The Way of Macroplastic through the Environment
Abstract
:1. Introduction
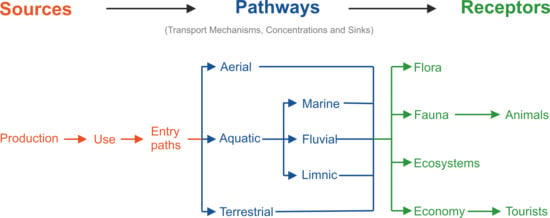
2. Sources of Macroplastic
2.1. Production of Macroplastic
2.2. Entry Paths of Macroplastic into the Environment
2.2.1. Land-Based Macroplastic: Coastal and Inland Mismanaged Plastic Waste
2.2.2. Ocean-Based Macroplastic
3. Pathways of Macroplastic
3.1. Transport Paths of Macroplastic
3.1.1. Transport in Aquatic Environments
3.1.2. Transport in Terrestrial Environments
3.1.3. Transport in Aerial Environments
3.2. Macroplastic Concentrations in the Environment
3.2.1. Freshwater Environments
3.2.2. Marine Environments
3.2.3. Terrestrial Environments
3.3. Sinks of Macroplastic
4. Receptors of Macroplastic
5. Conclusions
- How can MaP be meaningfully defined so that the high diversity of items is comprehensively represented?
- How can the individual sources and entry paths of MaP be quantified?
- Is the composition of plastic debris changing over time?
- What are the main influences on aquatic, terrestrial, and aerial transport of MaP?
- How do the temporal changes of the MaP (biofouling, fragmentation) and the surrounding systems (dry/rainfall season, tides, extreme events) influence the transport?
- What influence does the high diversity of MaP items have on ecotoxicology?
- Where and how can MaP be effectively removed from the environment?
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hopmann, C.; Michaeli, W. Einführung in die Kunststoffverarbeitung, 7th ed.; Hanser, C., Ed.; Carl Hanser Fachbuchverlag: Munich, Germany, 2015; ISBN 978-3-446-44627-4. [Google Scholar]
- Baur, E.; Osswald, T.A.; Rudolph, N.; Saechtling, H. Saechtling Kunststoff Taschenbuch; Saechtling, H., Ed.; Carl Hanser: Munich, Germany, 2013; ISBN 3446437290. [Google Scholar]
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.G.; McGonigle, D.; Russell, A.E. Lost at sea: Where is all the plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef] [PubMed]
- Billard, G.; Boucher, J. The Challenges of Measuring Plastic Pollution. Veolia Inst. Rev. Facts Rep. 2019, 68–75. [Google Scholar]
- Vriend, P.; van Calcar, C.; Kooi, M.; Landman, H.; Pikaar, R.; van Emmerik, T. Rapid Assessment of Floating Macroplastic Transport in the Rhine. Front. Mar. Sci. 2020, 7, 10. [Google Scholar] [CrossRef] [Green Version]
- Koelmans, A.A.; Besseling, E.; Foekema, E.; Kooi, M.; Mintenig, S.; Ossendorp, B.C.; Redondo-Hasselerharm, P.E.; Verschoor, A.; van Wezel, A.P.; Scheffer, M. Risks of Plastic Debris: Unravelling Fact, Opinion, Perception, and Belief. Environ. Sci. Technol. 2017, 51, 11513–11519. [Google Scholar] [CrossRef]
- Windsor, F.M.; Durance, I.; Horton, A.A.; Thompson, R.C.; Tyler, C.R.; Ormerod, S.J. A catchment-scale perspective of plastic pollution. Glob. Chang. Biol. 2019, 25. [Google Scholar] [CrossRef] [Green Version]
- Blettler, M.C.M.; Ulla, M.A.; Rabuffetti, A.P.; Garello, N. Plastic pollution in freshwater ecosystems: Macro-, meso-, and microplastic debris in a floodplain lake. Environ. Monit. Assess. 2017, 189, 581. [Google Scholar] [CrossRef]
- Van Emmerik, T.; Seibert, J.; Strobl, B.; Etter, S.; den Oudendammer, T.; Rutten, M.; bin Ab Razak, M.S.; van Meerveld, I. Crowd-Based Observations of Riverine Macroplastic Pollution. Front. Earth Sci. 2020, 8, 382. [Google Scholar] [CrossRef]
- Woodall, L.C.; Sanchez-Vidal, A.; Canals, M.; Paterson, G.L.J.; Coppock, R.; Sleight, V.; Calafat, A.; Rogers, A.D.; Narayanaswamy, B.E.; Thompson, R.C. The deep sea is a major sink for microplastic debris. R. Soc. Open Sci. 2014, 1, 140317. [Google Scholar] [CrossRef] [Green Version]
- Law, K.L.; Morét-Ferguson, S.; Maximenko, N.A.; Proskurowski, G.; Peacock, E.E.; Hafner, J.; Reddy, C.M. Plastic accumulation in the North Atlantic subtropical gyre. Science 2010, 329, 1185–1188. [Google Scholar] [CrossRef] [Green Version]
- Zettler, E.R.; Mincer, T.J.; Amaral-Zettler, L.A. Life in the plastisphere: Microbial communities on plastic marine debris. Environ. Sci. Technol. 2013, 47, 7137–7146. [Google Scholar] [CrossRef]
- Beer, S.; Garm, A.; Huwer, B.; Dierking, J.; Nielsen, T.G. No increase in marine microplastic concentration over the last three decades—A case study from the Baltic Sea. Sci. Total Environ. 2018, 621, 1272–1279. [Google Scholar] [CrossRef] [PubMed]
- Lebreton, L.; Egger, M.; Slat, B. A global mass budget for positively buoyant macroplastic debris in the ocean. Sci. Rep. 2019, 9, 1985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pabortsava, K.; Lampitt, R.S. High concentrations of plastic hidden beneath the surface of the Atlantic Ocean. Nat. Commun. 2020, 11, 339. [Google Scholar] [CrossRef] [PubMed]
- Waldschläger, K.; Lechthaler, S.; Stauch, G.; Schüttrumpf, H. The way of microplastic through the environment—Application of the source-pathway-receptor model (review). Sci. Total Environ. 2020, 713, 136584. [Google Scholar] [CrossRef]
- Narayan, S.; Hanson, S.; Nicholls, R.; Clarke, D.; Horrillo-Caraballo, J.M.; Reeve, D.E.; Simmonds, D.; Pan, S.; Fox, A.; Thompson, R.; et al. Use of the source-pathway-receptor-consequence model in coastal flood risk assessment: Application of a source-pathway-receptor-consequence (S-P-R-C) methodology to the Teign Estuary, UK. Eur. Geosci. Union Gen. Assess. 2011, 165, 1939–1944. [Google Scholar] [CrossRef]
- Li, W.; Tse, H.; Fok, L. Plastic waste in the marine environment: A review of sources, occurrence and effects. Sci. Total. Environ. 2016, 566, 333–349. [Google Scholar] [CrossRef]
- Barboza, L.G.A.; Cózar, A.; Gimenez, B.C.G.; Barros, T.L.; Kershaw, P.J.; Guilhermino, L. Macroplastics Pollution in the Marine Environment. In World Seas: An Environmental Evaluation; Elsevier: Amsterdam, The Netherlands, 2019; pp. 305–328. ISBN 9780128050521. [Google Scholar]
- Winton, D.J.; Anderson, L.G.; Rocliffe, S.; Loiselle, S. Macroplastic pollution in freshwater environments: Focusing public and policy action. Sci. Total Environ. 2020, 704, 135242. [Google Scholar] [CrossRef]
