1. Introduction
When metal cations interact with natural organic matter, metal ligand complexes of various strengths can form. Natural organic matter in soil and water can therefore have a significant influence on chemical speciation of various metals in the environment. For example, Grosell et al. [
1] demonstrated that humic acid can reduce the toxicity of lead for fathead minnows during laboratory tank exposure. García-Mina et al. [
2] reviewed the role that metal-humic complexes can play in plant micronutrient uptake. Senesi and Loffredo [
3] reviewed the role of humic substances on the speciation of metals in soils. Senesi and Lofferedo [
4] reviewed the various spectroscopic methods utilized for studying the influence of humic acid metal complexes on metal speciation in soil.
Some metal humate complexes have been demonstrated to be electrochemically inert [
5,
6,
7], while other metals that form complexes are still available for electrochemical reduction [
8]. These latter complexes may, for example, be reduced to their zero-valance forms by a cathodic scan on a mercury electrode. This reduction reaction, of the complexed metal, occurs at a more negative potential than for the simple metal ion. The shift in reduction potential is a function of the metal ligand stability constant and the ligand concentration in solution, and this shift can be rationalized by reference to the Lingane equation [
9].
When the ligand concentration is sufficiently higher than the metal concentration, and the metal ion equilibration with the ligand is sufficiently rapid, the shift in reduction potential is a linear function of the log of the ligand concentration, as predicted by the Lingane [
9] equation. The shift in the reduction potential will also be a function of the pH of the solution if the speciation of the ligand and its affinity for the metal is influenced by pH. This phenomenon was also observed by Greter et al. [
10]. These investigators noted the shift in apparent reduction potential in the presence of humic material and provided compelling evidence for the adsorption of the Pb
2+ humate complex onto the mercury electrode. Similar observations were made by Turner et al. (1987), where the differential pulse polarography (DPP) measurement in the presence of fulvic acids yielded peak maxima at a significantly more negative value than fulvic acid-free solutions [
11]. Wallman et al. [
12] also observed that Pb
2+ fulvic acid complexes adsorbed onto a static mercury electrode and were detectable by cyclic voltammetry (CV). The adsorbed Pb
2+ was reduced during the cathodic scan, and the peak current progressively shifted to more negative peak voltages with increased adsorption time. These authors noted that the Pb
2+ fulvic acid complexes were labile on the time scale of CV.
In a previous publication, using anodic stripping voltammetry (ASV), we demonstrated that copper is strongly bound by water-extractable soil organic matter and by humic acids [
13]. A significant fraction of the bound copper was electrochemically inert, and the formation of this inert fraction was time-dependent. The presence of this electrochemically inert fraction is manifested by a nonlinear Copper ASV response when organic matter is titrated with Cu
2+. Similar observations have been made by other investigators. Our investigations with with Pb
2+ using ASV indicate that the concentration of the inert Pb
2+ complexes is at least an order of magnitude lower than for Cu
2+ (results not shown). Thus, ASV results for Pb
2+ do not show the pronounced nonlinearity of response vs. concentration that was observed for Cu
2+. However, during our investigations, we noticed that there was a notable shift in the DPP peak maximum (E
p) potential for Pb
2+ in the presence of humic acids when compared to the E
p of Pb
2+ in humic-free buffer solutions. In the current investigation, we sought to further elucidate the interaction of Pb
2+ with various humic acids by examining the shift in the reduction potential of Pb
2+ in the presence of humic acids. Our results demonstrated that Pb
2+ interacts with humic material primarily by the formation of an electrochemically active (reducible) complex. This Pb
2+ humate complex, although in rapid equilibrium with the solution species, is reduced at a more negative reduction potential (100 to 200 mV) than uncomplexed Pb
2+.
3. Results and Discussion
Differential pulse polarography provides estimates of the reduction potentials for metals cations such as Pb
2+, Cd
2+, Zn
2+ and Cu
2+ [
15]. Our observations indicated that the presence of various ligating small molecules (3,4-dihyroxybenzoic acid and salicylic acid) and humic acids results in a shift in the E
p for Pb
2+ to a more negative voltage. The magnitude of these potential shifts increases with increasing concentrations of complex forming ligands.
The shift in the voltammogram peak maximum (E
p) can be understood utilizing an equation originally presented by Lingane [
9]. The principle assumption is that the metal cation (
M) is complexed by a ligand (
L) with the formation of
MLp and that this reaction is in rapid equilibrium with a stability constant
KL. The equilibrium expression (Equation (1)) is given by:
In Equation (1),
fc,
fm and
fL represent activity coefficients for the complex, the metal ion and the ligand, respectively, and
p is the stoichiometric coefficient for the complex. The Lingane equation for apparent reduction potential of the metal complex is given by Equation (2).
The
kc and the
ka terms are kinetic terms related to the diffusion of the complex in solution and the diffusion of the metal in the mercury electrode, respectively. The term
fa represents the activity coefficient of the metal on the mercury electrode. Expanding the
Log term (Equation (3)):
For a simple metal ion, the equation reduces to the following (Equation (4)):
The
km term is a kinetic term related to the diffusion of the uncomplexed metal in solution. The shift in the peak maximum is the difference in
E0′ between the complex metal ion and the simple metal ion in the buffer solution and is given by
f, Equation (5).
For low molecular weight complexing agents, the ratio
kc/
km is likely to be close to one, and the contribution of the activity coefficients to Δ
E is also small or, at least, constant. Therefore, the shift in the reduction potential measured by the differential pulse is primarily a function of the stability constant and the ligand concentration (Equation (6)).
The
kc/
km term may be important for humic substances, as diffusion rates for humic-bound Pb
2+ is likely to be slower than for lower molecular weight Pb
2+ species [
15].
The measurements of E
p made during this investigation can be used to estimate a value for Δ
E. If the concentration of the ligand (
L) and
p are available, it is then possible to estimate the value of
KL. If the natural abundance and stoichiometry of the complexing groups on the humic material were well-characterized, it would be possible to estimate the magnitude of the stability constant for a complex formation for Pb
2+ with the humic material. It is tempting to use the dissociable proton content of humic materials as an estimate of the abundance of complexing groups; however, past research has indicated that not all of these groups are available to complex metals [
3,
16,
17]. Furthermore, many of the humic substance-binding sites are likely to be multidentate, and some binding may involve the nonspecific accumulation of counter ions in the vicinity of the negatively charged humic substance [
3,
18,
19,
20].
