Application of Gas-Permeable Membranes For-Semi-Continuous Ammonia Recovery from Swine Manure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Origin of Manure
2.2. Semi-Continuous Recovery of Ammonia from Manure
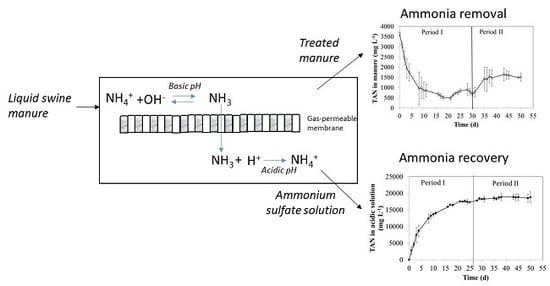
2.2.1. Experimental Set-Up
2.2.2. Manure and Acidic Solution Sampling
2.3. Effect of Differential Heating on Osmotic Distillation
2.4. Analytical Methods and Statistical Analysis
3. Results and Discussion
3.1. TAN Removal and Recovery by the Gas-Permeable System in Semi-Continuous Mode: Effect of Total Ammonia Nitrogen Loading Rate
3.2. Characterization of the Acidic Solution Containing the Concentrated Ammonia Product
3.3. Practical Considerations and Further Research
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Webb, J.; Menzi, H.; Pain, B.F.; Misselbrook, T.H.; Dämmgen, U.; Hendriks, H.; Döhler, H. Managing ammonia emissions from livestock production in Europe. Environ. Pollut. 2005, 135, 399–406. [Google Scholar] [CrossRef]
- European Environment Agency (EEA). Agriculture, Ammonia Emissions Statistics- Data Extracted in June 2015. Available online: http://ec.europa.eu/eurostat/statistics-explained/index.php/Agriculture_-_ammonia_emission_statistics (accessed on 30 June 2017).
- Wing, S.; Wolf, S. Intensive livestock operations, health, and quality of life among eastern North Carolina residents. Environ. Health. Perspect. 2000, 108, 233–238. [Google Scholar] [CrossRef]
- Directive (EU) 2016/2284 of the European Parlament and of the Council of 14 December 2016 on the reduction of national emissions of certain atmospheric pollutants, amending Directive 2003/35/EC and repeling Directive 2001/81/EC. Off. J. Eur. Commun. 2016, L344, 1–31.
- Dube, P.J.; Vanotti, M.B.; Szogi, A.A.; Garcia-González, M.C. Enhancing recovery of ammonia from swine manure anaerobic digester effluent using gas-permeable membrane technology. Waste Manag. 2016, 49, 372–377. [Google Scholar] [CrossRef]
- Funderburg, E. Why Are Nitrogen Prices So High? Agriculture News and Views 2001; The Samuel Roberts Noble Foundation: Ardmore, OK, USA, 2013; Available online: www.noble.org/ag/soils/nitrogenprices/ (accessed on 30 June 2017).
- Sareer, O.; Mazahar, S.; Khanum Al Akbari, W.M.; Umar, S. Nitrogen pollution, plants and human health. In Plants, Pollutants and Remediation; Springer: Dordrecht, The Netherlands, 2016; pp. 47–57. [Google Scholar]
- Xie, M.; Shon, H.K.; Gray, S.R.; Elimelech, M. Membrane-based processes for wastewater nutrient recovery: Technology, challenges, and future directions. Water Res. 2016, 89, 210–221. [Google Scholar] [CrossRef]
- Masse, L.; Massé, D.I.; Pellerin, Y.; Dubreuil, J. Osmotic pressure and substrate resistance during the concentration of manure nutrients by reverse osmosis membranes. J. Membr. Sci. 2010, 348, 28–33. [Google Scholar] [CrossRef]
- Bonmatí, A.; Flotats, X. Air stripping of ammonia from pig slurry: Characterization and feasibility as a pre-or post-treatment to mesophilic anaerobic digestion. Waste Manag. 2003, 23, 261–272. [Google Scholar] [CrossRef]
- Milan, Z.; Sánchez, E.; Weiland, P.; de Las Pozas, C.; Borja, R.; Mayari, R.; Rovirosa, N. Ammonia removal from anaerobically treated piggery manure by ion exchange in columns packed with homoionic zeolites. Chem. Eng. J. 1997, 66, 65–71. [Google Scholar] [CrossRef]
- Uludag-Demirer, S.; Demirer, G.N.; Chen, S. Ammonia removal from anaerobically digested dairy manure by struvite precipitation. Process Biochem. 2005, 40, 3667–3674. [Google Scholar] [CrossRef]
- Vanotti, M.B.; Szogi, A.A. Systems and Methods for Reducing Ammonia Emissions from Liquid Effluents and for Recovering the Ammonia. U.S. Patent 9,005,333 B1, 14 April 2015. [Google Scholar]