- Van Emmerik, T.; Schwarz, A. Plastic debris in rivers. WIREs Water 2020, 7, 116. [Google Scholar] [CrossRef] [Green Version]
- Liro, M.; van Emmerik, T.; Wyżga, B.; Liro, J.; Mikuś, P. Macroplastic Storage and Remobilization in Rivers. Water 2020, 12, 2055. [Google Scholar] [CrossRef]
- Chae, Y.; An, Y.-J. Current research trends on plastic pollution and ecological impacts on the soil ecosystem: A review. Environ. Pollut. 2018, 240, 387–395. [Google Scholar] [CrossRef]
- Bucci, K.; Tulio, M.; Rochman, C.M. What is known and unknown about the effects of plastic pollution: A meta-analysis and systematic review. Ecol. Appl. 2020, 30, e02044. [Google Scholar] [CrossRef] [PubMed]
- Amaral-Zettler, L.A.; Zettler, E.R.; Mincer, T.J. Ecology of the plastisphere. Nat. Rev. Microbiol. 2020, 18, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Arthur, C.; Baker, J.; Bamford, H. (Eds.) Proceedings of the International Research Workshop on the Occurrence, Effects and Fate of Microplastic Marine Debris, Tacoma, WA, USA, 9–11 September 2008; NOAA Technical Memorandum NOS-OR&R-30; NOAA Marine Debris Division: Silver Spring, MD, USA, 2009. [Google Scholar]
- Piehl, S.; Leibner, A.; Löder, M.G.J.; Dris, R.; Bogner, C.; Laforsch, C. Identification and quantification of macro- and microplastics on an agricultural farmland. Sci. Rep. 2018, 8, 17950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawecki, D.; Nowack, B. Polymer-Specific Modeling of the Environmental Emissions of Seven Commodity Plastics as Macro- and Microplastics. Environ. Sci. Technol. 2019, 53, 9664–9676. [Google Scholar] [CrossRef] [PubMed]
- Lebreton, L.; Andrady, A. Future scenarios of global plastic waste generation and disposal. Palgrave Commun. 2019, 5, 2922. [Google Scholar] [CrossRef] [Green Version]
- Barnes, D.K.A.; Galgani, F.; Thompson, R.C.; Barlaz, M. Accumulation and fragmentation of plastic debris in global environments. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1985–1998. [Google Scholar] [CrossRef] [Green Version]
- European Commission. Guidance on Monitoring of Marine Litter in European Seas. A Guidance Document within the Common Implementation Strategy for the Marine Strategy Framework Directive; MSFD Technical Subgroup on Marine Litter; Publications Office of the EU: Luxembourg, 2013; ISBN 978-92-79-32709-4. [Google Scholar]
- Van Emmerik, T.; Kieu-Le, T.-C.; Loozen, M.; van Oeveren, K.; Strady, E.; Bui, X.-T.; Egger, M.; Gasperi, J.; Lebreton, L.; Nguyen, P.-D.; et al. A Methodology to Characterize Riverine Macroplastic Emission into the Ocean. Front. Mar. Sci. 2018, 5, 3404. [Google Scholar] [CrossRef] [Green Version]
- Hartmann, N.B.; Hüffer, T.; Thompson, R.C.; Hassellöv, M.; Verschoor, A.; Daugaard, A.E.; Rist, S.; Karlsson, T.; Brennholt, N.; Cole, M.; et al. Are We Speaking the Same Language? Recommendations for a Definition and Categorization Framework for Plastic Debris. Environ. Sci. Technol. 2019, 53, 1039–1047. [Google Scholar] [CrossRef] [Green Version]
- UBA. Kunststoffe in der Umwelt. 2019. Available online: www.umweltbundesamt.de/publikationen (accessed on 4 August 2020).
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef]
- Chin, L.W.; Fung, T.H. Plastic in Marine Litter. In Plastics and the Environment; Hester, R.E., Harrison, R.M., Eds.; Royal Society of Chemistry: Cambridge, UK, 2019; pp. 21–59. ISBN 978-1-78801-241-6. [Google Scholar]
- Bond, T.; Ferrandiz-Mas, V.; Felipe-Sotelo, M.; van Sebille, E. The occurrence and degradation of aquatic plastic litter based on polymer physicochemical properties: A review. Crit. Rev. Environ. Sci. Technol. 2018, 48, 685–722. [Google Scholar] [CrossRef]
- Hengstmann, E.; Gräwe, D.; Tamminga, M.; Fischer, E.K. Marine litter abundance and distribution on beaches on the Isle of Rügen considering the influence of exposition, morphology and recreational activities. Mar. Pollut. Bull. 2017, 115, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Barnes, D.K.A.; Morley, S.A.; Bell, J.; Brewin, P.; Brigden, K.; Collins, M.; Glass, T.; Goodall-Copestake, W.P.; Henry, L.; Laptikhovsky, V.; et al. Marine plastics threaten giant Atlantic Marine Protected Areas. Curr. Biol. 2018, 28, R1137–R1138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Derraik, J.G.B. The pollution of the marine environment by plastic debris: A review. Mar. Pollut. Bull. 2002, 44, 842–852. [Google Scholar] [CrossRef]
- Nakashima, E.; Isobe, A.; Kako, S.; Itai, T.; Takahashi, S. Quantification of toxic metals derived from macroplastic litter on Ookushi Beach, Japan. Environ. Sci. Technol. 2012, 46, 10099–10105. [Google Scholar] [CrossRef]
- PlasticsEurope. Plastics—The Facts 2019. An Analysis of European Plastics Production, Demand and Waste Data; PlasticsEurope: Brussels, Belgium, 2019. [Google Scholar]
- Lechthaler, S. Makroplastik in der Umwelt. Betrachtung Terrestrischer und Aquatischer Bereiche; Springer Vieweg: Wiesbaden, Germany, 2020; ISBN 978-3-658-30336-5. [Google Scholar]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoornweg, D.; Bhada-Tata, P. What a Waste: A Global Review of Solid Waste Management; World Bank: Washington, DC, USA, 2012. [Google Scholar]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Marine pollution. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Ryberg, M.; Laurent, A.; Hauschild, M. Mapping of Global Plastic Value Chain and Plastics Losses to the Environment. With a Particular Focus on Marine Environment; UNEP: Nairobi, Kenya, 2018. [Google Scholar]
- Mehlhart, G.; Blepp, M. Study on Land-Sources Litter (LSL) in the Marine Environment. Review of Sources and Literature. In the Context of the Initiative of the Declaration of the Global Plastics Associations for Solutions of Marine Litter; Öko-Institut: Freiburg, Germany, 2012; Available online: https://www.kunststoffverpackungen.de/show.php?ID=5262&PHPSESSID=lp8dmvipkks4ajl1htvf5j5907 (accessed on 21 February 2019).
