Seasonal Variation and Retention of Ammonium in Small Agricultural Streams in Central Thailand
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area Description
2.2. Seasonal Variations in Inorganic Nitrogen Concentration
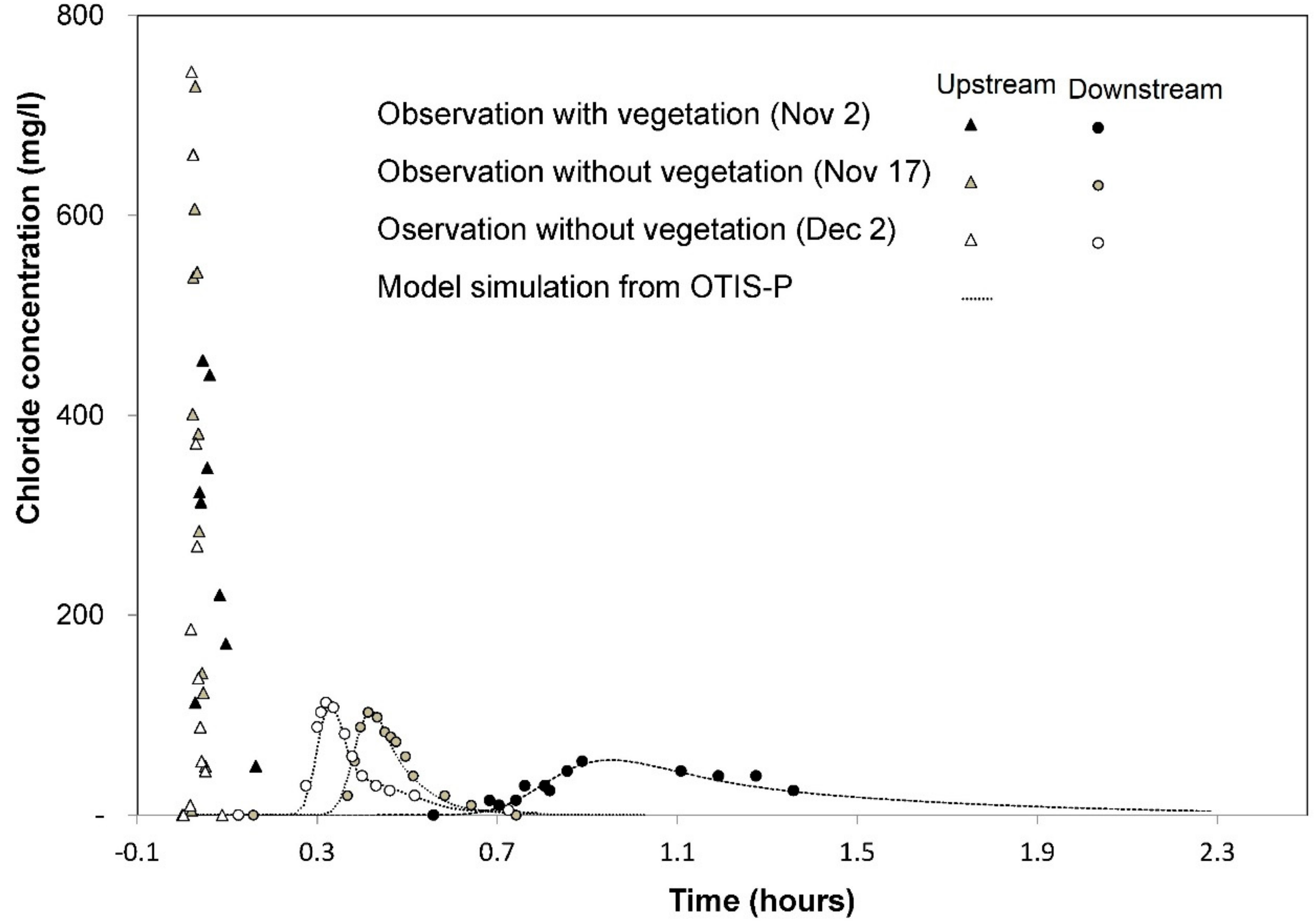
2.3. Tracer Injection Experiment
2.4. Estimation of the Hydraulic and Nutrient Retention
3. Results and Discussion
3.1. Seasonal Variation in Nitrogen Concentration in the Small Agricultural Streams
3.2. In-Stream Hydraulic and Nitrogen Retention
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Smith, V.H. Eutrophication of freshwater and coastal marine ecosystems: A global problem. Environ. Sci. Pollut. Res. Int. 2003, 10, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Ta, T.T.; Trinh, D.A.; Do, N.T. Nitrogen flow assessment in rapidly urbanizing Hai Duong province, downstream of Cau River Basin, Vietnam. J. Mater. Cycles Waste Manag. 2018, 20, 533–542. [Google Scholar] [CrossRef]
- Smith, V.H.; Tilman, G.D.; Nekola, J.C. Eutrophication: Impacts of excess nutrient inputs on freshwater, marine, and terrestrial ecosystems. Environ. Pollut. 1999, 100, 179–196. [Google Scholar] [CrossRef]
- Carpenter, S.R.; Caraco, N.F.; Correll, D.L.; Howarth, W.R.; Sharpley, N.A.; Smith, H.V. Nonpoint pollution of surface waters with phosphorus and nitrogen. Ecol. Appl. 1998, 8, 559–568. [Google Scholar] [CrossRef]
- Nijboer, R.C.; Verdonschot, P.F.M. Variable selection for modelling effects of eutrophication on stream and river ecosystems. Ecol. Model. 2004, 177, 17–39. [Google Scholar] [CrossRef]
- Nyenje, P.M.; Foppen, J.W.; Uhlenbrook, S.; Kulabako, R.; Muwanga, A. Eutrophication and Nutrient Release in Urban Areas of Sub-Saharan Africa—A Review. Sci. Total Environ. 2010, 408, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Hilton, J.; O’Hare, M.; Bowes, M.J.; Jones, J.I. How green is my river? A new paradigm of eutrophication in rivers. Sci. Total Environ. 2006, 365, 66–83. [Google Scholar] [CrossRef] [PubMed]
- Meksumpun, C.; Meksumpun, S. Integration of aquatic ecology and biological oceanographic knowledge for development of area-based eutrophication assessment criteria leading to water resource remediation and utilization management: A case study in Tha Chin, the most eutrophic river of Thailand. Water Sci. Technol. 2008, 58, 2303–2311. [Google Scholar] [PubMed]
- Mahujchariyawong, J.; Ikeda, S. Modelling of environmental phytoremediation in eutrophic river—The case of water hyacinth harvest in Tha-chin River, Thailand. Ecol. Model. 2001, 142, 121–134. [Google Scholar] [CrossRef]
- Salehin, M.; Packman, A.I.; Worman, A. Comparison of transient storage in vegetated and unvegetated reaches of a small agricultural stream in Sweden: Seasonal variation and anthropogenic manipulation. Adv. Water Resour. 2003, 26, 951–964. [Google Scholar] [CrossRef]