Using the stability constants imbedded in Visual Minteq [
21] and the conditions of these experiments, we calculated the speciation of Pb
2+. At pH 8, and in the absence of humic matter, Pb
2+ would exist primarily as Pb
2+, PbOH
+ and Pb(OH)
2 (44.7%, 53.9% and 1.31%), respectively. The HEPES, MOPS and PIPES buffering agents utilized were selected because of their minimal interactions with metal species and should not impact this distribution [
22]. The shift in E
p that we measured represents a change in Pb
2+ speciation, where there is some hydrolysis to a ligated form of Pb
2+. The ligated species may be mono- or multidentate and could involve mixed ligand complexes as well.
The differential DPP method was tested with two low molecular weight humic acid analogs (3,4-dihydroxybenzoic acid and salicylic acid) and a polyacrylic acid sample of 5100 g/mol. The carboxylic acid and phenol functionalities on these compounds are similar to the ligating functionalities on humic materials [
23,
24,
25].
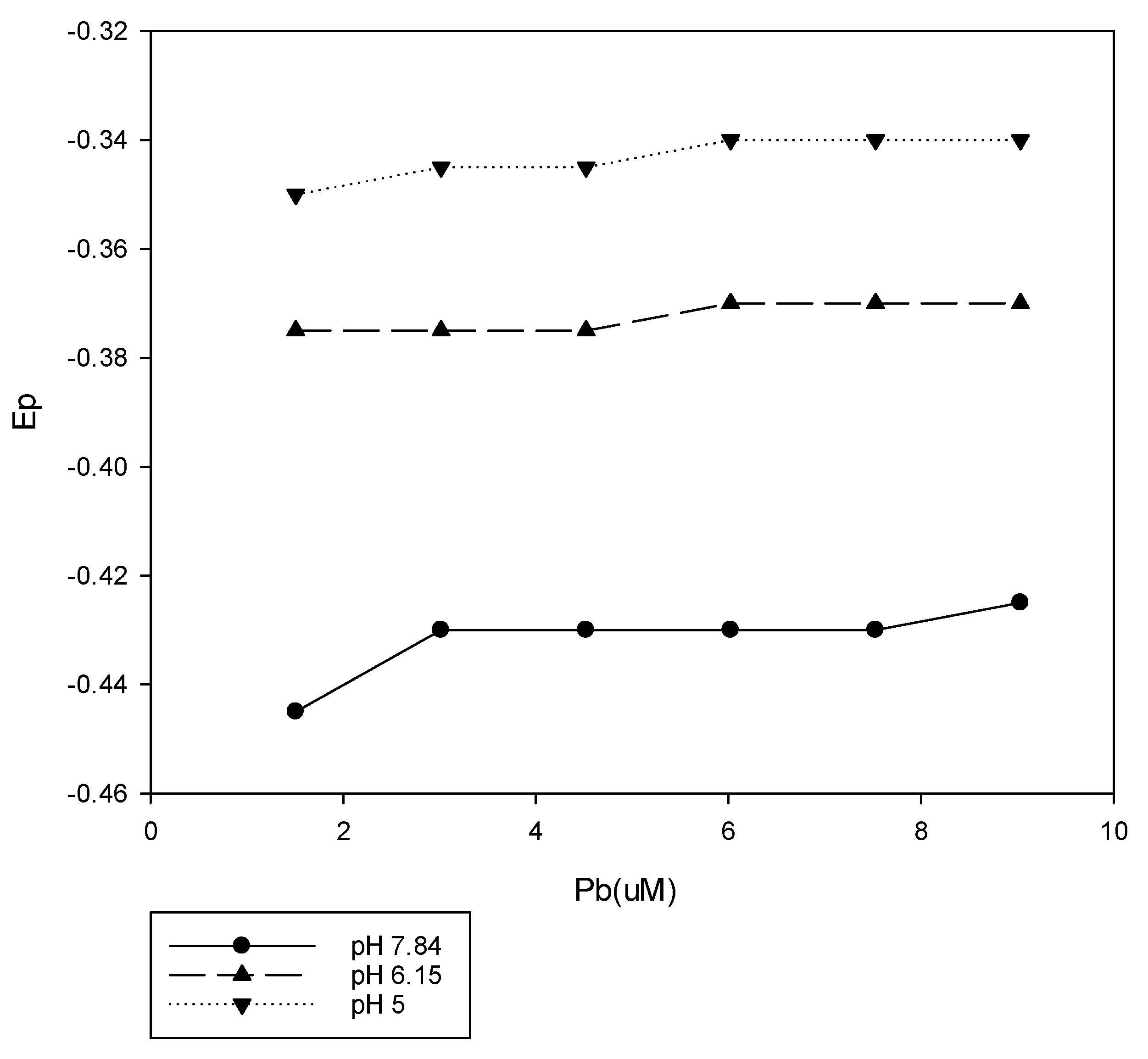
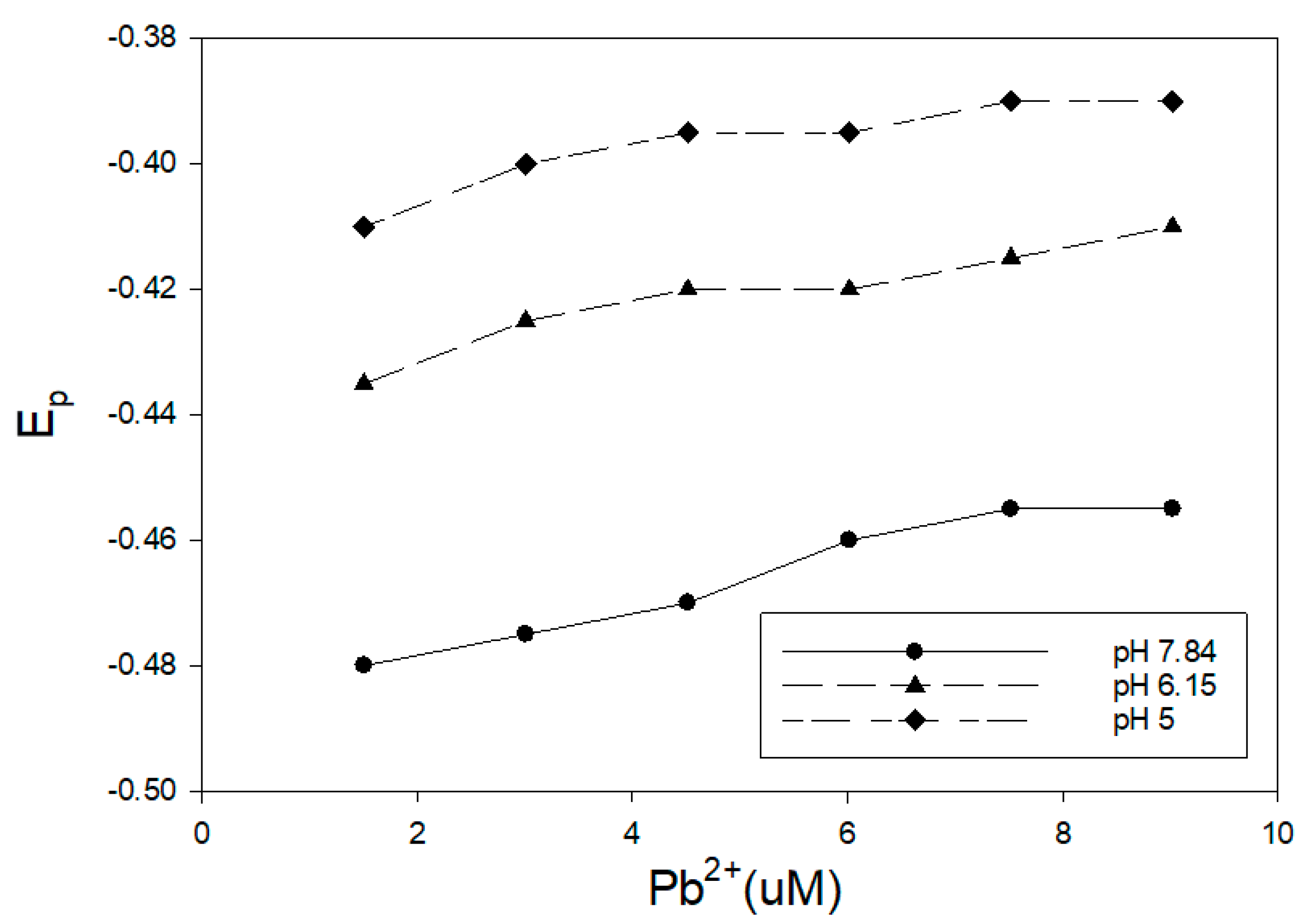
3,4-dihydroxybenzoic acid was utilized in this study as a proxy for humic materials. The effect of this organic acid on the reduction of Pb
2+ (2–10 uM) was examined at three different pH values, and the results are presented in
Figure 1. The concentration of 3,4-dihydroxybenzoic acid was 1.25 mM for all of these measurements. This ligand has been reported to react with various metals through the ring hydroxyl groups [
26]. The pH of the solution was maintained with 1 mL of buffer in 15 mL of sample. The impact of this ligand and pH on the reduction of Pb
2+ is clear. The pH 5 sample showed minimal shift from the reduction potential of uncomplexed Pb
2+, and the observed E
p was very close to a ligand-free buffer solution. Measurements at pH 6.15 and 7.84 showed a shift toward a more negative voltage. The greater shift for the pH 7.84 measurements was consistent with the increased ionization of the ring hydroxyl groups, making a complex formation more favorable. On the other hand, Khayat et al. [
27] examined the interaction of 3,4-dihydroxybenzoic acid and Pb
2+ using a potentiometric approach at much higher Pb
2+ and ligand concentrations. In their study, a precipitate was formed at pH 3 to 4.5 that appeared to be consistent with the formation of a complex through the ionized carboxylic acid group. Khayat et al. [
27] did note that the precipitate appeared to dissolve at a higher pH, with the possible formation of a strong complexation through the phenolic groups.
Salicylic acid has been used by other investigators as a low molecular weight humic acid analog [
23,
24,
