Germination and Seedling Growth Responses of Zygophyllum fabago, Salsola kali L. and Atriplex canescens to PEG-Induced Drought Stress
Abstract
:1. Introduction
2. Material and Methods
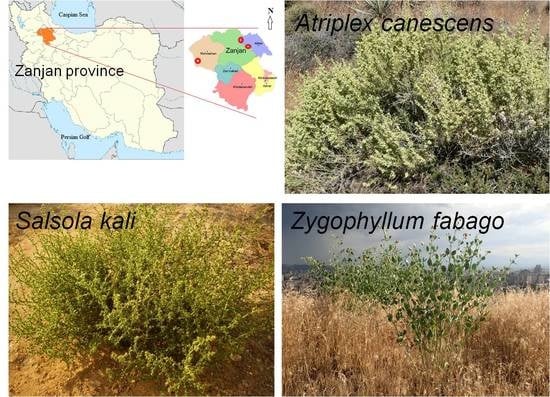
2.1. Plant Materials
2.2. Data Analysis
3. Results
3.1. Germination Percentage
3.2. Mean Germination Time
3.3. Germination Rate
3.4. Seedling Vigor Index
3.5. Root and Shoot Length
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Farooq, M.; Wahid, A.; Lee, D.-J.; Ito, O.; Siddique, K.H. Advances in drought resistance of rice. Crit. Rev. Plant Sci. 2009, 28, 199–217. [Google Scholar] [CrossRef]
- Mahdavi, A.; Moradi, P.; Mastinu, A. Variation in Terpene Profiles of Thymus vulgaris in Water Deficit Stress Response. Molecules 2020, 25, 1091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, D.; Wang, S.; Cao, B.; Cao, D.; Leng, G.; Li, H.; Yin, L.; Shan, L.; Deng, X. Genotypic variation in growth and physiological response to drought stress and re-watering reveals the critical role of recovery in drought adaptation in maize seedlings. Front. Plant Sci. 2016, 6, 1241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Gall, H.; Philippe, F.; Domon, J.-M.; Gillet, F.; Pelloux, J.; Rayon, C. Cell wall metabolism in response to abiotic stress. Plants 2015, 4, 112–166. [Google Scholar] [CrossRef]
- Kumar, A.; Memo, M.; Mastinu, A. Plant behaviour: An evolutionary response to the environment? Plant Biol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Flexas, J.; Bota, J.; Galmes, J.; Medrano, H.; Ribas-Carbó, M. Keeping a positive carbon balance under adverse conditions: Responses of photosynthesis and respiration to water stress. Physiol. Plant. 2006, 127, 343–352. [Google Scholar] [CrossRef]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ge, T.; Sui, F.; Bai, L.; Tong, C.; Sun, N. Effects of water stress on growth, biomass partitioning, and water-use efficiency in summer maize (Zea mays L.) throughout the growth cycle. Acta Physiol. Plant. 2012, 34, 1043–1053. [Google Scholar] [CrossRef]
- Rahmati, M.; Mirás-Avalos, J.M.; Valsesia, P.; Lescourret, F.; Génard, M.; Davarynejad, G.H.; Bannayan, M.; Azizi, M.; Vercambre, G. Disentangling the effects of water stress on carbon acquisition, vegetative growth, and fruit quality of peach trees by means of the QualiTree model. Front. Plant Sci. 2018, 9, 3. [Google Scholar] [CrossRef] [Green Version]
- Rad, S.V.; Valadabadi, S.A.R.; Pouryousef, M.; Saifzadeh, S.; Zakrin, H.R.; Mastinu, A. Quantitative and Qualitative Evaluation of Sorghum bicolor L. under Intercropping with Legumes and Different Weed Control Methods. Horticulturae 2020, 6, 78. [Google Scholar] [CrossRef]
- Rauf, M.; Munir, M.; ul Hassan, M.; Ahmad, M.; Afzal, M. Performance of wheat genotypes under osmotic stress at germination and early seedling growth stage. Afr. J. Biotechnol. 2007, 6, 971–975. [Google Scholar]
- Khayatnezhad, M.; Gholamin, R.; Jamaatie-Somarin, S.; Zabihi-Mahmoodabad, R. Effects of peg stress on corn cultivars (Zea mays L.) at germination stage. World Appl. Sci. J. 2010, 11, 504–506. [Google Scholar]
- Gupta, A.K.; Rather, M.A.; Kumar Jha, A.; Shashank, A.; Singhal, S.; Sharma, M.; Pathak, U.; Sharma, D.; Mastinu, A. Artocarpus lakoocha Roxb. and Artocarpus heterophyllus Lam. Flowers: New Sources of Bioactive Compounds. Plants 2020, 9, 1329. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, E.A. Seed priming to alleviate salinity stress in germinating seeds. J. Plant Physiol. 2016, 192, 38–46. [Google Scholar] [CrossRef]