- Macfadyen, G.; Huntington, T.; Cappell, R. Abandoned, Lost or Ortherwise Discarded Fishing Gear; Food and Agriculture Organization of the United Nations: Rome, Italy, 2009; ISBN 978-92-5-106196-1. [Google Scholar]
- Carlton, J.T.; Chapman, J.W.; Geller, J.B.; Miller, J.A.; Carlton, D.A.; McCuller, M.I.; Treneman, N.C.; Steves, B.P.; Ruiz, G.M. Tsunami-driven rafting: Transoceanic species dispersal and implications for marine biogeography. Science 2017, 357, 1402–1406. [Google Scholar] [CrossRef] [Green Version]
- Lebreton, L.C.M.; van der Zwet, J.; Damsteeg, J.-W.; Slat, B.; Andrady, A.; Reisser, J.; Reisser, J. River plastic emissions to the world’s oceans. Nat. Commun. 2017, 8, 15611. [Google Scholar] [CrossRef]
- Ryberg, M.W.; Hauschild, M.Z.; Wang, F.; Averous-Monnery, S.; Laurent, A. Global environmental losses of plastics across their value chains. Resour. Conserv. Recycl. 2019, 151, 104459. [Google Scholar] [CrossRef]
- Novotny, T.E.; Slaughter, E. Tobacco Product Waste: An Environmental Approach to Reduce Tobacco Consumption. Curr. Environ. Health Rep. 2014, 1, 208–216. [Google Scholar] [CrossRef] [Green Version]
- Scarascia-Mugnozza, G.; Sica, C.; Russo, G. Plastic Materials in European Agriculture: Actual Use and Perspectives. J. Agric. Eng. 2011, 42, 15. [Google Scholar] [CrossRef]
- Bos, U.; Makishi, C.; Fischer, M. Life Cycle Assessment of common used agricultural plastic products in the EU. Acta Hortic. 2008, 341–350. [Google Scholar] [CrossRef]
- Eriksen, M.; Lebreton, L.C.M.; Carson, H.S.; Thiel, M.; Moore, C.J.; Borerro, J.C.; Galgani, F.; Ryan, P.G.; Reisser, J. Plastic Pollution in the World’s Oceans: More than 5 Trillion Plastic Pieces Weighing over 250,000 Tons Afloat at Sea. PLoS ONE 2014, 9, e111913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koelmans, A.A.; Kooi, M.; Law, K.L.; van Sebille, E. All is not lost: Deriving a top-down mass budget of plastic at sea. Environ. Res. Lett. 2017, 12, 114028. [Google Scholar] [CrossRef] [Green Version]
- Turrell, W.R. Estimating a regional budget of marine plastic litter in order to advise on marine management measures. Mar. Pollut. Bull. 2020, 150, 110725. [Google Scholar] [CrossRef] [PubMed]
- Nihei, Y.; Yoshida, T.; Kataoka, T.; Ogata, R. High-Resolution Mapping of Japanese Microplastic and Macroplastic Emissions from the Land into the Sea. Water 2020, 12, 951. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, C.; Krauth, T.; Wagner, S. Export of Plastic Debris by Rivers into the Sea. Environ. Sci. Technol. 2017, 51. [Google Scholar] [CrossRef]
- Europäische Kommission. Vorschlag für eine Richtlinie des Europäischen Parlaments und des Rates über die Verringerung der Auswirkungen Bestimmter Kunststoffprodukte auf die Umwelt; COM(2018) 340 Final; Europäische Kommission: Brussels, Belgium, 2018. [Google Scholar]
- Bertling, J.; Hamann, L.; Bertling, R. Kunststoffe in der Umwelt; Fraunhofer UMSICHT: Oberhausen, Germany, 2018. [Google Scholar]
- Slaughter, E.; Gersberg, R.M.; Watanabe, K.; Rudolph, J.; Stransky, C.; Novotny, T.E. Toxicity of cigarette butts, and their chemical components, to marine and freshwater fish. Tob. Control. 2011, 20, i25–i29. [Google Scholar] [CrossRef]
- Patrício Silva, A.L.; Prata, J.C.; Walker, T.R.; Duarte, A.C.; Ouyang, W.; Barcelò, D.; Rocha-Santos, T. Increased plastic pollution due to Covid-19 pandemic: Challenges and recommendations. Chem. Eng. J. 2020, 405, 126683. [Google Scholar] [CrossRef]
- Canning-Clode, J.; Sepúlveda, P.; Almeida, S.; Monteiro, J. Will COVID-19 Containment and Treatment Measures Drive Shifts in Marine Litter Pollution? Front. Mar. Sci. 2020, 7, 125708. [Google Scholar] [CrossRef]
- Liu, E.K.; He, W.Q.; Yan, C.R. ‘White revolution’ to ‘white pollution’—Agricultural plastic film mulch in China. Environ. Res. Lett. 2014, 9, 91001. [Google Scholar] [CrossRef] [Green Version]
- Bläsing, M.; Amelung, W. Plastics in soil: Analytical methods and possible sources. Sci. Total Environ. 2018, 612, 422–435. [Google Scholar] [CrossRef]
- Carr, S.A.; Liu, J.; Tesoro, A.G. Transport and fate of microplastic particles in wastewater treatment plants. Water Res. 2016, 91, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Talvitie, J.; Heinonen, M.; Pääkkönen, J.-P.; Vahtera, E.; Mikola, A.; Setälä, O.; Vahala, R. Do wastewater treatment plants act as a potential point source of microplastics? Preliminary study in the coastal Gulf of Finland, Baltic Sea. Water Sci. Technol. 2015, 72, 1495–1504. [Google Scholar] [CrossRef] [PubMed]
- Lechthaler, S.; Dolny, R.; Spelthahn, V.; Pinnekamp, J.; Linnemann, V. Sampling concept for microplastics in combined sewage-affected freshwater and freshwater sediments. Fundam. Appl. Limnol. 2019. [Google Scholar] [CrossRef]
- He, P.; Chen, L.; Shao, L.; Zhang, H.; Lü, F. Municipal solid waste (MSW) landfill: A source of microplastics? —Evidence of microplastics in landfill leachate. Water Res. 2019, 159, 38–45. [Google Scholar] [CrossRef]
- Kazour, M.; Terki, S.; Rabhi, K.; Jemaa, S.; Khalaf, G.; Amara, R. Sources of microplastics pollution in the marine environment: Importance of wastewater treatment plant and coastal landfill. Mar. Pollut. Bull. 2019, 146, 608–618. [Google Scholar] [CrossRef]
- Eurostat. Behandlung der Abfälle nach Abfallkategorie, Gefährlichkeit und Abfallbewirtschaftungsmaßnahmen: Deponierung Kunststoffabfälle. Available online: https://ec.europa.eu/eurostat/de/web/waste/data/database (accessed on 25 February 2020).
