, T.; Hills, T. CCS from Industrial Sources. Sustain. Technol. Syst. Policies 2012. [Google Scholar] [CrossRef] [Green Version]
- Jin, H.; Gao, L.; Li, S.; van Sembeek, E.; Porter, R.; Mikunda, T.; Dijkstra, J.W.; de Coninck, H.; Jansen, D. Supporting Early Carbon Capture Utilization and Storage Development in Non-Power Industrial Sectors, Shaanxi Province, China; Report No. 12; The Centre for Low Carbon Futures: Birmingham, UK, 2012. [Google Scholar]
- CLCF. Carbon Capture and Utilization in the Green Economy: Using CO2 to Manufacture Fuel, Chemicals and Materials; The Centre for Low Carbon Futures: New York, NY, USA, 2011; ISBN 978-0-9572588-1-5. [Google Scholar]
- Angelis-Dimakis, A.; Castillo-Castillo, A. Enabling new values chains for CO2 reuse. In Proceedings of the 13th International Conference on Protection and Restoration of the Environment, Mykonos, Greece, 3–8 July 2016; Kungolos, A., Ed.; Grafima Publications: Thessaloniki, Greece, 2016; pp. 489–496, ISBN 978-960-6865-94-7. [Google Scholar]
- D’Amore, F.; Bezzo, F. Economic optimization of European supply chains for CO2 capture, transport and sequestration. Int. J. Greenh. Gas Control 2017, 65, 99–116. [Google Scholar] [CrossRef]
- Boot-Handford, M.E.; Abanades, J.C.; Anthony, E.J.; Blunt, M.J.; Brandani, S.; Mac Dowell, N.; Fernandez, J.R.; Ferrari, M.C.; Gross, R.; Hallett, J.P.; et al. Carbon capture and storage update. Energy Environ. Sci. 2014, 7, 130–189. [Google Scholar] [CrossRef]
- Leung, D.Y.C.; Caramanna, G.; Maroto-Valer, M.M. An overview of current status of carbon dioxide capture and storage technologies. Renew. Sustain. Energy Rev. 2014, 39, 426–443. [Google Scholar] [CrossRef] [Green Version]
- GCCSI. The Global Status of CCS; Global CCS Institute: Canberra, Australia, 2011. [Google Scholar]
- Spigarelli, B.P.; Kawatra, K.S. Opportunities and challenges in carbon dioxide capture. J. CO2 Util. 2013, 1, 69–87. [Google Scholar] [CrossRef]
- Muller, C. CO2 Capture and Storage CCS and the Industry of Carbon-Based Resources; ETH Zurich: Sonneggstrasse, Switzerland, 2017. [Google Scholar]
- Creamer, A.E.; Gao, B. Carbon Dioxide Capture: An Effective Way to Combat Global Warming, 1st ed.; Springer International Publishing: Berlin, Germany, 2015; pp. 17–24. ISBN 978-3-319-17009-1. [Google Scholar]
- Lyngfelt, A. Chemical Looping Reforming a and s; Department of Energy and Environmenta Chalmers University of Technology: Goteborg, Sweden, 2007. [Google Scholar]
- Wong, S. Module 2 CO2 capture: Post Combustions Flue Gas Separation. Retrieved from Global CCS Institute. Available online: https://hub.globalccsinstitute.com/publications/building-capacity-co2-capture-and-storage-apec-region-training-manual-policy-makers-and-practitioners/module-2-co2-capture-post-combustion-flue-gas-separation (accessed on 31 May 2018).