25]. The effect of salicylic acid on the reduction of lead was, therefore, investigated. Since the sodium salicylate is fairly soluble, it was possible to vary the concentration over a fairly large concentration range and, therefore, test the prediction of the Lingane equation.
Measurements at two different pH values are summarized in
Figure 2 and illustrate that the salicylate complexation can shift the reduction potential for Pb
2+ to more negative values. It is interesting that the E
p at pH 6.15 and 7.84 are very close. This observation possibly indicates that the phenolic hydroxyl group of salicylic acid does not ionize when the Pb
2+ salicylate (Sal) complex forms. The shift in the E
p increased with the logarithmic concentration of salicylic acid in the solution, as predicted by the Lingane equation. The slopes of the two lines are 34.4 and 36.8 mV/log (Sal), respectively. This differs from the slope predicted by the Nernst equation (29.6 at 25 °C) but is still reasonably close. There are very few measurements of the stability constants between salicylate and Pb
2+ in the literature. Furia and Porto [
28] investigated the interaction of Pb
2+ with hydrogen salicylate using potentiometric titration. These investigators measured the stability constants at a rather high ionic strength (3-M KClO
4) and extrapolated the constant to 0 ionic strength. Their results indicated that, at pH 7.84, PbSal and Pb(Sal)
22− should be the dominant species. Using the Furia and Porto [
28] stability constants and a variant of the DeFord Hume equation [
29,
30,
31], which is similar to the Lingane equation, we calculated that the shift in reduction potential should be closer to a factor of two times greater at pH 7.84 than what we observed. Furthermore, there should be a significant difference between pH 6.15 and pH 7.84. The calculation indicated that the shift for pH 7.84 should be about four times greater than for pH 6.15. Clearly, the salicylate ligation of Pb
2+ requires additional investigation.
Previous workers have considered polycarboxylic acids to be viable models for humic substances and have examined the interaction of metal cations with polycarboxylic acids of various molecular weights [
32]. In recent years, this polymer view of humic substances has been challenged by several groups [
33,
34]. Nevertheless, we examined the interaction of Pb
2+ with 100-mg/L sodium polyacrylate of 5100 g/mol at both pH 6.12 and pH 8. The results presented in
Figure 3 show little difference between the two pH values. In addition, the presence of the 1-M NaNO
3 background electrolyte made little difference in the negative shift of the Pb
2+ reduction peak (results are not shown).