- Larson, M.; Schubert, G.H. Effect of osmotic water stress on germination and initial development of ponderosa pine seedlings. For. Sci. 1969, 15, 30–36. [Google Scholar]
- Falusi, M.; Calamassi, R.; Tocci, A. Sensitivity of seed germination and seedling root growth to moisture stress in four provenances of Pinus halepensis Mill. Silvae Genet. 1983, 32, 4–9. [Google Scholar]
- Larher, F.; Leport, L.; Petrivalsky, M.; Chappart, M. Effectors for the osmoinduced proline response in higher plants. Plant Physiol. Biochem. (Paris) 1993, 31, 911–922. [Google Scholar]
- Kaur, S.; Gupta, A.K.; Kaur, N. Gibberellic acid and kinetin partially reverse the effect of water stress on germination and seedling growth in chickpea. Plant Growth Regul. 1998, 25, 29–33. [Google Scholar] [CrossRef]
- Sen, A.; Alikamanoglu, S. Antioxidant enzyme activities, malondialdehyde, and total phenolic content of PEG-induced hyperhydric leaves in sugar beet tissue culture. In Vitro Cell. Dev. Biol.-Plant 2013, 49, 396–404. [Google Scholar] [CrossRef]
- Sanderson, S.C.; Stutz, H.C. High chromosome numbers in Mojavean and Sonoran desert Atriplex canescens (Chenopodiaceae). Am. J. Bot. 1994, 81, 1045–1053. [Google Scholar] [CrossRef]
- Hao, G.Y.; Lucero, M.E.; Sanderson, S.C.; Zacharias, E.H.; Holbrook, N.M. Polyploidy enhances the occupation of heterogeneous environments through hydraulic related trade-offs in Atriplex canescens (Chenopodiaceae). New Phytol. 2013, 197, 970–978. [Google Scholar] [CrossRef] [PubMed]
- Toderich, K.; Shuyskaya, E.; Taha, F.; Ismail, S.; Gismatullina, L.; Li, E. Adaptive fruit structural mechanisms of Asiatic Salsola species and its germplasm conservation and utilization. J. Arid Land Stud. 2012, 22, 73–76. [Google Scholar]
- Akhani, H. Notes on the Flora of Iran: 1. Asparagus (Asparagaceae) and Nitraria (Zygophyllaceae). Edinb. J. Bot. 2002, 59, 295. [Google Scholar] [CrossRef]
- Mozaffarian, V. A Dictionary of Iranian Plant Names; Farhang Mosavar Publication: Tehran, Iran, 2006. [Google Scholar]
- Blank, R.R.; Young, J.A.; Martens, E.; Palmquist, D.E. Influence of temperature and osmotic potential on germination of Allenrolfea occidentalis seeds. J. Arid Environ. 1994, 26, 339–347. [Google Scholar] [CrossRef]
- Sy, A.; Grouzis, M.; Danthu, P. Seed germination of seven Sahelian legume species. J. Arid Environ. 2001, 49, 875–882. [Google Scholar] [CrossRef]
- Zheng, Y.; Xie, Z.; Gao, Y.; Jiang, L.; Xing, X.; Shimizu, H.; Rimmington, G.M. Effects of light, temperature and water stress on germination of Artemisia sphaerocephala. Ann. Appl. Biol. 2005, 146, 327–335. [Google Scholar] [CrossRef]
- Stevens, J.; Barrett-Lennard, E.; Dixon, K. Enhancing the germination of three fodder shrubs (Atriplex amnicola, A. nummularia, A. undulata; Chenopodiaceae): Implications for the optimisation of field establishment. Aust. J. Agric. Res. 2006, 57, 1279–1289. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, J.; Li, X.; Zhang, S.; Lan, H. Effects of environmental stress on seed germination and seedling growth of Salsola ferganica (Chenopodiaceae). Acta Ecol. Sin. 2016, 36, 456–463. [Google Scholar] [CrossRef]
- Michel, B.E.; Kaufmann, M.R. The osmotic potential of polyethylene glycol 6000. Plant Physiol. 1973, 51, 914–916. [Google Scholar] [CrossRef]