- Rubin, E.S.; Mantripragada, H.; Marks, A.; Vesteeg, P.; Kitchin, J. The outlook for improved carbon capture technology. Prog. Energy Combust. Sci. 2011, 38, 630–671. [Google Scholar] [CrossRef]
- Jansen, D.; Gazzani, M.; Manzolini, G.; van Dijk, E. Pre-combustion CO2 capture. Int. J. Greenh. Gas Control 2015, 40, 167–187. [Google Scholar] [CrossRef]
- Toftegaard, M.B.; Brix, J.; Jensen, P.A.; Glarborg, P.; Jensen, A.D. Oxy-fuel combustion of solid fuels. Prog. Energy Combust. 2010, 36, 581–625. [Google Scholar] [CrossRef]
- Kolster, C.; Mechleri, E.; Krevor, S.; Dowell, N.M. The role of CO2 purification and transport networks in carbon capture and storage cost reduction. Int. J. Greenh. Gas Control 2015, 58, 127–141. [Google Scholar] [CrossRef]
- Porter, R.T.; Fairweather, M.; Pourkashanian, M.; Wooley, R.M. The range and level of impurities in CO2 streams from different carbon capture sources. Int. J. Greenh. Gas Control 2015, 36, 161–174. [Google Scholar] [CrossRef]
- ZEP. The Costs of CO2 Capture, Transport and Storage-Post-Demonstration CCS in the EU; European Technology Platform for Zero Emission Fossil Fuel Power Plants: Brussels, Belgium, 2011. [Google Scholar]
- Summary of CO2 Stream Composition. Retrieved from Global CCS Institute. Available online: https://hub.globalccsinstitute.com/publications/hazard-analysis-offshore-carbon-capture-platforms-and-offshore-pipelines/e6-summary-co2-stream-composition (accessed on 31 May 2018).
- Abbas, Z.; Mezher, T.; Abu-Zahra, M. CO2 purification. Part I: Purification requirement review and the selection of impurities deep removal technologies. Int. J. Greenh. Gas Control 2013, 16, 324–334. [Google Scholar] [CrossRef]
- Abbas, Z.; Mezher, T.; Abu-Zahra, M. CO2 purification. Part II: Techno-economic evaluation of oxygen and water deep removal processes. Int. J. Greenh. Gas. Control 2013, 16, 335–341. [Google Scholar] [CrossRef]
- Aspelund, A.; Jordal, K. Gas conditioning—The interface between CO2 capture and transport. Int. J. Greenh. Gas Control 2007, 1, 343–354. [Google Scholar] [CrossRef]
- Lee, J.Y.; Keener, T.C.; Yang, Y.J. Potential flue gas impurities in carbon dioxide streams separated from coal-fired power plants. J. Air Waste Manag. Assoc. 2009, 59, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Wetenhall, B. The effect of CO2 Purity on the development of pipeline Networks for carbon capture and storage schemes. Int. J. Greenh. Gas Control 2014, 30, 197–211. [Google Scholar] [CrossRef] [Green Version]
- Ivan, S. Corrosion of pipelines used for CO2 transport in CCS: Is it a real problem? Int. J. Greenh. Gas Control 2011, 5, 749–756. [Google Scholar]
- De Visser, E.; Hendriks, C.; Barrio, M.; Mølnvik, M.J.; de Koeijer, G.; Liljemark, S.; Le Gallo, Y. Dynamis CO2 quality recommendations. Int. J. Greenh. Gas Control 2008, 2, 478–484. [Google Scholar] [CrossRef]
- Aspelund, A.; Molnvik, M.J.; De Koeijer, G. Ship Transport of CO2: Technical Solutions and analysis of Costs, Energy Utilization, Exergy efficiency and CO2 Emissions. Chem. Eng. Res. Des. 2006, 84, 847–855. [Google Scholar] [CrossRef]
- Wong, S. Module 4 CO2 Compression and Transportation to Storage Site. Retrieved from Global CCS Institute. Available online: https://hub.globalccsinstitute.com/publications/building-capacity-co2-capture-and-storage-apec-region-training-manual-policy-makers-and-practitioners/module-4-co2-compression-and-transportation-storage-site (accessed on 31 May 2018).
- Global CCS Institute. Accelerating the Uptake of CCS: Industrial Use of Captured Carbon Dioxide; Parsons Brickerhoff: New York, NY, USA, 2011. [Google Scholar]
- Girdon, P.; Gloger, C.; Gonzalez, D.; Henneqiun, J.; Krinninger, K.; de Lorenzi, L.; Wilyman, P. Minimum Specifications for Food Gas Applications; European Industrial Gases Association AISBL: Brussels, Belgium, 2006. [Google Scholar]
- Linde. Available online: http://www.linde-gas.com/en/processes/freezing_and_cooling/metal_cooling/index.html (accessed on 4 July 2018).