The small difference in results for the two pH values may indicate that there is little change in the ionization of the carboxylate groups between pH 6 and 8. The carboxylate concentration of the polyacrylate should be about 10.6 mM/g. This is somewhat larger than the carboxylate concentrations of the humic acids examined in this study but not largely so. The lack of pH dependence probably results from the absence of phenolic groups in polyacrylate.
The Pahokee peat humic acid and Pb
2+ interaction was examined at four different pH values and at different Pb
2+ concentrations. The Pahokee peat humic acid concentration was 100 mg/L, and the results are presented in
Figure 4. As expected, the impact of humic acid on the E
p for Pb
2+ increased with the pH, which increased the ionization of the humic acid and the availability of metal complexing ligands. It was also noted that the shift in E
p decreased as the Pb
2+ concentration in the solution was increased. This concentration dependence for E
p indicates that there is a spectrum of binding interactions of Pb
2+ with the humic material, with the more stable complexes formed at low Pb
2+ concentrations. We also observed that the shift in the E
p was a function of the concentration of humic acid (
Figure 5).
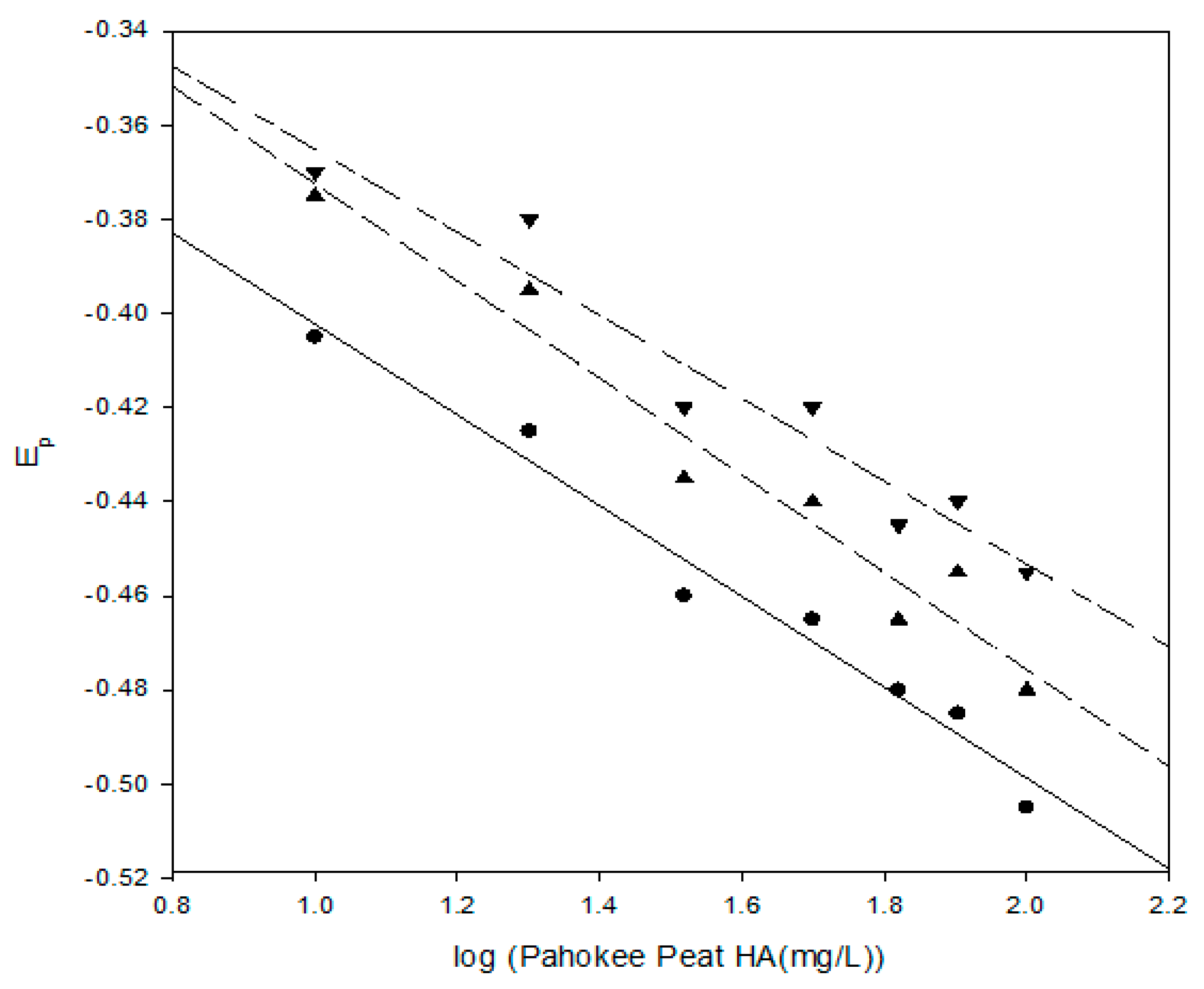
These experiments with the Pahokee peat humic acid were conducted at pH 8 in a 0.100-M HEPES buffer with 1.0-M NaNO
3 as a background electrolyte. These results illustrate that the shift in reduction potential for the Pb
2+ humic acid complex is a function of the humic acid concentration. These results are plotted in
Figure 6 as a function of the Log of the humic acid concentration in mg/L for three different Pb
2+ concentrations. The linearity of these plots conforms to the general form of the Lingane equation.
A similar observation is illustrated in
Figure 7 for Pb
2+ in the presence of a commercially available TeraVita leonardite humic acid. These experiments were conducted at pH 8 in 0.100-M HEPES buffer with 1.0-M NaNO
3 as a background electrolyte. It is clear that the shift of the reduction potential for Pb
2+ is a function of the humic acid concentration.