- Wang, C.; Liu, J.; Xiao, H.; Du, D. Response of Leaf Functional Traits of Cerasus yedoensis (Mats.) Yü Li to Serious Insect Attack. Pol. J. Environ. Stud. 2016, 25. [Google Scholar] [CrossRef]
- Agrawal, R. Seed Technology; Oxford IBH Publishing: New Delhi, India, 1991; 658p. [Google Scholar]
- Kulkarni, M.; Sparg, S.; Van Staden, J. Germination and post-germination response of Acacia seeds to smoke-water and butenolide, a smoke-derived compound. J. Arid Environ. 2007, 69, 177–187. [Google Scholar] [CrossRef]
- Scott, S.; Jones, R.; Williams, W. Review of Data Analysis Methods for Seed Germination 1. Crop Sci. 1984, 24, 1192–1199. [Google Scholar] [CrossRef]
- Bao, A.-K.; Wang, S.-M.; Wu, G.-Q.; Xi, J.-J.; Zhang, J.-L.; Wang, C.-M. Overexpression of the Arabidopsis H+-PPase enhanced resistance to salt and drought stress in transgenic alfalfa (Medicago sativa L.). Plant Sci. 2009, 176, 232–240. [Google Scholar] [CrossRef]
- Carmo-Silva, A.E.; Gore, M.A.; Andrade-Sanchez, P.; French, A.N.; Hunsaker, D.J.; Salvucci, M.E. Decreased CO2 availability and inactivation of Rubisco limit photosynthesis in cotton plants under heat and drought stress in the field. Environ. Exp. Bot. 2012, 83, 1–11. [Google Scholar] [CrossRef]
- Hussain, M.I.; Lyra, D.-A.; Farooq, M.; Nikoloudakis, N.; Khalid, N. Salt and drought stresses in safflower: A review. Agron. Sustain. Dev. 2016, 36, 4. [Google Scholar] [CrossRef] [Green Version]
- Mastinu, A.; Bonini, S.A.; Rungratanawanich, W.; Aria, F.; Marziano, M.; Maccarinelli, G.; Abate, G.; Premoli, M.; Memo, M.; Uberti, D. Gamma-oryzanol Prevents LPS-induced Brain Inflammation and Cognitive Impairment in Adult Mice. Nutrients 2019, 11, 728. [Google Scholar] [CrossRef] [Green Version]
- Mastinu, A.; Kumar, A.; Maccarinelli, G.; Bonini, S.A.; Premoli, M.; Aria, F.; Gianoncelli, A.; Memo, M. Zeolite Clinoptilolite: Therapeutic Virtues of an Ancient Mineral. Molecules 2019, 24, 1517. [Google Scholar] [CrossRef] [Green Version]
- Delachiave, M.; De Pinho, S. Scarification, temperature and light in germination of Senna occidentalis seed (Caesalpinaceae). Seed Sci. Technol. 2003, 31, 225–230. [Google Scholar] [CrossRef]
- Guerrier, G. Fluxes of Na+, K+ and Cl−, and osmotic adjustment in Lycopersicon pimpinellifolium and L. esculentum during short-and long-term exposures to NaCl. Physiol. Plant. 1996, 97, 583–591. [Google Scholar]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Gu, M.; Li, N.; Shao, T.; Long, X.; Brestič, M.; Shao, H.; Li, J. Accumulation capacity of ions in cabbage (Brassica oleracea L.) supplied with sea water. Plant Soil Environ. 2016, 62, 314–320. [Google Scholar]
- Bajji, M.; Kinet, J.-M.; Lutts, S. Salt stress effects on roots and leaves of Atriplex halimus L. and their corresponding callus cultures. Plant Sci. 1998, 137, 131–142. [Google Scholar] [CrossRef]
- Toosi, A.F.; Bakar, B.B.; Azizi, M. Effect of drought stress by using PEG 6000 on germination and early seedling growth of Brassica juncea Var. Ensabi. Sci. Pap. Ser. A Agron. 2014, LVII, 360–363. [Google Scholar]
- Turk, M.A.; Tawaha, A.R.M.; Lee, K.D. Seed germination and seedling growth of three lentil cultivars under moisture stress. Asian J. Plant Sci. 2004. [Google Scholar] [CrossRef]
- Khayatnezhad, M.; Gholamin, R. Effects of water and salt stresses on germination and seedling growth in two durum wheat (Triticum durum Desf.) genotypes. Sci. Res. Essays 2011, 6, 4597–4603. [Google Scholar]