- AHDB Horticulture. Available online: https://horticulture.ahdb.org.uk/sources-co2 (accessed on 4 July 2018).
- AWS Committee on Filler Metals. Specification for Welding Shielding Gases; American Welding Society: Miami, FL, USA, 1997. [Google Scholar]
- Linde. Gas Applications for the Pulp and Paper Industry; Linde North America Inc.: New York, NY, USA, 2012. [Google Scholar]
- R&D Magazine. Available online: https://www.rdmag.com/article/2013/06/cleaning-electronics-manufacturing (accessed on 13 September 2018).
- Leeson, D.; Mac Dowell, N.; Shah, N.; Petit, C.; Fennell, P.S. A Techno-economic analysis and systematic review of carbon capture and storage (CCS) applied to the iron and steel, cement, oil refining and pulp and paper industries, as well as other high purity source. Int. J. Greenh. Gas Control 2017, 61, 71–84. [Google Scholar] [CrossRef]
- Kuramochi, T.; Ramírez, A.; Turkenburg, W.; Faaij, Y. Techno-economic prospects for CO2 capture from distributed energy systems. Renew. Sustain. Energy Rev. 2013, 19, 328–347. [Google Scholar] [CrossRef]
- Vermeulen, P.C.M. Alternative sources of CO2 for the greenhouse horticulture. In Proceedings of the 2nd International Symposium on Energy Challenges and Mechanics (ECM2), Aberdeen, UK, 19–21 August 2014. [Google Scholar]
- Bodor, M.; Santos, R.M.; Van Gerven, T.; Vlad, M. Recent developments and perspectives on the treatment of industrial wastes by mineral carbonation—A review. Cent. Eur. J. Eng. 2013, 3, 566–584. [Google Scholar] [CrossRef]
- Global CCS Institute. CCS for Iron and Steel Production. Available online: https://www.globalccsinstitute.com/insights/authors/dennisvanpuyvelde/2013/08/23/ccs-iron-and-steel-production (accessed on 31 May 2018).
- Rutberg, P.G.; Kuznetsov, V.A.; Bratsev, A.N.; Popov, V.E.; Shtengel, S.V.; Ufimtsev, A.A. Use of carbon dioxide in the chemical synthesis technologies, plasma gasification and carbon production. Mater. Sci. Eng. 2011, 19, 012003. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, A.S.; Zhai, Y.; Hill, D.; Sridhar, N. The Electrochemical Reduction of Carbon Dioxide to Formate/Formic Acid: Engineering and Economic Feasibility. ChemSusChem 2011, 4, 1301–1310. [Google Scholar] [CrossRef] [PubMed]
- Posten, C.; Schaub, G. Microalgae and terrestrial biomass as source for fuels—A process view. J. Biotechnol. 2009, 142, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Ritter, J.; Ebner, A. State-of-the-Art Adsorption and Membrane Separation Processes for Hydrogen Production in the Chemical and Petrochemical Industries. Sep. Sci. Technol. 2007, 42, 1123–1193. [Google Scholar] [CrossRef]
- Kuramochi, K.; Ramírez, A.; Turkenburg, W.; Faaij, A. Comparative assessment of CO2 capture technologies for carbon-intensive industrial processes. Prog. Energy Combust. 2012, 38, 87–112. [Google Scholar] [CrossRef]
- Energy, Element, and Carbon Counts. Demonstrating CO2 Capture in the UK Cement, Chemicals, Iron and Steel and Oil Refining Sectors by 2025: A Techno-Economic Study; PSCE, Imperial College, and University of Sheffield: Cambridge, UK, 2014. [Google Scholar]
- Nazmul Hassan, S.M.; Douglas, P.; Croiset, E. Techno-Economic Study of CO2 Capture from an Existing Cement Plant Using MEA Scrubbing. Int. J. Green Energy 2007, 4, 197–220. [Google Scholar] [CrossRef]