- Good, T.P.; June, J.A.; Etnier, M.A.; Broadhurst, G. Derelict fishing nets in Puget Sound and the Northwest Straits: Patterns and threats to marine fauna. Mar. Pollut. Bull. 2010, 60, 39–50. [Google Scholar] [CrossRef]
- Link, J.; Segal, B.; Casarini, L.M. Abandoned, lost or otherwise discarded fishing gear in Brazil: A review. Perspect. Ecol. Conserv. 2019, 17, 1–8. [Google Scholar] [CrossRef]
- 7Krüger, L.; Casado-Coy, N.; Valle, C.; Ramos, M.; Sánchez-Jerez, P.; Gago, J.; Carretero, O.; Beltran-Sanahuja, A.; Sanz-Lazaro, C. Plastic debris accumulation in the seabed derived from coastal fish farming. Environ. Pollut. 2020, 257, 113336. [Google Scholar] [CrossRef]
- Lebreton, L.; Slat, B.; Ferrari, F.; Sainte-Rose, B.; Aitken, J.; Marthouse, R.; Hajbane, S.; Cunsolo, S.; Schwarz, A.; Levivier, A.; et al. Evidence that the Great Pacific Garbage Patch is rapidly accumulating plastic. Sci. Rep. 2018, 8, 4666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Rivera, S.; Lizaso, J.L.S.; Millán, J.M.B. Composition, spatial distribution and sources of macro-marine litter on the Gulf of Alicante seafloor (Spanish Mediterranean). Mar. Pollut. Bull. 2017, 121, 249–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebbesmeyer, C.C.; Ingraham, W.J. Pacific toy spill fuels ocean current pathways research. Eos Trans. AGU 1994, 75, 425. [Google Scholar] [CrossRef]
- Panama Maritime Authority; Dutch Safety Board; Bundesstelle für Seeunfalluntersuchung. Joint Investigation Report: Loss of Containers Overboard from MSC Zoe. 1–2 January 2019; PMA: Panama City, Panama; DSB: The Hague, The Netherlands; BSU: Hamburg, Germany, 2020. [Google Scholar]
- IMO. Resolution MEPC.36(28) Marine Environment Protection Committee Adoption of Amendments to the Annex of the Protocol of 1987 Relating to the International Convention for the Prevention of Pollution from Ships (Amendment to Annex V of MARPOL 73/78); IMO: London, UK, 1989. [Google Scholar]
- Monteiro, R.C.P.; do Ivar Sul, J.A.; Costa, M.F. Plastic pollution in islands of the Atlantic Ocean. Environ. Pollut. 2018, 238, 103–110. [Google Scholar] [CrossRef]
- Herz, M.; Davis, J. Cruise Control. A Report on How Cruise Ships Affect. the Marine Environment; The Ocean Conservancy: Washington, DC, USA, 2002. [Google Scholar]
- Meyer, T. Ökologie Mitteleuropäischer Flussauen; Spektrum Akademischer: Berlin/Heidelberg, Germany, 2017; ISBN 978-3-662-55454-8. [Google Scholar]
- Schwarz, A.E.; Ligthart, T.N.; Boukris, E.; van Harmelen, T. Sources, transport, and accumulation of different types of plastic litter in aquatic environments: A review study. Mar. Pollut. Bull. 2019, 143, 92–100. [Google Scholar] [CrossRef]
- Ballent, A.; Pando, S.; Purser, A.; Juliano, M.F.; Thomsen, L. Modelled transport of benthic marine microplastic pollution in the Nazaré Canyon. Biogeosciences 2013, 10, 7957–7970. [Google Scholar] [CrossRef] [Green Version]
- Van Emmerik, T.; Strady, E.; Kieu-Le, T.-C.; Nguyen, L.; Gratiot, N. Seasonality of riverine macroplastic transport. Sci Rep. 2019, 9, 13549. [Google Scholar] [CrossRef]
- Hurley, R.; Woodward, J.; Rothwell, J.J. Microplastic contamination of river beds significantly reduced by catchment-wide flooding. Nat. Geosci. 2018, 10, 124006. [Google Scholar] [CrossRef]
- Kurniawan, S.B.; Imron, M.F. Seasonal variation of plastic debris accumulation in the estuary of Wonorejo River, Surabaya, Indonesia. Environ. Technol. Innov. 2019, 16, 100490. [Google Scholar] [CrossRef]
- Castrop, E.; van Emmerik, T.; van den Berg, S.; Kosten, S.; Strady, E.; Kieu-Le, T.-C. Plants, plastic and rivers: Do water hyacinths play a role in riverine macroplastic transport? In Proceedings of the 22nd EGU General Assembly, Lund, Sweden, 4–8 May 2020. [Google Scholar]
- Driedger, A.G.J.; Dürr, H.H.; Mitchell, K.; van Cappellen, P. Plastic debris in the Laurentian Great Lakes: A review. J. Great Lakes Res. 2015, 41, 9–19. [Google Scholar] [CrossRef]
- Ghaffari, S.; Bakhtiari, A.R.; Ghasempouri, S.M.; Nasrolahi, A. The influence of human activity and morphological characteristics of beaches on plastic debris distribution along the Caspian Sea as a closed water body. Environ. Sci. Pollut. Res. Int. 2019, 26, 25712–25724. [Google Scholar] [CrossRef]
- Chen, X.; Xiong, X.; Jiang, X.; Shi, H.; Wu, C. Sinking of floating plastic debris caused by biofilm development in a freshwater lake. Chemosphere 2019, 222, 856–864. [Google Scholar] [CrossRef] [PubMed]
- Tramoy, R.; Gasperi, J.; Colasse, L.; Tassin, B. Transfer dynamic of macroplastics in estuaries—New insights from the Seine estuary: Part 1. Long term dynamic based on date-prints on stranded debris. Mar. Pollut. Bull. 2020, 152, 110894. [Google Scholar] [CrossRef] [PubMed]
- Ryan, P.G. The transport and fate of marine plastics in South Africa and adjacent oceans. S. Afr. J. Sci 2020, 116. [Google Scholar] [CrossRef]
- Van Sebille, E.; Aliani, S.; Law, K.L.; Maximenko, N.; Alsina, J.; Bagaev, A.; Bergmann, M.; Chapron, B.; Chubarenko, I.; Cózar, A.; et al. The physical oceanography of the transport of floating marine debris. Environ. Res. Lett. 2020, 15. [Google Scholar] [CrossRef] [Green Version]
- Isobe, A.; Kubo, K.; Tamura, Y.; Kako, S.; Nakashima, E.; Fujii, N. Selective transport of microplastics and mesoplastics by drifting in coastal waters. Mar. Pollut. Bull. 2014, 89, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, M.; Maximenko, N.; Thiel, M.; Cummins, A.; Lattin, G.; Wilson, S.; Hafner, J.; Zellers, A.; Rifman, S. Plastic pollution in the South Pacific subtropical gyre. Mar. Pollut. Bull. 2013, 68, 71–76. [Google Scholar] [CrossRef]
- Poulain, M.; Mercier, M.J.; Brach, L.; Martignac, M.; Routaboul, C.; Perez, E.; Desjean, M.C.; Ter Halle, A. Small Microplastics As a Main Contributor to Plastic Mass Balance in the North Atlantic Subtropical Gyre. Environ. Sci. Technol. 2019, 53, 1157–1164. [Google Scholar] [CrossRef] [Green Version]