- Bahrami, H.; Razmjoo, J.; Jafari, A.O. Effect of drought stress on germination and seedling growth of sesame cultivars (Sesamum indicum L.). Int. J. AgriSci. 2012, 2, 423–428. [Google Scholar]
- Zandi Esfahan, E.; Azarnivand, H. Effect of water stress on seed germination of Agropyron elongatum, Agropyron desertourm & Secale montanum. Desert 2012, 17, 249–253. [Google Scholar]
- Kasera, P.K.; Mohammed, S. Ecology of inland saline plants. In Desert Plants; Springer: Berlin/Heidelberg, Germany, 2010; pp. 299–320. [Google Scholar]
- Liu, K.; Baskin, J.M.; Baskin, C.C.; Du, G. Very fast-germinating seeds of desert species are cryptoviparous-like. Seed Sci. Res. 2013, 23, 163–167. [Google Scholar] [CrossRef]
- Richards, R. Selectable traits to increase crop photosynthesis and yield of grain crops. J. Exp. Bot. 2000, 51, 447–458. [Google Scholar] [CrossRef]
- Botwright, T.; Condon, A.; Rebetzke, G.; Richards, R. Field evaluation of early vigour for genetic improvement of grain yield in wheat. Aust. J. Agric. Res. 2002, 53, 1137–1145. [Google Scholar] [CrossRef]
- Siddique, K.; Tennant, D.; Perry, M.; Belford, R. Water use and water use efficiency of old and modern wheat cultivars in a Mediterranean-type environment. Aust. J. Agric. Res. 1990, 41, 431–447. [Google Scholar] [CrossRef]
- Turner, N.C. Further progress in crop water relations. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 1996; Volume 58, pp. 293–338. [Google Scholar]
- Abd Allah, A.; Badawy, S.A.; Zayed, B.; El-Gohary, A. The role of root system traits in the drought tolerance of rice (Oryza sativa L.). J. Plant Prod. 2010, 1, 621–631. [Google Scholar] [CrossRef]
- Fraser, T.E.; Silk, W.K.; Rost, T.L. Effects of low water potential on cortical cell length in growing regions of maize roots. Plant Physiol. 1990, 93, 648–651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Razmjoo, K.; Heydarizadeh, P.; Sabzalian, M.R. Effect of salinity and drought stresses on growth parameters and essential oil content of Matricaria chamomile. Int. J. Agric. Biol. 2008, 10, 451–454. [Google Scholar]
- Kosturkova, G.; Todorova, R.; Sakthivelu, G.; Akitha Devi, M.; Giridhar, P.; Rajasekaran, T.; Ravishankar, G. Response of Bulgarian and Indian soybean genotypes to drought and water deficiency in field and laboratory conditions. Gen. Appl. Plant Physiol. 2008, 34, 239–250. [Google Scholar]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reza Yousefi, A.; Rashidi, S.; Moradi, P.; Mastinu, A. Germination and Seedling Growth Responses of Zygophyllum fabago, Salsola kali L. and Atriplex canescens to PEG-Induced Drought Stress. Environments 2020, 7, 107. https://0-doi-org.brum.beds.ac.uk/10.3390/environments7120107
Reza Yousefi A, Rashidi S, Moradi P, Mastinu A. Germination and Seedling Growth Responses of Zygophyllum fabago, Salsola kali L. and Atriplex canescens to PEG-Induced Drought Stress. Environments. 2020; 7(12):107. https://0-doi-org.brum.beds.ac.uk/10.3390/environments7120107
Chicago/Turabian StyleReza Yousefi, Ali, Sakineh Rashidi, Parviz Moradi, and Andrea Mastinu. 2020. "Germination and Seedling Growth Responses of Zygophyllum fabago, Salsola kali L. and Atriplex canescens to PEG-Induced Drought Stress" Environments 7, no. 12: 107. https://0-doi-org.brum.beds.ac.uk/10.3390/environments7120107