- García-González, M.C.; Vanotti, M.B. Recovery of ammonia from swine manure using gas-permeable membranes: Effect of waste strength and pH. Waste Manag. 2015, 38, 455–461. [Google Scholar] [CrossRef]
- García-González, M.C.; Vanotti, M.B.; Szogi, A.A. Recovery of ammonia from swine manure using gas-permeable membranes: Effect of aeration. J. Environ. Manag. 2015, 152, 19–26. [Google Scholar] [CrossRef]
- Zarebska, A.; Romero Nieto, D.; Chirstensen, K.V.; Fjerbaek Sotoft, L.; Norddahl, B. Ammonium fertilizers production from manure: A critical review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 1469–1521. [Google Scholar] [CrossRef]
- Daguerre-Martini, S.; Vanotti, M.B.; Rodríguez-Pastor, M.; Rosal, A.; Moral, R. Nitrogen recovery from wastewater using gas-permeable membranes: Impact of inorganic carbon content and natural organic matter. Water Res. 2018, 137, 2010–2210. [Google Scholar] [CrossRef]
- Samani Majd, A.M.; Mukhtar, S. Ammonia recovery enhancement using a tubular gar-permeable membrane system in laboratory and field-scale studies. Trans. ASABE 2013, 56, 1951–1958. [Google Scholar]
- Ahn, Y.T.; Hwang, Y.H.; Shin, H.S. Application of PTFE membrane for ammonia removal in a membrane contactor. Water Sci. Technol. 2011, 63, 2944–2948. [Google Scholar] [CrossRef]
- Rothrock, M.J.; Szögi, A.A.; Vanotti, M.B. Recovery of ammonia from poultry litter using gas-permeable membranes. Trans. ASABE 2010, 53, 1267–1275. [Google Scholar] [CrossRef]
- Samani Majd, A.M.; Mukhtar, S. Ammonia diffusion and capture into a tubular gas-permeable membrane using diluted acids. Trans. ASABE 2013, 56, 1943–1950. [Google Scholar]
- García-González, M.C.; Vanotti, M.B.; Szogi, A.A. Recovery of ammonia from anaerobically digested manure using gas-permeable membranes. Sci. Agric. 2016, 73, 434–438. [Google Scholar] [CrossRef]
- Oliveira Filho, J.D.S.; Daguerre-Martini, S.; Vanotti, M.B.; Saez-Tovar, J.; Rosal, A.; Pérez-Murcia, M.D.; Bustamante, M.A.; Moral, R. Recovery of ammonia in raw and co-digested swine manure using gas-permeable membrane technology. Front. Sustain. Food Syst. 2018, 2, 30. [Google Scholar] [CrossRef]
- Vanotti, M.B.; Szogi, A.A.; Dube, P.J. Systems and Methods for Recovering Ammonium and Phosphorous from Liquid Effluents. U.S. Patent 20160347630 A1, 1 December 2016. [Google Scholar]
- American Public Health Association. Standard Methods for the Examination of Water, Wastewater APHA. In American Water Works Association and Water Environment Federation, 21st ed.; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- US Environmental Protection Agency (EPA). Methods for Chemical Analysis of Water and Waste, EPA/600/4-79/020; US Environmental Protection Agency: Cincinnati, Ohio, 1983.
- Hansen, K.H.; Angelidaki, I.; Ahring, B.K. Anaerobic digestion of swine manure. Inhibition by ammonia. Water Res. 1998, 32, 5–12. [Google Scholar] [CrossRef]
- Vanotti, M.B.; Dube, P.J.; Szogi, A.A.; García-González, M.C. Recovery of ammonia and phosphate minerals from swine wastewater using gas-permeable membranes. Water Res. 2017, 112, 137–146. [Google Scholar] [CrossRef]
- Darestani, M.; Haigh, V.; Couperthwaite, S.J.; Millar, G.J.; Nghiem, L.D. Hollow fibre membrane contactors for ammonia recovery: Current status and future development. J. Environ. Chem. Eng. 2017, 5, 1349–1359. [Google Scholar] [CrossRef]
- Wang, G.; Shi, H.; Shen, Z. Influence of osmotic distillation on membrane absorption for the treatment of high strength ammonia wastewater. J. Environ. Sci. 2004, 16, 651–655. [Google Scholar]
- Zarebska, A.; Romero Nieto, D.; Christensen, K.V.; Norddahl, B. Ammonia recovery from agricultural wastes by membrane distillation: Fouling characterization and mechanism. Water Res. 2014, 56, 1–10. [Google Scholar] [CrossRef]