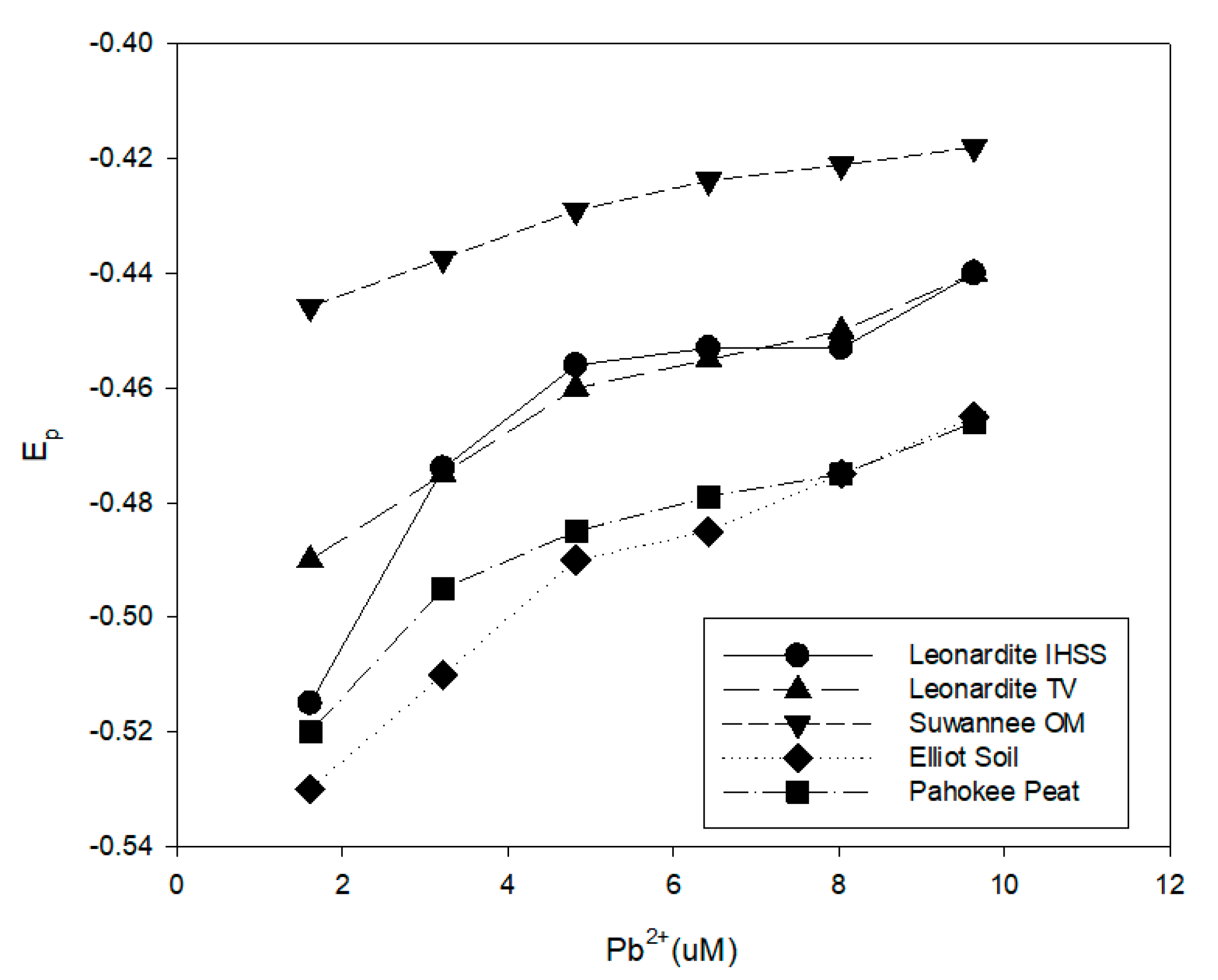
In
Figure 8, the E
p values for Pb
2+ in the presence of various humic acids, at a concentration of 100 mg/L, at pH 8 are compared. The HEPES buffer was 0.100 M, and the NaNO
3 was 1.00 M. The results indicate the greatest shift in the Pb
2+ reduction potential was observed for the Elliot soil humic acid samples, and the lowest shift was observed for the Suwannee River natural organic matter. The two leonardite samples displayed roughly the same behavior. For all of the humic acids, the E
p shifts a more negative voltages at lower Pb
2+ concentrations. The E