- Gooseff, M.N.; LaNier, J.; Haggerty, R.; Kokkeler, K. Determining in-channel (dead zone) transient storage by comparing solute transport in a bedrock channel-alluvial channel sequence, Oregon. Water Resour. Res. 2005, 41. [Google Scholar] [CrossRef] [Green Version]
- Lawrence, J.E.; Skold, M.E.; Hussain, F.A.; Silverman, D.R.; Resh, V.H.; Sedlak, D.L.; Luthy, R.G.; McCray, J.E. Hyporheic Zone in Urban Streams: A Review and Opportunities for Enhancing Water Quality and Improving Aquatic Habitat by Active Management. Environ. Eng. Sci. 2013, 30, 480–501. [Google Scholar] [CrossRef]
- Stream, S.W. Concepts and methods for assessing solute dynamics in stream ecosystems. J. N. Am. Benthol. Soc. 1990, 9, 95–119. [Google Scholar]
- Valett, H.M.; Morrice, J.A.; Dahm, C.N.; Campana, M.E. Parent lithology, surface-groundwater exchange, and nitrate retention in headwater streams. Limnol. Oceanogr. 1996, 41, 333–345. [Google Scholar] [CrossRef] [Green Version]
- Gücker, B.; Boëchat, I.G. Stream Morphology Controls Ammonium Retention in Tropical Headwaters. Ecology 2004, 85, 2818–2827. [Google Scholar] [CrossRef]
- Dodds, W.K.; Jones, J.R.; Welch, E.B. Suggested classification of stream trophic state: Distributions of temperate stream types by chlorophyll, total nitrogen, and phosphorus. Water Res. 1998, 32, 1455–1462. [Google Scholar] [CrossRef]
- Hall, R.O.J.; Tank, J.L. Ecosystem metabolism controls nitrogen uptake in streams in Grand Teton National Park, Wyoming. Limnol. Oceanogr. 2003, 48, 1120–1128. [Google Scholar] [CrossRef] [Green Version]
- Schaffner, M.; Bader, H.; Scheidegger, R. Modeling the contribution of point sources and non-point sources to Thachin River water pollution. Sci. Total Environ. 2009, 407, 4902–4915. [Google Scholar] [CrossRef] [PubMed]
- Schaffner, M.; Wittmer, I. The Thachin River is overloaded with nutrients. Eawag 2007, 62, 18–20. [Google Scholar]
- Le, T.T.A. Estimation of In-Channel Nitrogen Retention in a Small Tropical Stream Using One-Dimensional Transport with Inflow and Storage (OTIS) Model. Master’s Thesis, Mahidol University, Chang Wat Nakhon Pathom, Thailand, 2011. [Google Scholar]
- Mulholland, P.J.; Tank, J.L.; Webster, J.R.; Bowden, W.B.; Dodds, W.K.; Gregory, S.V.; Grimm, N.B.; Hamilton, S.K.; Johnson, S.L.; Martí, E.; et al. Can uptake length in streams be determined by nutrient addition experiments? Results from an interbiome comparison study. J. N. Am. Benthol. Soc. 2002, 21, 544–560. [Google Scholar] [CrossRef]
- Fetter, C.W. Applied Hydrogeology, 3rd ed.; Prentice-Hall, Inc.: Upper Saddle River, NJ, USA, 1994; p. 691. [Google Scholar]
- Harvey, J.W.; Wagner, B.J. Quantifying hydrologic interactions between streams and their subsurface hyporheic zones. In Streams and Ground Waters; Jones, J.B., Mulholland, P.J., Eds.; Academic Press: San Diego, CA, USA, 2000. [Google Scholar]
- Moore, R.D. Slug Injection Using Salt in Solution. Stream Water Manag. Bull. 2005, 8, 1–6. [Google Scholar]