- Cokgor, E.U.; Zengin, G.E.; Tas, D.O.; Oktay, S.; Randall, C.; Orhon, D. Respirometric assessment of primary sludge fermentation product. J. Environ. Eng. 2006, 132, 68–74. [Google Scholar] [CrossRef]
- Antezana, W.; De Blas, C.; García-Rebollar, P.; Rodríguez, C.; Beccaccia, A.; Ferrer, P.; Cerisuelo, A.; Moset, V.; Estellés, F.; Cambra-López, M.; et al. Composition, potential emissions and agriculture value of pig slurry from Spanish commercial farms. Nutr. Cycl. Agroecosyst. 2016, 104, 159–173. [Google Scholar] [CrossRef]
- Lauterböck, B.; Nikolausz, M.; Lv, Z.; Baumgartner, M.; Liebhard, G.; Fuchs, W. Improvement of anaerobic digestion performance by continuous nitrogen removal with a membrane contactor treating substrate rich in ammonia and sulfide. Bioresour. Technol. 2014, 158, 209–216. [Google Scholar] [CrossRef]
- Lauterböck, B.; Ortner, M.; Haider, R.; Fuchs, W. Counteracting ammonia inhibition in anaerobic digestion by removal with a hollow fiber membrane contactor. Water Res. 2012, 46, 4861–4869. [Google Scholar] [CrossRef]





| Membranes Properties | First Experiment (Section 2.2) | Second Experiment (Section 2.3) |
|---|---|---|
| Inner diameter (mm) | 8.64 | 4.08 |
| Wall thickness (mm) | 0.76 | 0.56 |
| Pore size (µm) | 2.5 | - |
| Bubble point (kPa) | 207 | - |
| Density (g cm3) | 0.45 | 0.95 |
| Length (m) | 0.8 | 0.61 |
| Surface area (m2) | 0.026 | 0.091 |
| Period | TKNin (mg L−1) | TANin (mg L−1) | N orgin (mg L−1) | TKNeff (mg L−1) | TANeff (mg L−1) | N orgeff (mg L−1) |
|---|---|---|---|---|---|---|
| I | 4481 ± 182 | 3425 ± 122 | 1029 | 1830 ± 381 | 748 ± 153 | 1082 |
| II | 4685 ± 222 | 3486 ± 134 | 1199 | 2745 ± 345 | 1525 ± 83 | 1220 |
| Type of Wastewater | Operation Mode a | Treatment Time (d) | Ratio Membrane Surface/Manure Volume (m2 L−1) | Aeration Rate (Lair Lwaste−1 min−1) | Initial TAN Concentration (mg L−1) | TAN Removal (%) | TAN Recovery over Removed (%) | Average TAN Recovery (g m−2 d−1) b | Initial pH | Final pH | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Digested swine effluent | Batch | 6 | 0.013 | 0.12 | 2350 | 97 | 93 c | 25.1 c | 8.36 | 9.47 | [28] |
| Anaerobically digested swine manure | Batch | 5 | 0.013 | 0.12 | 2097 | 97 | 98 | 30.7 | 8.71 | 9.26 | [5] |
| Anaerobically digested swine manure | Batch | 5 | 0.013 | 0.12 | 1465 | 99 | 96 | 22.7 | 8.47 | 9.17 | [5] |
| Raw swine manure | Batch | 18 | 0.013 | 0.24 | 2390 | 99 | 99 | 9.5 | 7.50 | 9.20 | [15] |
| Raw swine manure centrate | Semi-continuous | 7 | 0.013 | 0.24 | 3451 | 79 | 90 | 27.1 | 7.60 | 8.46 | Present study (period I) |
| No heating | Heating | |
|---|---|---|
| Water recovery (g m−2 d−1) | 921 ± 185 | 612 ± 22 |
| Ammonia recovery (g m−2 d−1) | 49.5 ± 0.3 | 51.8 ± 2.6 |
| Period I | Period II | |
|---|---|---|
| COD removal (%) | 36.6 ± 11.6 | 26.5 ± 3.2 |
| TS removal (%) | 15.4 ± 7.1 | 6.2 ± 3.7 |
| VS removal (%) | 17. 1 ± 7.5 | 6.6 ± 5.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riaño, B.; Molinuevo-Salces, B.; Vanotti, M.B.; García-González, M.C. Application of Gas-Permeable Membranes For-Semi-Continuous Ammonia Recovery from Swine Manure. Environments 2019, 6, 32. https://0-doi-org.brum.beds.ac.uk/10.3390/environments6030032
Riaño B, Molinuevo-Salces B, Vanotti MB, García-González MC. Application of Gas-Permeable Membranes For-Semi-Continuous Ammonia Recovery from Swine Manure. Environments. 2019; 6(3):32. https://0-doi-org.brum.beds.ac.uk/10.3390/environments6030032
Chicago/Turabian StyleRiaño, Berta, Beatriz Molinuevo-Salces, Matías B. Vanotti, and María Cruz García-González. 2019. "Application of Gas-Permeable Membranes For-Semi-Continuous Ammonia Recovery from Swine Manure" Environments 6, no. 3: 32. https://0-doi-org.brum.beds.ac.uk/10.3390/environments6030032