- Van Sebille, E.; England, M.H.; Froyland, G. Origin, dynamics and evolution of ocean garbage patches from observed surface drifters. Environ. Res. Lett. 2012, 7, 44040. [Google Scholar] [CrossRef]
- Fazey, F.M.C.; Ryan, P.G. Biofouling on buoyant marine plastics: An experimental study into the effect of size on surface longevity. Environ. Pollut. 2016, 210, 354–360. [Google Scholar] [CrossRef]
- Ryan, P.G. Does size and buoyancy affect the long-distance transport of floating debris? Environ. Res. Lett. 2015, 10, 84019. [Google Scholar] [CrossRef]
- Kooi, M.; van Nes, E.H.; Scheffer, M.; Koelmans, A.A. Ups and Downs in the Ocean: Effects of Biofouling on Vertical Transport of Microplastics. Environ. Sci. Technol. 2017, 51, 7963–7971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, S.; Andrady, A.L. Fouling of floating plastic debris under Biscayne Bay exposure conditions. Mar. Pollut. Bull. 1991, 22, 608–613. [Google Scholar] [CrossRef]
- Pohl, F.; Eggenhuisen, J.T.; Kane, I.A.; Clare, M.A. Transport and Burial of Microplastics in Deep-Marine Sediments by Turbidity Currents. Environ. Sci. Technol. 2020, 54, 4180–4189. [Google Scholar] [CrossRef] [PubMed]
- Kane, I.A.; Clare, M.A.; Miramontes, E.; Wogelius, R.; Rothwell, J.J.; Garreau, P.; Pohl, F. Seafloor microplastic hotspots controlled by deep-sea circulation. Science 2020, 368. [Google Scholar] [CrossRef]
- Browne, M.A.; Crump, P.; Niven, S.J.; Teuten, E.; Tonkin, A.; Galloway, T.; Thompson, R. Accumulation of microplastic on shorelines woldwide: Sources and sinks. Environ. Sci. Technol. 2011, 45, 9175–9179. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.; Park, Y.-G. Destination of floating plastic debris released from ten major rivers around the Korean Peninsula. Environ. Int. 2020, 138, 105655. [Google Scholar] [CrossRef]
- Collins, C.; Hermes, J.C. Modelling the accumulation and transport of floating marine micro-plastics around South Africa. Mar. Pollut. Bull. 2019, 139, 46–58. [Google Scholar] [CrossRef]
- Ho, N.H.E.; Not, C. Selective accumulation of plastic debris at the breaking wave area of coastal waters. Environ. Pollut. 2019, 245, 702–710. [Google Scholar] [CrossRef]
- Lee, J.; Hong, S.; Song, Y.K.; Hong, S.H.; Jang, Y.C.; Jang, M.; Heo, N.W.; Han, G.M.; Lee, M.J.; Kang, D.; et al. Relationships among the abundances of plastic debris in different size classes on beaches in South Korea. Mar. Pollut. Bull. 2013, 77, 349–354. [Google Scholar] [CrossRef]
- Kataoka, T.; Hinata, H.; Kato, S. Analysis of a beach as a time-invariant linear input/output system of marine litter. Mar. Pollut. Bull. 2013, 77, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, T.; Hinata, H.; Kato, S. Backwash process of marine macroplastics from a beach by nearshore currents around a submerged breakwater. Mar. Pollut. Bull. 2015, 101, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Hinata, H.; Mori, K.; Ohno, K.; Miyao, Y.; Kataoka, T. An estimation of the average residence times and onshore-offshore diffusivities of beached microplastics based on the population decay of tagged meso- and macrolitter. Mar. Pollut. Bull. 2017, 122, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Bowman, D.; Manor-Samsonov, N.; Golik, A. Dynamics of litter pollution on Israeli Mediterranean beaches: A budgetary, litter flux approach. J. Coast. Res. 1998, 14, 418–432. [Google Scholar]
- Garrity, S.D.; Levings, S.C. Marine debris along the Caribbean coast of Panama. Mar. Pollut. Bull. 1993, 26, 317–324. [Google Scholar] [CrossRef]
- Turrell, W.R. A simple model of wind-blown tidal strandlines: How marine litter is deposited on a mid-latitude, macro-tidal shelf sea beach. Mar. Pollut. Bull. 2018, 137, 315–330. [Google Scholar] [CrossRef]
- Lechner, A.; Keckeis, H.; Lumesberger-Loisl, F.; Zens, B.; Krusch, R.; Tritthart, M.; Glas, M.; Schludermann, E. The Danube so colourful: A potpourri of plastic litter outnumbers fish larvae in Europe’s second largest river. Environ. Pollut. 2014, 188, 177–181. [Google Scholar] [CrossRef] [Green Version]
- Critchell, K.; Lambrechts, J. Modelling accumulation of marine plastics in the coastal zone; what are the dominant physical processes? Estuar. Coast. Shelf Sci. 2016, 171, 111–122. [Google Scholar] [CrossRef]
- Sadri, S.S.; Thompson, R.C. On the quantity and composition of floating plastic debris entering and leaving the Tamar Estuary, Southwest England. Mar. Pollut. Bull. 2014, 81, 55–60. [Google Scholar] [CrossRef]
- Vermeiren, P.; Muñoz, C.C.; Ikejima, K. Sources and sinks of plastic debris in estuaries: A conceptual model integrating biological, physical and chemical distribution mechanisms. Mar. Pollut. Bull. 2016, 113, 7–16. [Google Scholar] [CrossRef]
- Cozzolino, L.; Nicastro, K.R.; Zardi, G.I.; Los Santos, C.B. Species-specific plastic accumulation in the sediment and canopy of coastal vegetated habitats. Sci. Total Environ. 2020, 723, 138018. [Google Scholar] [CrossRef] [PubMed]
- Allen, S.; Allen, D.; Phoenix, V.R.; Le Roux, G.; Durántez Jiménez, P.; Simonneau, A.; Binet, S.; Galop, D. Atmospheric transport and deposition of microplastics in a remote mountain catchment. Nat. Geosci. 2019, 71, 299. [Google Scholar] [CrossRef]
- Zylstra, E.R. Accumulation of wind-dispersed trash in desert environments. J. Arid Environ. 2013, 89, 13–15. [Google Scholar] [CrossRef]
- van Calcar, C.J.; van Emmerik, T.H.M. Abundance of plastic debris across European and Asian rivers. Environ. Res. Lett. 2019, 14, 124051. [Google Scholar] [CrossRef] [Green Version]
- Crosti, R.; Arcangeli, A.; Campana, I.; Paraboschi, M.; González-Fernández, D. ‘Down to the river’: Amount, composition, and economic sector of litter entering the marine compartment, through the Tiber river in the Western Mediterranean Sea. Rend. Lincei Sci. Fis. Nat. 2018, 29, 859–866. [Google Scholar] [CrossRef]