- Runkel, R.L.; McKnight, D.M.; Andrews, E.D. Analysis of transient storage subject to unsteady flow: Diel flow variation in an Antarctic stream. J. N. Am. Benthol. Soc. 1998, 17, 145–154. [Google Scholar] [CrossRef]
- Morrice, J.A.; Valett, H.M.; Dahm, C.N.; Champana, M.E. Alluvial characteristics, groundwater-surface water exchange and hydrological retention in headwater streams. Hydrol. Process. 1997, 11, 253–267. [Google Scholar] [CrossRef]
- Covino, T.P.; McGlynn, B.L.; McNamara, R.A. Tracer Additions for Spiraling Curve Characterization (TASCC): Quantifying stream nutrient uptake kinetics from ambient to saturation. Limnol. Oceanogr. Methods 2010, 8, 484–498. [Google Scholar] [CrossRef] [Green Version]
- García, V.J.; Gantes, P.; Giménez, L.; Hegoburu, C.; Ferreiro, N.; Sabater, F.; Feijoó, C. High nutrient retention in chronically nutrient-rich lowland streams. Freshw. Sci. 2017, 36, 26–40. [Google Scholar] [CrossRef]
- Kasahara, T.; Hill, A.R. Lateral Hyporheic Zone Chemistry in an Artificially Constructed Gravel Bar and a Re-Meandered Stream Channel, Southern Ontario, Canada. J. Am. Water Resour. Assoc. 2007, 43, 1257–1269. [Google Scholar] [CrossRef]
- Johnson, L.B.; Richards, C.; Host, G.E.; Authur, J.W. Landscape influences on water chemistry in Midwestern stream ecosystems. Freshw. Biol. 1997, 37, 193–208. [Google Scholar] [CrossRef]
- Goolsby, D.A.; Battaglin, W.A.; Aulenbach, B.T.; Hooper, R.P. Nitrogen flux and sources in the Mississippi River Basin. Sci. Total Environ. 2000, 248, 75–86. [Google Scholar] [CrossRef] [Green Version]
- Collins, R.; Jenkins, A. The impact of agricultural land use on stream chemistry in the Middle Hills of the Himalayas, Nepal. J. Hydrol. 1996, 185, 71–86. [Google Scholar] [CrossRef]
- Knight, R.L.; Payne, V.W.E.; Borer, R.E.; Clarke, R.A.; Pries, J.H. Constructed wetlands for livestock wastewater management. Ecol. Eng. 2000, 15, 41–55. [Google Scholar] [CrossRef]
- Ensign, S.H.; Doyle, M.W. In-channel transient storage and associated nutrient retention: Evidence from experimental manipulations. Limnol. Oceanogr. 2005, 50, 1740–1751. [Google Scholar] [CrossRef] [Green Version]
- Schulz, M.; Kozerski, H.P.; Pluntke, T.; Rinke, K. The influence of macrophytes on sedimentation and nutrient retention in the lower river Spree (Germany). Water Res. 2003, 37, 569–578. [Google Scholar] [CrossRef]
- Stofleth, J.M.J.; Shields, F.D.; Fox, G.A. Hyporheic and total transient storage in small, sand-bed streams. Hydrol. Process. 2008, 22, 1885–1894. [Google Scholar] [CrossRef]
- Harvey, J.W.; Conklin, M.H.; Koelsch, R.S. Predicting changes in hydrologic retention in an evolving semi-arid alluvial stream. Adv. Water Resour. 2003, 26, 939–950. [Google Scholar] [CrossRef]
- Triska, F.J.; Jackman, A.P.; Duff, J.H.; Avanzino, R.J. Ammonium sorption to channel and riparian sediments—A transient storage pool for dissolved inorganic nitrogen. Biogeochemistry 1994, 26, 67–83. [Google Scholar] [CrossRef]