p for the various humic acids did not correlate with the average molecular weight or IHSS carboxylic acid and phenolic hydroxyl contents. For example, according to results published by the IHSS, the Suwanee River NOM had the highest phenolic content yet exhibited the least negative Pb
2+ reduction potentials [
35].
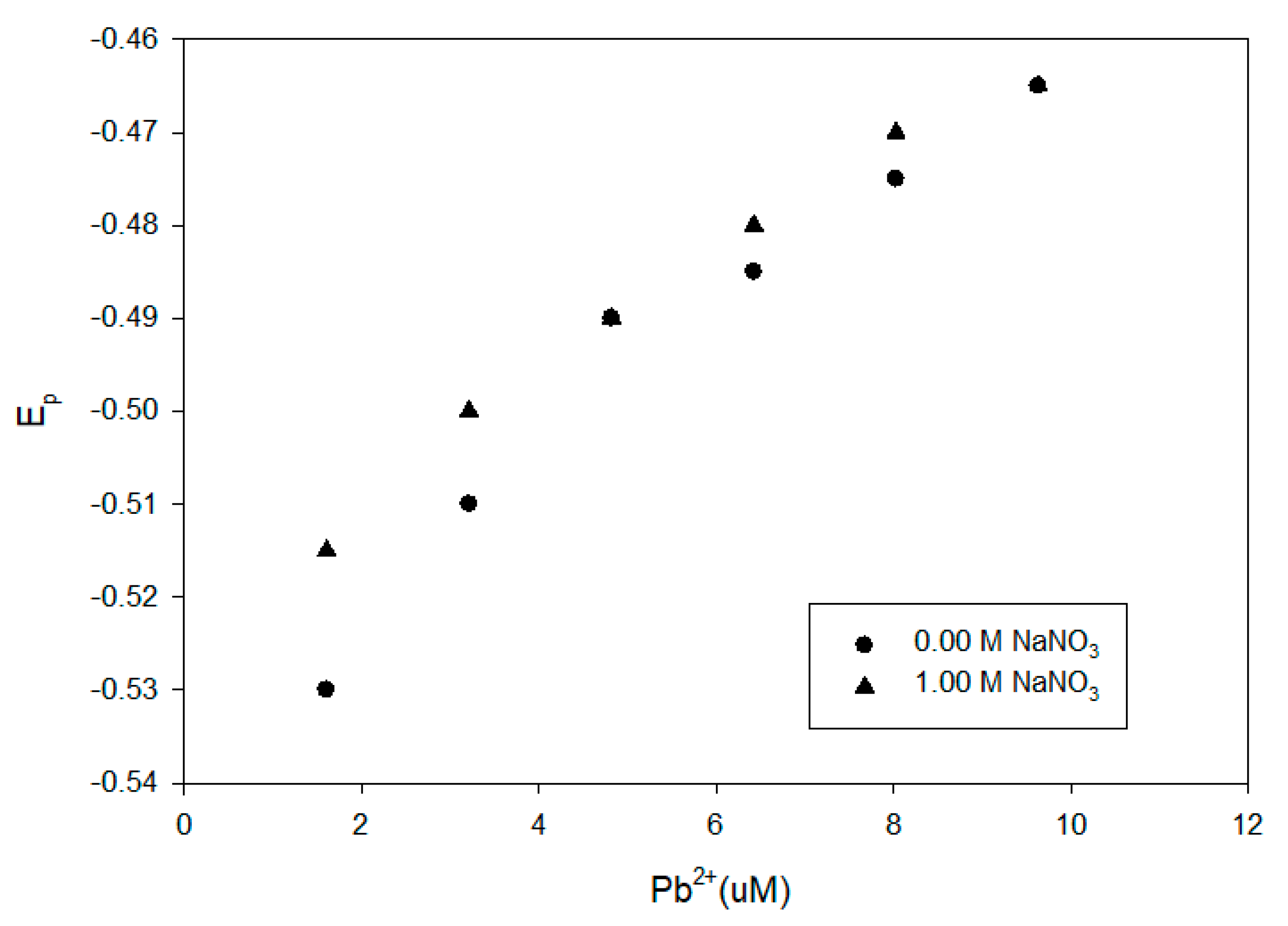
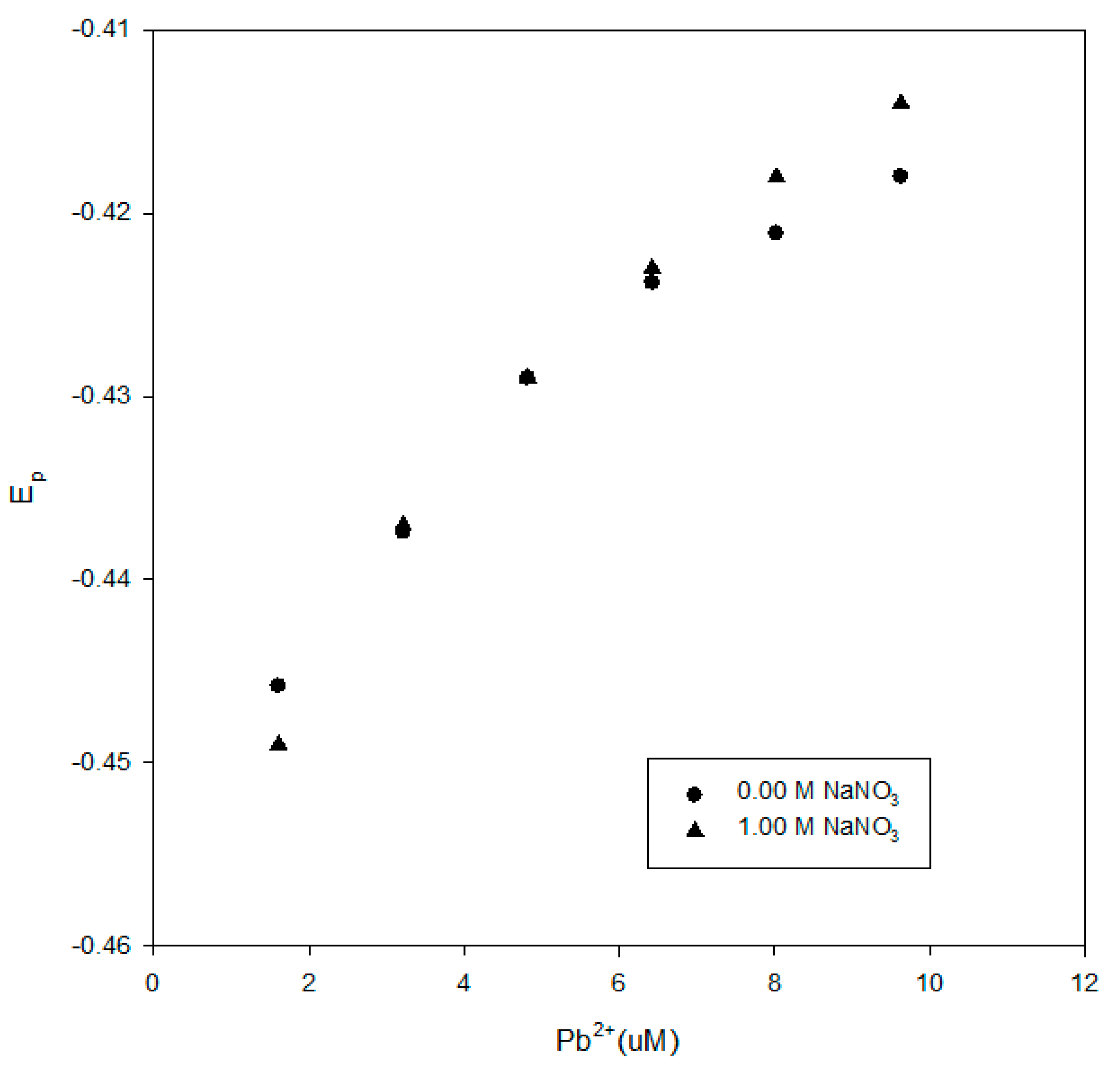
We further examined the impact of the 1.0-M NaNO
3 background electrolyte on the DPP reduction potentials. We compared the results for the Elliot soil humic acid, the Suwannee River NOM and the IHSS leonardite with and without 1.0-M NaNO
3 in
Figure 9,
Figure 10 and
Figure 11. The presence of the background electrolyte did not induce a major difference or consistent impact on the reduction potentials.