- Castro-Jiménez, J.; González-Fernández, D.; Fornier, M.; Schmidt, N.; Sempéré, R. Macro-litter in surface waters from the Rhone River: Plastic pollution and loading to the NW Mediterranean Sea. Mar. Pollut. Bull. 2019, 146, 60–66. [Google Scholar] [CrossRef]
- Gasperi, J.; Dris, R.; Bonin, T.; Rocher, V.; Tassin, B. Assessment of floating plastic debris in surface water along the Seine River. Environ. Pollut. 2014, 195, 163–166. [Google Scholar] [CrossRef] [Green Version]
- Van der Wal, M.; van der Meulen, M.; Tweehuijsen, G.; Peterlin, M.; Palatinus, A.; Kovač Viršek, M.; Coscia, L.; Kržan, A. SFRA0025: Identification and Assessment of Riverine Input of (Marine) Litter; Final Report for the European Commission DG Environment under Framework Contract No. ENV.D.2/FRA/2012/0025; Eunomia Research & Consulting: Bristol, UK, 2015. [Google Scholar]
- Schöneich-Argent, R.I.; Dau, K.; Freund, H. Wasting the North Sea—A field-based assessment of anthropogenic macrolitter loads and emission rates of three German tributaries. Environ. Pollut. 2020, 263, 114367. [Google Scholar] [CrossRef]
- Schirinzi, G.F.; Köck-Schulmeyer, M.; Cabrera, M.; González-Fernández, D.; Hanke, G.; Farré, M.; Barceló, D. Riverine anthropogenic litter load to the Mediterranean Sea near the metropolitan area of Barcelona, Spain. Sci. Total Environ. 2020, 714, 136807. [Google Scholar] [CrossRef]
- Van Emmerik, T.; Loozen, M.; van Oeveren, K.; Buschman, F.; Prinsen, G. Riverine plastic emission from Jakarta into the ocean. Environ. Res. Lett. 2019, 14, 84033. [Google Scholar] [CrossRef] [Green Version]
- Ebere, E.C.; Wirnkor, V.A.; Ngozi, V.E.; Chukwuemeka, I.S. Macrodebris and microplastics pollution in Nigeria: First report on abundance, distribution and composition. Environ. Anal. Health Toxicol. 2019, 34, e2019012. [Google Scholar] [CrossRef] [Green Version]
- Blettler, M.C.M.; Garello, N.; Ginon, L.; Abrial, E.; Espinola, L.A.; Wantzen, K.M. Massive plastic pollution in a mega-river of a developing country: Sediment deposition and ingestion by fish (Prochilodus lineatus). Environ. Pollut. 2019, 255, 113348. [Google Scholar] [CrossRef] [PubMed]
- Faure, F.; Demars, C.; Wieser, O.; Kunz, M.; Alencastro, L.F. Plastic pollution in Swiss surface waters: Nature and concentrations, interaction with pollutants. Environ. Chem. 2015, 12, 582. [Google Scholar] [CrossRef]
- Boucher, J.; Faure, F.; Pompini, O.; Plummer, Z.; Wieser, O.; Felippe de Alencastro, L. (Micro) plastic fluxes and stocks in Lake Geneva basin. TrAC Trends Anal. Chem. 2019, 112, 66–74. [Google Scholar] [CrossRef]
- Corcoran, P.L.; Norris, T.; Ceccanese, T.; Walzak, M.J.; Helm, P.A.; Marvin, C.H. Hidden plastics of Lake Ontario, Canada and their potential preservation in the sediment record. Environ. Pollut. 2015, 204, 17–25. [Google Scholar] [CrossRef]
- Egessa, R.; Nankabirwa, A.; Basooma, R.; Nabwire, R. Occurrence, distribution and size relationships of plastic debris along shores and sediment of northern Lake Victoria. Environ. Pollut. 2020, 257, 113442. [Google Scholar] [CrossRef]
- Browne, M.A.; Galloway, T.S.; Thompson, R.C. Spatial patterns of plastic debris along Estuarine shorelines. Environ. Sci. Technol. 2010, 44, 3404–3409. [Google Scholar] [CrossRef]
- Yao, W.; Di, D.; Wang, Z.; Liao, Z.; Huang, H.; Mei, K.; Dahlgren, R.A.; Zhang, M.; Shang, X. Micro- and macroplastic accumulation in a newly formed Spartina alterniflora colonized estuarine saltmarsh in southeast China. Mar. Pollut. Bull. 2019, 149, 110636. [Google Scholar] [CrossRef] [Green Version]
- Carpenter, E.J.; Smith, K.L. Plastics on the Sargasso sea surface. Science 1972, 175, 1240–1241. [Google Scholar] [CrossRef]
- Colton, J.B.; Burns, B.R.; Knapp, F.D. Plastic particles in surface waters of the northwestern atlantic. Science 1974, 185, 491–497. [Google Scholar] [CrossRef]
- Suaria, G.; Aliani, S. Floating debris in the Mediterranean Sea. Mar. Pollut. Bull. 2014, 86, 494–504. [Google Scholar] [CrossRef]
- Galgani, F.; Hanke, G.; Maes, T. Global Distribution, Composition and Abundance of Marine Litter. In Marine Anthropogenic Litter; Bergmann, M., Gutow, L., Klages, M., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 29–56. ISBN 978-3-319-16509-7. [Google Scholar]
- Galgani, F.; Leaute, J.P.; Moguedet, P.; Souplet, A.; Verin, Y.; Carpentier, A.; Goraguer, H.; Latrouite, D.; Andral, B.; Cadiou, Y.; et al. Litter on the Sea Floor Along European Coasts. Mar. Pollut. Bull. 2000, 40, 516–527. [Google Scholar] [CrossRef]
- Thiel, S.; Thomé-Kozmiensky, E.; Quicker, P.; Gosten, A. (Eds.) Energie aus Abfall; Thomé-Kozmiensky: Neuruppin, Germany, 2018; ISBN 978-3-944310-39-8. [Google Scholar]
- Rech, S.; Thiel, M.; Borrell Pichs, Y.J.; García-Vazquez, E. Travelling light: Fouling biota on macroplastics arriving on beaches of remote Rapa Nui (Easter Island) in the South Pacific Subtropical Gyre. Mar. Pollut. Bull. 2018, 137, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Suaria, G.; Perold, V.; Lee, J.R.; Lebouard, F.; Aliani, S.; Ryan, P.G. Floating macro- and microplastics around the Southern Ocean: Results from the Antarctic Circumnavigation Expedition. Environ. Int. 2020, 136, 105494. [Google Scholar] [CrossRef]
- Gündoğdu, S.; Çevik, C. Mediterranean dirty edge: High level of meso and macroplastics pollution on the Turkish coast. Environ. Pollut. 2019, 255, 113351. [Google Scholar] [CrossRef] [PubMed]
- European Commission. The 2018 Annual Economic Report on the EU Blue Economy; Publications Office of the EU: Luxembourg, 2018; ISBN 978-92-79-81757-1. [Google Scholar]
- Gündoğdu, S.; Yeşilyurt, İ.N.; Erbaş, C. Potential interaction between plastic litter and green turtle Chelonia mydas during nesting in an extremely polluted beach. Mar. Pollut. Bull. 2019, 140, 138–145. [Google Scholar] [CrossRef]
- Portman, M.E.; Brennan, R.E. Marine litter from beach-based sources: Case study of an Eastern Mediterranean coastal town. Waste Manag. 2017, 69, 535–544. [Google Scholar] [CrossRef] [Green Version]