- Barker, D.J.; Turner, S.A.; Napier-Moore, P.A.; Clark, M.; Davison, J.E. CO2 Capture in the Cement Industry. Energy Procedia 2009, 1, 87–94. [Google Scholar] [CrossRef]
- EEL. The Costs of Carbon Capture and Storage (CCS) for UK Industry—A High Level Review; Element Energy Ltd.: Cambridge, UK, 2013. [Google Scholar]
- Davison, J.; Mancuso, L.; Ferrari, N. Costs of CO2 capture technologies in coal fired power and hydrogen plants. Energy Procedia 2014, 63, 7598–7607. [Google Scholar] [CrossRef]
- Kolstad, C.; Young, D. Cost Analysis of Carbon Capture and Storage for the Latrobe Valley; University of California: Santa Barbara, CA, USA, 2010. [Google Scholar]
- Borgert, K.J.; Rubin, E.S. Oxyfuel combustion: Technical and economic considerations for the development of carbon capture from pulverized coal power plants. Energy Procedia 2013, 37, 1291–1300. [Google Scholar] [CrossRef]
- Rubin, E.; Davison, J.; Herzog, H. The cost of CO2 capture and storage. Int. J. Greenh. Gas Control 2015, 40, 378–400. [Google Scholar] [CrossRef]
- Morgan, D.; Grant, T. FE/NETL CO2 Transport Cost Model: Model Overview, Presentation, DOE/NETL-2014/1668; US Dept. of Energy, National Energy, Technology Laboratory: Pittsburgh, PA, USA, 2014.
- Knoope, M.M.J.; Ramirez, A.; Faiij, A.P. A state of the art review of techno-economic models predicting the costs of CO2 pipeline transport. Int. J. Greenh. Gas Control 2013, 16, 241–270. [Google Scholar] [CrossRef]
- Mallon, W.; Buit, L.; van Wingerden, J.; Lemmens, H.; Eldrup, N.H. Costs of CO2 transportation infrastructures. Energy Procedia 2013, 37, 2969–2980. [Google Scholar] [CrossRef]
- Brownsort, P. Ship Transport of CO2 for Enhanced Oil Recovery—Literature Survey; Scottish Carbon Capture & Storage: Edinburgh, UK, 2015. [Google Scholar]
- Kjarstad, J.; Skagestad, R.; Eldrup, N.H.; Johnsson, F. Ship transport—A low cost and low risk CO2 transport option in the Nordic countries. Int. J. Greenh. Gas Control 2016, 54, 168–184. [Google Scholar] [CrossRef]
- Weihs, G.F.; Kumar, K.; Wiley, D.E. Understanding the economic feasibility of ship transport of CO2 within the CCS chain. Energy Procedia 2014, 63, 2630–2637. [Google Scholar] [CrossRef]
- Tapia, J.F.; Lee, J.Y.; Ooi, R.; Foo, D.; Tan, R. A review of optimization and decision-making models for the planning of CO2 capture, utilization and storage (CCUS) systems. Sustain. Prod. Consum. 2018, 13, 1–15. [Google Scholar] [CrossRef]
- Hasan, F.; First, E.; Boukouvala, F.; Floudas, C. A multi-scale framework for CO2 capture, utilization, and sequestration: CCUS and CCU. Comput. Chem. Eng. 2015, 81, 2–21. [Google Scholar] [CrossRef]
- Hassiba, R.; Al-Mohannadi, D.; Linke, P. Carbon dioxide and heat integration of industrial parks. J. Clean. Prod. 2017, 155, 47–56. [Google Scholar] [CrossRef]
- Agrali, S.; Uctug, F.G.; Turkmen, B.A. An optimization model for carbon capture & storage/utilization vs. carbon trading: A case study of fossil-fired power plants in Turkey. J. Environ. Manag. 2018, 215, 305–315. [Google Scholar]