Our previous work investigated the impact of soluble organic matter in arid soil extracts on copper speciation [
13]. DPP results for Pb
2+ in a water extract from a soil sampled from the root zone of a creosote bush is shown in
Figure 12. The location of this soil was from a roadside site located in Halloran Springs, California, USA. This soil was discussed in Steinberg and Hodge [
13]. The total organic carbon content of this soil sample water extract was measured at 35 mg/L. Assuming that humic material is approximately 50% carbon indicates that this extract would have approximately 70-mg/L humic material. This creosote soil extract was examined using the DPP method. The results are similar to the humic materials examined in this study. The reduction potential of Pb
2+ in these soil extracts shows similar pH dependence to that observed in the humic acid solution. In addition, the Ep shift was dependent on the Pb
2+ concentration, indicating a heterogeneous distribution of lead binding groups in the extract.
The results from size exclusion chromatography of the various humic materials are shown in
Figure 13. The number-averaged (M
n) and weight-averaged (M
w) molecular weights derived from the SEC are tabulated in
Table 1. The most striking results are the large differences in number-averaged vs. weight-averaged molecular weights and that all of the samples had rather low average molecular weights and an overall very broad molecular weight distribution. Furthermore, all of the humic materials examined contained a large contribution from low molecular weight components. These observations are consistent with the results of other studies [
36,
37,
38]. The E
p results did not show any apparent correlation with M
n or M
w of the humic substance.
The observed Ep may be partially a function of unknown electrode kinetic factors and instrumental settings, as well as the reduction potential of the complexed species. Therefore, we examined the influence of the various DPP parameters on the position of the observed reduction peak for Pahokee peat humic acid in order to gauge the magnitude of the influence of various instrumental parameters on the observations. All of these experiments were done with 15 mL of the Pahokee peat HA 100 mg/L in 100-mM HEPES pH 8 and 1.00-M NaNO
3 and with 4.8-uM Pb
2+.
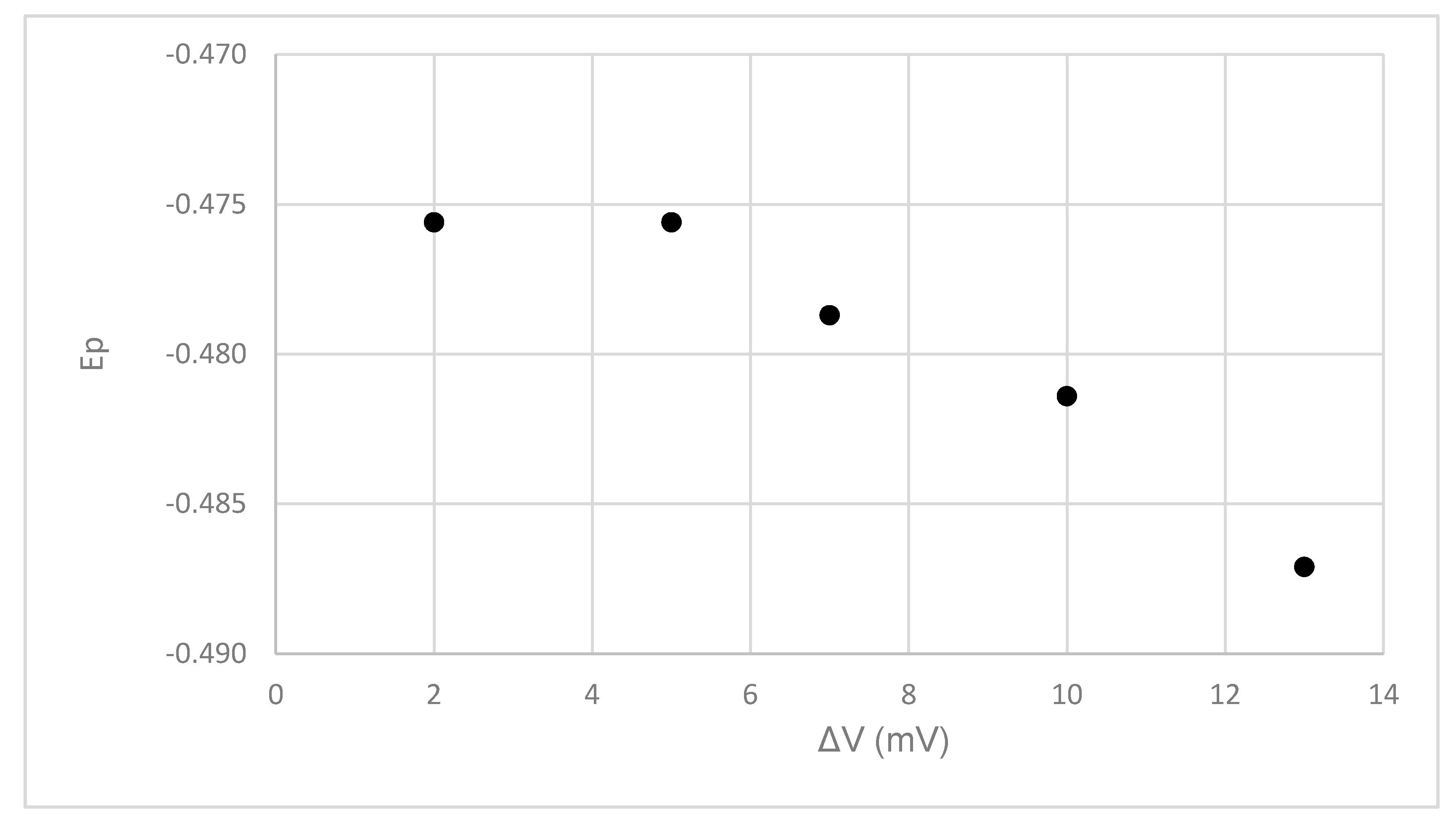
Figure 14 indicates that increasing the voltage increment (ΔV) between mercury drops tends to yield a more negative E
p, although there was little change in the peak position between the 2- and 4-mV increments.
The results presented in
Figure 15 indicate that increasing the pulse amplitude leads to a less negative E
p value.