- Mansui, J.; Molcard, A.; Ourmières, Y. Modelling the transport and accumulation of floating marine debris in the Mediterranean basin. Mar. Pollut. Bull. 2015, 91, 249–257. [Google Scholar] [CrossRef]
- Gómez, V.; Pozo, K.; Nuñez, D.; Přibylová, P.; Audy, O.; Baini, M.; Fossi, M.C.; Klánová, J. Marine plastic debris in Central Chile: Characterization and abundance of macroplastics and burden of persistent organic pollutants (POPs). Mar. Pollut. Bull. 2020, 152, 110881. [Google Scholar] [CrossRef]
- Jeyasanta, K.I.; Sathish, N.; Patterson, J.; Edward, J.K.P. Macro-, meso- and microplastic debris in the beaches of Tuticorin district, Southeast coast of India. Mar. Pollut. Bull. 2020, 154, 111055. [Google Scholar] [CrossRef]
- Maharana, D.; Saha, M.; Dar, J.Y.; Rathore, C.; Sreepada, R.A.; Xu, X.-R.; Koongolla, J.B.; Li, H.-X. Assessment of micro and macroplastics along the west coast of India: Abundance, distribution, polymer type and toxicity. Chemosphere 2020, 246, 125708. [Google Scholar] [CrossRef] [PubMed]
- Isyrini, R.; La Nafie, Y.A.; Ukkas, M.; Rachim, R.; Cordova, M.R. Marine Macro Debris from Makassar Strait Beaches with Three Different Designations. IOP Conf. Ser. Earth Environ. Sci. 2019, 253, 12039. [Google Scholar] [CrossRef]
- Vlachogianni, T.; Fortibuoni, T.; Ronchi, F.; Zeri, C.; Mazziotti, C.; Tutman, P.; Varezić, D.B.; Palatinus, A.; Trdan, Š.; Peterlin, M.; et al. Marine litter on the beaches of the Adriatic and Ionian Seas: An assessment of their abundance, composition and sources. Mar. Pollut. Bull. 2018, 131, 745–756. [Google Scholar] [CrossRef]
- Aydin, C.; Guven, O.; Salihoglu, B.; Kideys, A.E. The Influence of Land Use on Coastal Litter: An Approach to Identify Abundance and Source in the Coastal Area of Cilician Basin, Turkey. Turk. J. Fish. Aquat. Sci. 2016, 16. [Google Scholar] [CrossRef]
- Laglbauer, B.J.L.; Franco-Santos, R.M.; Andreu-Cazenave, M.; Brunelli, L.; Papadatou, M.; Palatinus, A.; Grego, M.; Deprez, T. Macrodebris and microplastics from beaches in Slovenia. Mar. Pollut. Bull. 2014, 89, 356–366. [Google Scholar] [CrossRef] [Green Version]
- Prevenios, M.; Zeri, C.; Tsangaris, C.; Liubartseva, S.; Fakiris, E.; Papatheodorou, G. Beach litter dynamics on Mediterranean coasts: Distinguishing sources and pathways. Mar. Pollut. Bull. 2018, 129, 448–457. [Google Scholar] [CrossRef]
- Munari, C.; Corbau, C.; Simeoni, U.; Mistri, M. Marine litter on Mediterranean shores: Analysis of composition, spatial distribution and sources in north-western Adriatic beaches. Waste Manag. 2016, 49, 483–490. [Google Scholar] [CrossRef]
- Pasternak, G.; Zviely, D.; Ribic, C.A.; Ariel, A.; Spanier, E. Sources, composition and spatial distribution of marine debris along the Mediterranean coast of Israel. Mar. Pollut. Bull. 2017, 114, 1036–1045. [Google Scholar] [CrossRef]
- Andrady, A. Persistence of Plastic Litter in the Oceans. In Marine Anthropogenic Litter; Bergmann, M., Gutow, L., Klages, M., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 57–72. ISBN 978-3-319-16509-7. [Google Scholar]
- JAMSTEC. Japan Agency for Marine-Earth Science and Technology. Available online: http://www.godac.jamstec.go.jp/catalog/dsdebris/e/index.html (accessed on 16 January 2020).
- Galgani, F.; Souplet, A.; Cadiou, Y. Accumulation of debris on the deep sea floor off the French Mediterranean coast. Mar. Ecol. Prog. Ser. 1996, 142, 225–234. [Google Scholar] [CrossRef] [Green Version]
- Pham, C.K.; Ramirez-Llodra, E.; Alt, C.H.S.; Amaro, T.; Bergmann, M.; Canals, M.; Company, J.B.; Davies, J.; Duineveld, G.; Galgani, F.; et al. Marine litter distribution and density in European seas, from the shelves to deep basins. PLoS ONE 2014, 9, e95839. [Google Scholar] [CrossRef] [Green Version]
- Chiba, S.; Saito, H.; Fletcher, R.; Yogi, T.; Kayo, M.; Miyagi, S.; Ogido, M.; Fujikura, K. Human footprint in the abyss: 30 year records of deep-sea plastic debris. Mar. Policy 2018, 96, 204–212. [Google Scholar] [CrossRef]
- Consoli, P.; Scotti, G.; Romeo, T.; Fossi, M.C.; Esposito, V.; D’Alessandro, M.; Battaglia, P.; Galgani, F.; Figurella, F.; Pragnell-Raasch, H.; et al. Characterization of seafloor litter on Mediterranean shallow coastal waters: Evidence from Dive Against Debris®, a citizen science monitoring approach. Mar. Pollut. Bull. 2020, 150, 110763. [Google Scholar] [CrossRef]
- Barnes, D.K.A.; Walters, A.; Gonçalves, L. Macroplastics at sea around Antarctica. Mar. Environ. Res. 2010, 70, 250–252. [Google Scholar] [CrossRef]
- Huerta Lwanga, E.; Mendoza Vega, J.; Ku Quej, V.; Chi, J.d.L.A.; Sanchez Del Cid, L.; Chi, C.; Escalona Segura, G.; Gertsen, H.; Salánki, T.; van der Ploeg, M.; et al. Field evidence for transfer of plastic debris along a terrestrial food chain. Sci. Rep. 2017, 7, 14071. [Google Scholar] [CrossRef]
- Basnet, K. Solid waste pollution versus sustainable development in high mountain environment: A case study of Sagarmatha National Park of Khumbu Region, Nepal. Contrib. Nepal Stud. 1993, 20, 131–139. [Google Scholar]
- Corcoran, P.L.; Moore, C.J.; Jazvac, K. An anthropogenic marker horizon in the future rock record. GSAT 2014, 24, 4–8. [Google Scholar] [CrossRef]
- Gestoso, I.; Cacabelos, E.; Ramalhosa, P.; Canning-Clode, J. Plasticrusts: A new potential threat in the Anthropocene’s rocky shores. Sci. Total Environ. 2019, 687, 413–415. [Google Scholar] [CrossRef]
- Ehlers, S.M.; Ellrich, J.A. First record of ‘plasticrusts’ and ‘pyroplastic’ from the Mediterranean Sea. Mar. Pollut. Bull. 2020, 151, 110845. [Google Scholar] [CrossRef]
- Egger, M.; Sulu-Gambari, F.; Lebreton, L. First evidence of plastic fallout from the North Pacific Garbage Patch. Sci. Rep. 2020, 10, 7495. [Google Scholar] [CrossRef]
- Bergmann, M.; Klages, M. Increase of litter at the Arctic deep-sea observatory HAUSGARTEN. Mar. Pollut. Bull. 2012, 64, 2734–2741. [Google Scholar] [CrossRef]
- Gall, S.C.; Thompson, R.C. The impact of debris on marine life. Mar. Pollut. Bull. 2015, 92, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Kenyon, K.W.; Kridler, E. Laysan Albatrosses swallow indigestible matter. Auk 1969, 86, 339–343. [Google Scholar] [CrossRef]