- Pérez-Fortes, M.; Bocin-Dumitriu, A.; Tzimas, E. Techno-economic assessment of carbon utilization Assessment of Carbon Utilization Potential in Europe. In Chemical Engineering Transactions; AIDIC—The Italian Association of Chemical Engineering: Milano, Italy, 2014. [Google Scholar]
- Duraccio, V.; Gnoni, M.; Elia, G. Carbon capture and reuse in an industrial district: A technical and economic feasibility study. J. CO2 Util. 2015, 10, 23–29. [Google Scholar] [CrossRef]
- Reiter, G.; Lindorfer, J. Evaluating CO2 sources for power-to-gas applications—A case study for Austria. J. CO2 Util. 2015, 10, 40–49. [Google Scholar] [CrossRef]
- Karjunen, H.; Tynjälä, T.; Hyppänen, T. A method for assessing infrastructure for CO2 utilization: A case study of Finland. Appl. Energy 2017, 205, 33–43. [Google Scholar] [CrossRef]
- Patricio, J.; Angelis-Dimakis, A.; Castillo-Castillo, A.; Kalmykova, Y.; Rosado, L. Region prioritization for the development of carbon capture and utilization technologies. J. CO2 Util. 2017, 17, 50–59. [Google Scholar] [CrossRef]
- Von der Assen, N.; Müller, L.J.; Steingrube, A.; Voll, P.; Bardow, A. Selecting CO2 sources for CO2 utilization by environmental-merit-order curves. Environ. Sci. Technol. 2016, 50, 1093–1101. [Google Scholar] [CrossRef] [PubMed]


| Source CO2 Purity | |||
|---|---|---|---|
| Technique | High CO2 % | Medium CO2 % | Low CO2 % |
| General Gas Conditioning | Fermentation processes | ||
| Beer (100%) | |||
| Wine (100%) | |||
| Bioethanol (100%) | |||
| Biogas production (99%) | |||
| Refineries gas sweetening (96–99%) | |||
| Absorption with Chemical Solvents or Combustion using pure oxygen | Iron and steel Industries | Pulp industry | |
| Recovery boiler (13%) | |||
| Corex (30%) | Paper industry | ||
| Energy production (13%) | |||
| TGRBF (22–38%) | Power generation | ||
| Oil fired boilers (11–13%) | |||
| Blast furnace (22%) | Coal fired boilers (11%) | ||
| Natural gas fired boilers (7–10%) | |||
| Lime production | Incineration of waste (10%) | ||
| Glass industries (10%) | |||
| Combustion of fuels in lime kilns (24–32%) | Aluminium production (3–10%) | ||
| Polyethylene production | |||
| Cement industries | Cracker furnaces (5%) | ||
| Gas turbines (3–4%) | |||
| Cement kilns (22%) | Carbon black manufacturing (2–5%) | ||
| Oil refineries (8–24%) | Brick dryers and kilns (1.5–4%) | ||
| Absorption with Physical Solvents | Hydrogen production (100%) | Integrated gasification combined cycles (12–14%) | |
| Ammonia production | |||
| Haber-Bosch process (30–99%) | |||
| Ethylene oxide production (30–100%) | |||
| Natural gas processing (2–70%) | |||
| CO2 Receiver | Required Purity | TRL [11] | Reference |
|---|---|---|---|
| Direct Use | |||
| Beverage carbonation | High > 99.0% | 9 | [43] |
| Wine making | High > 99.0% | 9 | [43] |
| Food processing, preservation and packaging | High > 99.0% | 9 | [43] |
| Coffee decaffeination | High > 99.0% | 9 | [43] |
| Supercritical CO2 as a solvent | High > 99.0% | 9 | [43] |
| Steel manufacture | High | 9 | [53] |
| Metal working | High | 9 | [44] |
| Welding | High > 99.8% | [46] | |
| Pulp and paper processing | High | [47] | |
| Water treatment | High > 99% | [43] | |
| Electronics | Supercritical phase | [48] | |
| Power generation (as power cycle working fluid) | [42] | ||
| Pneumatics | 9 | [45] | |