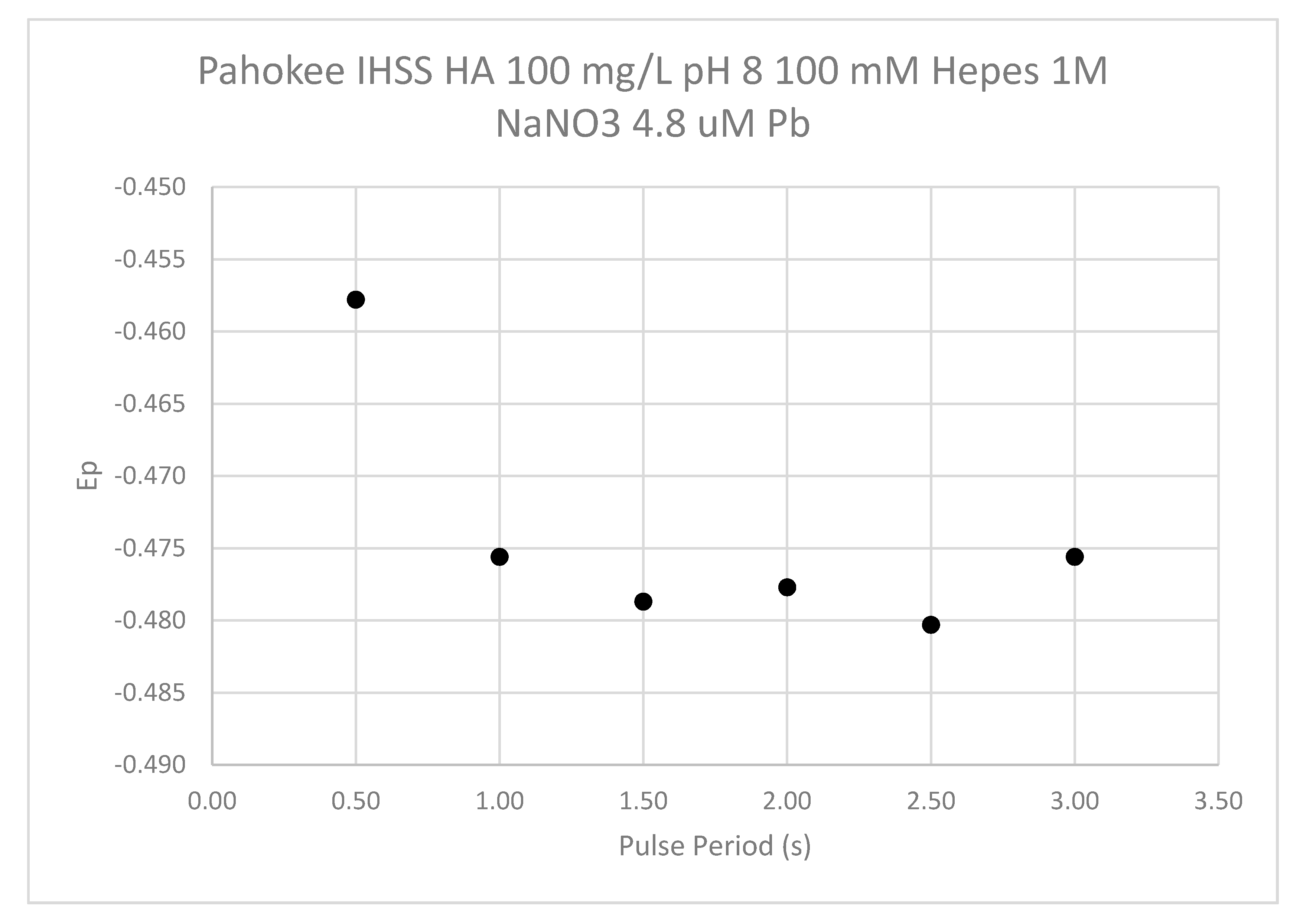
The influence of the pulse period (drop time) is shown in
Figure 16. The shortest pulse period examined had a less negative E
p value than the procedure used for sample analysis. Pulse periods from 1 to 3 s showed little change in the E
p.
It is clear that the observed E
p values are a function of the DPP conditions selected. Thus, caution in using a strictly thermodynamic interpretation of DPP observations is in order. Nevertheless, we used these results to estimate the magnitude of the Pb
2+ humic acid stability constants. To form this estimate, we utilized Equation (6), along with the observed E
p from humic-free solutions, to estimate the value for a conditional
logKL for the Pb
2+ binding by the various humic substances examined in this study. For the purposes of this calculation, we assumed that the shift in E
p from a humic-free to a humic-containing solution was equivalent to Δ
E in Equation (6). We also assumed that
p in Equation (6) was equal to 1. We estimated the molar concentration of the humic substance (
L) by dividing the mass concentration by either M
n or M
w. Using these highly simplified assumptions, we rearranged Equation (6) into Equation (7) to produce an estimate for log
KL. These estimates were performed with the results from lowest concentration of Pb
2+ used in the study (1.6 uM).
The results of this very preliminary calculation of conditional stability constants are reported in
Table 2. These calculations assumed a 1:1 stoichiometry for the Pb
2+ humic acid complex. The calculation ignores the heterogeneity of humic acids and the potential contribution of electrostatic effects to metal binding. It is quite possible that the binding capacity of these humic acids is much higher than this calculated molarity. If so, the estimated log
KL value could be considerably reduced. The metal-binding capacity for humic substances has not been well-established. For example, Logan et al. [
39] and Sahu and Banjeree [
40] utilized ion-selective electrodes with a graphical analysis to estimate both the metal-binding capacities and metal humic stability constants. Both parameters were found to be pH-dependent.
Schnitzer and Skinner [
41] and Schnitzer and Hansen [
42] examined metal binding by a soil fulvic acid at low pH (5 and lower) using Schubert’s ion exchange method. The molecular weight (Mn) of the fulvic acid was estimated using vapor pressure osmometry. Using the estimated molecular weight and the method of continuous variation, Schnitzer and Hansen [
42] determined that the complex involved a metal-to-fulvic acid ratio of 1:1 for Pb
2+ and other divalent metals at an ionic strength of 0.1, although, at lower ionic strength, this ratio decreased. These authors indicated that, at a lower ionic strength, mixed or polynuclear complexes were likely forming. The Log
KL (~6) values for Pb
2+ estimated in these studies was much lower than those reported here; however, the pH of these measurements was considerably lower as well. The assumptions used for our estimates of the Log
KL are consistent with Schnitzer and Hansen’s observation of 1:1 stoichiometry.