- Patterson Edward, J.K.; Mathews, G.; Raj, K.D.; Laju, R.L.; Bharath, M.S.; Kumar, P.D.; Arasamuthu, A.; Grimsditch, G. Marine debris—An emerging threat to the reef areas of Gulf of Mannar, India. Mar. Pollut. Bull. 2020, 151, 110793. [Google Scholar] [CrossRef]
- Kühn, S.; Bravo Rebolledo, E.L.; van Franeker, J.A. Deleterious Effects of Litter on Marine Life. In Marine Anthropogenic Litter; Bergmann, M., Gutow, L., Klages, M., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 75–116. ISBN 978-3-319-16509-7. [Google Scholar]
- Schuyler, Q.; Hardesty, B.D.; Wilcox, C.; Townsend, K. Global analysis of anthropogenic debris ingestion by sea turtles. Conserv. Biol. 2014, 28, 129–139. [Google Scholar] [CrossRef]
- Hyrenbach, K.D.; Hester, M.; Adams, J.; Titmus, A.; Michael, P.; Wahl, T.; Chang, C.-W.; Marie, A.; Vanderlip, C. Plastic ingestion by black-footed albatross phoebastria nigripes from kure atoll, Hawai’i: Linking chick diet remains and parental at-sea foraging distribution. Mar. Ornithol. 2017, 45, 225–236. [Google Scholar]
- Woods, J.S.; Rødder, G.; Verones, F. An effect factor approach for quantifying the entanglement impact on marine species of macroplastic debris within life cycle impact assessment. Ecol. Indic. 2019, 99, 61–66. [Google Scholar] [CrossRef]
- Naidoo, T.; Rajkaran, A. Impacts of plastic debris on biota and implications for human health: A South African perspective. S. Afr. J. Sci 2020, 116. [Google Scholar] [CrossRef]
- Rios, L.M.; Moore, C.; Jones, P.R. Persistent organic pollutants carried by synthetic polymers in the ocean environment. Mar. Pollut. Bull. 2007, 54, 1230–1237. [Google Scholar] [CrossRef]
- Rios, L.M.; Jones, P.R.; Moore, C.; Narayan, U.V. Quantitation of persistent organic pollutants adsorbed on plastic debris from the Northern Pacific Gyre’s “eastern garbage patch”. J. Environ. Monit. 2010, 12, 2226–2236. [Google Scholar] [CrossRef]
- Koelmans, A.A.; Besseling, E.; Foekema, E.M. Leaching of plastic additives to marine organisms. Environ. Pollut. 2014, 187, 49–54. [Google Scholar] [CrossRef]
- Hahladakis, J.N.; Velis, C.A.; Weber, R.; Iacovidou, E.; Purnell, P. An overview of chemical additives present in plastics: Migration, release, fate and environmental impact during their use, disposal and recycling. J. Hazard. Mater. 2018, 344, 179–199. [Google Scholar] [CrossRef]
- Steinmetz, Z.; Wollmann, C.; Schaefer, M.; Buchmann, C.; David, J.; Tröger, J.; Muñoz, K.; Frör, O.; Schaumann, G.E. Plastic mulching in agriculture. Trading short-term agronomic benefits for long-term soil degradation? Sci. Total Environ. 2016, 550, 690–705. [Google Scholar] [CrossRef] [PubMed]
- Araújo, M.C.B.; Costa, M.F. A critical review of the issue of cigarette butt pollution in coastal environments. Environ. Res. 2019, 172, 137–149. [Google Scholar] [CrossRef]
- Kurmus, H.; Mohajerani, A. The toxicity and valorization options of cigarette butts. Waste Manag. 2020, 104, 104–118. [Google Scholar] [CrossRef]
- Kalogerakis, N.; Karkanorachaki, K.; Kalogerakis, G.C.; Triantafyllidi, E.I.; Gotsis, A.D.; Partsinevelos, P.; Fava, F. Microplastics Generation: Onset of Fragmentation of Polyethylene Films in Marine Environment Mesocosms. Front. Mar. Sci. 2017, 4, 84. [Google Scholar] [CrossRef]
- Fath, A. Mikroplastik. Eine Drohende Gefahr? 1st ed.; Springer: Berlin/Heidelberg, Germany, 2019; ISBN 978-3-662-57851-3. [Google Scholar]
- Masó, M.; Garcés, E.; Pagès, F.; Camp, J. Drifting plastic debris as a potential vector for dispersing Harmful Algal Bloom (HAB) species. Sci. Mar. 2003, 67, 107–111. [Google Scholar] [CrossRef] [Green Version]
- Qi, Y.; Yang, X.; Pelaez, A.M.; Huerta Lwanga, E.; Beriot, N.; Gertsen, H.; Garbeva, P.; Geissen, V. Macro- and micro- plastics in soil-plant system: Effects of plastic mulch film residues on wheat (Triticum aestivum) growth. Sci. Total Environ. 2018, 645, 1048–1056. [Google Scholar] [CrossRef] [PubMed]
- Royer, S.-J.; Ferrón, S.; Wilson, S.T.; Karl, D.M. Production of methane and ethylene from plastic in the environment. PLoS ONE 2018, 13, e0200574. [Google Scholar] [CrossRef]
- Beaumont, N.J.; Aanesen, M.; Austen, M.C.; Börger, T.; Clark, J.R.; Cole, M.; Hooper, T.; Lindeque, P.K.; Pascoe, C.; Wyles, K.J. Global ecological, social and economic impacts of marine plastic. Mar. Pollut. Bull. 2019, 142, 189–195. [Google Scholar] [CrossRef]
- Gregory, M. Plastics and South Pacific Island shores: Environmental implications. Ocean Coast. Manag. 1999, 42, 603–615. [Google Scholar] [CrossRef]
- Hardesty, B.D.; Harari, J.; Isobe, A.; Lebreton, L.; Maximenko, N.; Potemra, J.; van Sebille, E.; Vethaak, A.D.; Wilcox, C. Using Numerical Model Simulations to Improve the Understanding of Micro-plastic Distribution and Pathways in the Marine Environment. Front. Mar. Sci. 2017, 4, 1985. [Google Scholar] [CrossRef] [Green Version]








| Land-Based Entry | Ocean-Based Entry |
|---|---|
| Littering | Littering |
|
|
| Industry | Industry |
|
|
| Natural storm events | Natural storm events |
|
|
Waste management
| Waste management (ship-generated)
|
| River transport | Offshore oil and gas platforms |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lechthaler, S.; Waldschläger, K.; Stauch, G.; Schüttrumpf, H. The Way of Macroplastic through the Environment. Environments 2020, 7, 73. https://0-doi-org.brum.beds.ac.uk/10.3390/environments7100073
Lechthaler S, Waldschläger K, Stauch G, Schüttrumpf H. The Way of Macroplastic through the Environment. Environments. 2020; 7(10):73. https://0-doi-org.brum.beds.ac.uk/10.3390/environments7100073
Chicago/Turabian StyleLechthaler, Simone, Kryss Waldschläger, Georg Stauch, and Holger Schüttrumpf. 2020. "The Way of Macroplastic through the Environment" Environments 7, no. 10: 73. https://0-doi-org.brum.beds.ac.uk/10.3390/environments7100073