| Fire suppression technology | High > 99.5% | 9 | |
| Refrigerant gas | High > 95.5% | 9 | [43] |
| Horticulture | High | 9 | [45] |
| Pharmaceutical processes | High > 99.5% | [43] | |
| Mineral Carbonation | |||
| Calcium carbonate and magnesium carbonate | Medium-Low | 7–8 | [42] |
| CO2 concrete curing | Medium-Low | 7–8 | [42] |
| Baking soda (sodium bicarbonate) | Medium-Low | [42] | |
| Bauxite residue treatment (“red mud”) | High | 7–9 | [42] |
| Fuels Production | |||
| Renewable methanol | High | 7–8 | [42] |
| Formic acid | High | [42] | |
| Algae cultivation | Medium-Low | 4–7 | [42] |
| CO2 injection to conventional methanol synthesis | High | [42] | |
| Genetically engineered micro-organisms | High > 99.5% | [45] | |
| Enhanced oil recovery (EOR) | High 95.0% | 9 | [45] |
| Enhanced coal bed methane recovery (ECBM) | High-Medium-low | 7 | [42] |
| Chemicals Production | |||
| Urea yield boosting | High | 9 | [42] |
| Polymer processing | High | 7 | [42] |
| Chemical synthesis (excludes polymers and fuels) | High | 6–8 | [54] |
| Reference | Pipelines Offshore | Pipelines Onshore | Ships | Truck Tankers | Railroad Tankers |
|---|---|---|---|---|---|
| [66] | Yes | Yes | No | No | No |
| [10] | Yes | Yes | Yes | No | No |
| [35] | No | No | Yes | No | No |
| [31] | Yes | Yes | Yes | Yes | No |
| [68] | Yes | Yes | No | No | No |
| [69] | Yes | Yes | Yes | No | No |
| [72] | Yes | Yes | No | No | No |
| [70] | No | No | Yes | No | No |
| [71] | No | Yes | No | No | No |
| Reference | CCU/CCS | Type of Receivers | Approach | Application | Economic Assessment | Environmental Impact Assessment | Social Impact Assessment |
|---|---|---|---|---|---|---|---|
| [74] | Both | EOR only | Optimization (MILP) | USA | Yes | No | No |
| [75] | Both | Three Selected | Optimization (Pinch) | Qatar | Yes | No | No |
| [76] | Both | EOR only | Optimization (MILP) | Turkey | Yes | No | No |
| [77] | CCU | Two Selected | Techno economic Analysis | Europe | Yes | No | No |
| [78] | CCU | Sugar Production | Techno economic Analysis | Industrial District | Yes | No | No |
| [79] | CCU | P2G | Techno economic Analysis | Austria | Yes | No | No |
| [80] | CCU | P2G | Simulation (Node Modelling) | Finland | Yes | No | No |
| [11] | CCU | Five Selected | Simulation (MFA) | Regional | Yes | No | |
| [81] | CCU | Nine Selected | Simulation (MFA) | Europe | Yes | No | No |
| [82] | CCU | - | Environmental Merit-Order | Europe | No | Yes | No |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pieri, T.; Nikitas, A.; Castillo-Castillo, A.; Angelis-Dimakis, A. Holistic Assessment of Carbon Capture and Utilization Value Chains. Environments 2018, 5, 108. https://0-doi-org.brum.beds.ac.uk/10.3390/environments5100108
Pieri T, Nikitas A, Castillo-Castillo A, Angelis-Dimakis A. Holistic Assessment of Carbon Capture and Utilization Value Chains. Environments. 2018; 5(10):108. https://0-doi-org.brum.beds.ac.uk/10.3390/environments5100108
Chicago/Turabian StylePieri, Tryfonas, Alexandros Nikitas, Arturo Castillo-Castillo, and Athanasios Angelis-Dimakis. 2018. "Holistic Assessment of Carbon Capture and Utilization Value Chains" Environments 5, no. 10: 108. https://0-doi-org.brum.beds.ac.uk/10.3390/environments5100108