- Ventura, W.B.; Yoshida, T. Ammonia volatilization from a flooded tropical soil. Plant Soil 1977, 46, 521–531. [Google Scholar] [CrossRef]
- Wondzell, S.M. Effect of morphology and discharge on hyporheic exchange flows in two small streams in the Cascade Mountains of Oregon, USA. Hydrol. Process. 2006, 20, 267–278. [Google Scholar] [CrossRef]




| Caption | Distance (m) | K (cm s−1) | Width (m) | Depth (cm) | Width (m) | Depth (cm) |
|---|---|---|---|---|---|---|
| Before removal | After removal | |||||
| CS1 | 10 | 1.7 × 10−3 | 2.10 | 9.4 | 1.90 | 13.0 |
| CS2 | 35 | 1.1 × 10−4 | 2.80 | 4.7 | 2.60 | 9.2 |
| CS3 | 80 | 9.6 × 10−5 | 1.80 | 8.2 | 1.40 | 12.2 |
| CS4 | 102 | - | 1.50 | 12.1 | 1.20 | 18.4 |
| - | 145 | 3.7 × 10−6 | - | - | - | - |
| CS5 | 160 | - | 1.80 | 14.5 | 1.80 | 18.6 |
| CS6 | 210 | 8.6 × 10−6 | 1.90 | 11.8 | 1.60 | 19.0 |
| Average | - | 1.98 | 10.1 | 1.75 | 15.1 | |
| Caption | With Vegetation | Without Vegetation | ||
|---|---|---|---|---|
| 2 November | 17 November | 9 December | ||
| Discharge | Q (L s−1) | 12 | 18 | 30 |
| Damköhler number | DaI | 1.1 | 1.8 | 3.9 |
| Hydraulic retention | ||||
| Longitudinal dispersion | D (m2 s−1) | 0.130 | 0.040 | 0.119 |
| Storage exchange coefficient | α (10−4 s−1) | 3.40 | 2.50 | 3.00 |
| Relative size of transient storage zone | As/A | 0.39 | 0.16 | 0.07 |
| Stream residence time | Tstr (h) | 49.0 | 66.7 | 55.6 |
| Storage zone residence time | Tsto (h) | 19.0 | 10.4 | 4.1 |
| Hydraulic uptake length | Shyd (m) | 198 | 400 | 504 |
| Retention factor | Rh (s m−1) | 5.75 | 1.56 | 0.49 |
| Ammonium retention | ||||
| Uptake length | Sw (m) | 505 | 558 | 1461 |
| Uptake velocity | Vf (mm h−1) | 0.05 | 0.12 | 0.06 |
| Uptake rate | U (mg m−2 h−1) | 0.08 | 0.05 | 0.02 |
| Date | n | Max | Min | Average | Median | |
|---|---|---|---|---|---|---|
| With vegetation | 2 November | 13 | 11.02 | 9.45 | 10.22 | 10.23 |
| Without vegetation | 17 November | 13 | 5.36 | 0.71 | 2.94 | 2.86 |
| 9 December | 14 | 5.38 | 1.73 | 3.59 | 3.41 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
T. T. Le, A.; Kasahara, T.; Vudhivanich, V. Seasonal Variation and Retention of Ammonium in Small Agricultural Streams in Central Thailand. Environments 2018, 5, 78. https://0-doi-org.brum.beds.ac.uk/10.3390/environments5070078
T. T. Le A, Kasahara T, Vudhivanich V. Seasonal Variation and Retention of Ammonium in Small Agricultural Streams in Central Thailand. Environments. 2018; 5(7):78. https://0-doi-org.brum.beds.ac.uk/10.3390/environments5070078
Chicago/Turabian StyleT. T. Le, Anh, Tamao Kasahara, and Varawoot Vudhivanich. 2018. "Seasonal Variation and Retention of Ammonium in Small Agricultural Streams in Central Thailand" Environments 5, no. 7: 78. https://0-doi-org.brum.beds.ac.uk/10.3390/environments5070078




