Proton Competition and Free Ion Activities Drive Cadmium, Copper, and Nickel Accumulation in River Biofilms in a Nordic Ecosystem
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
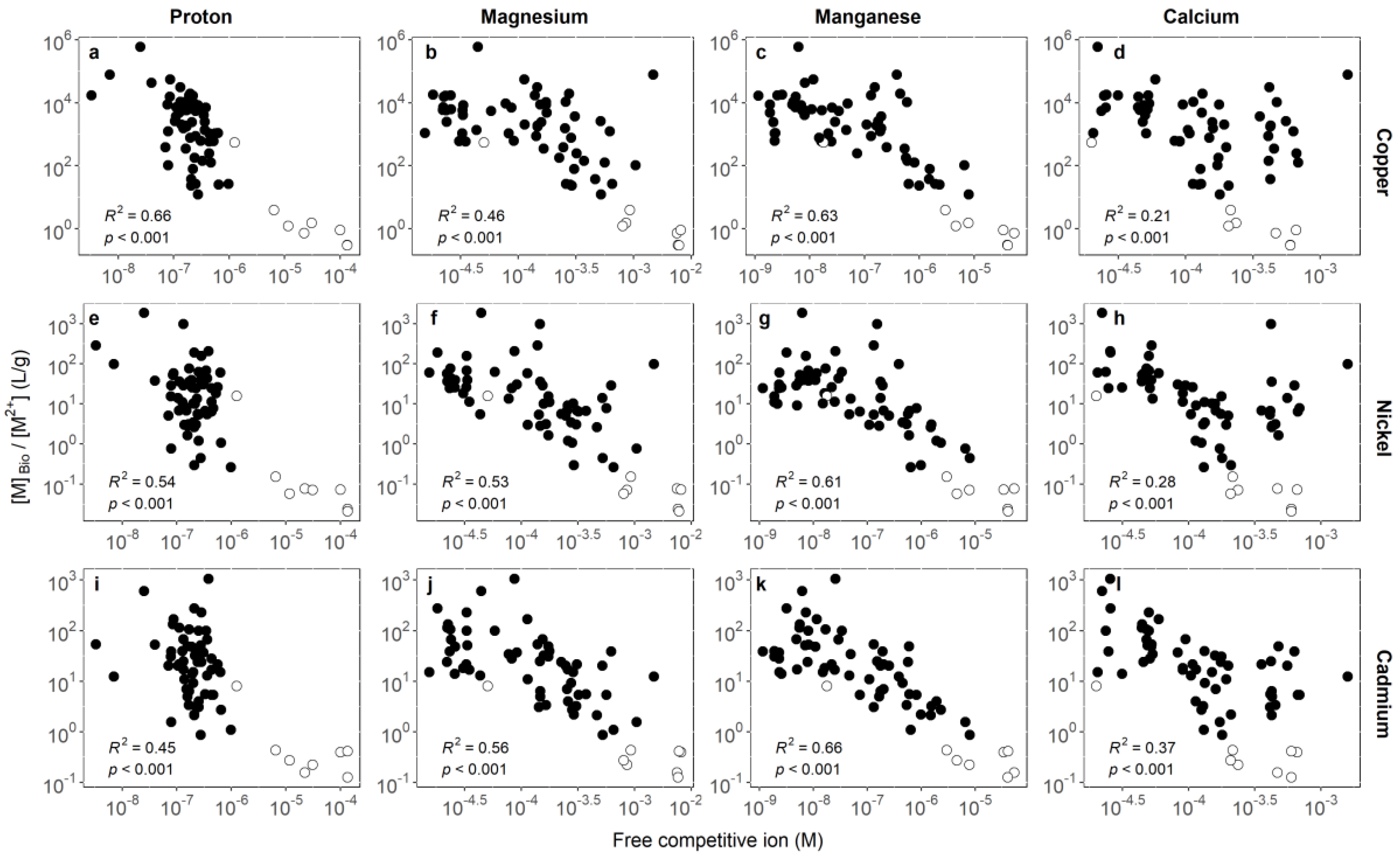
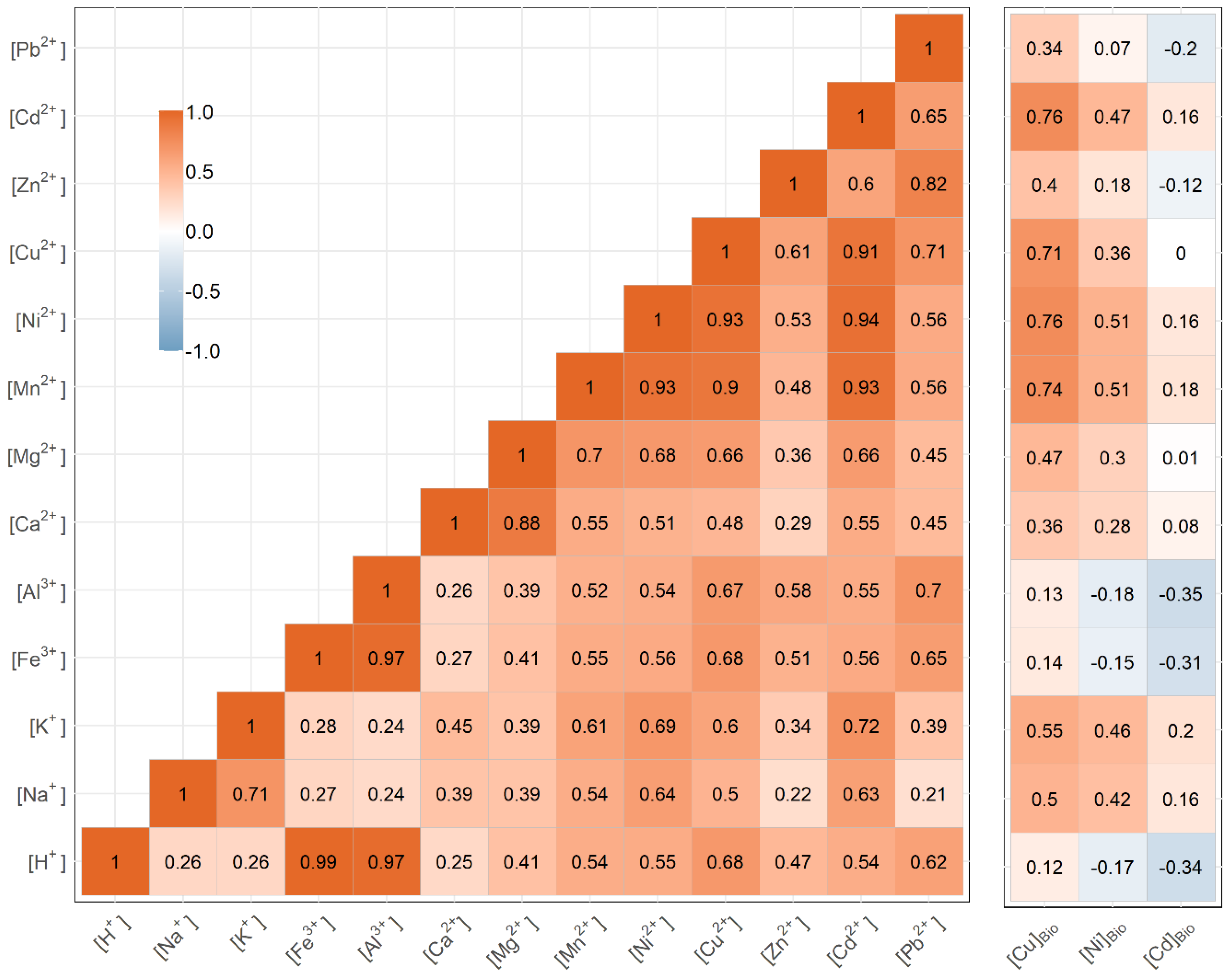
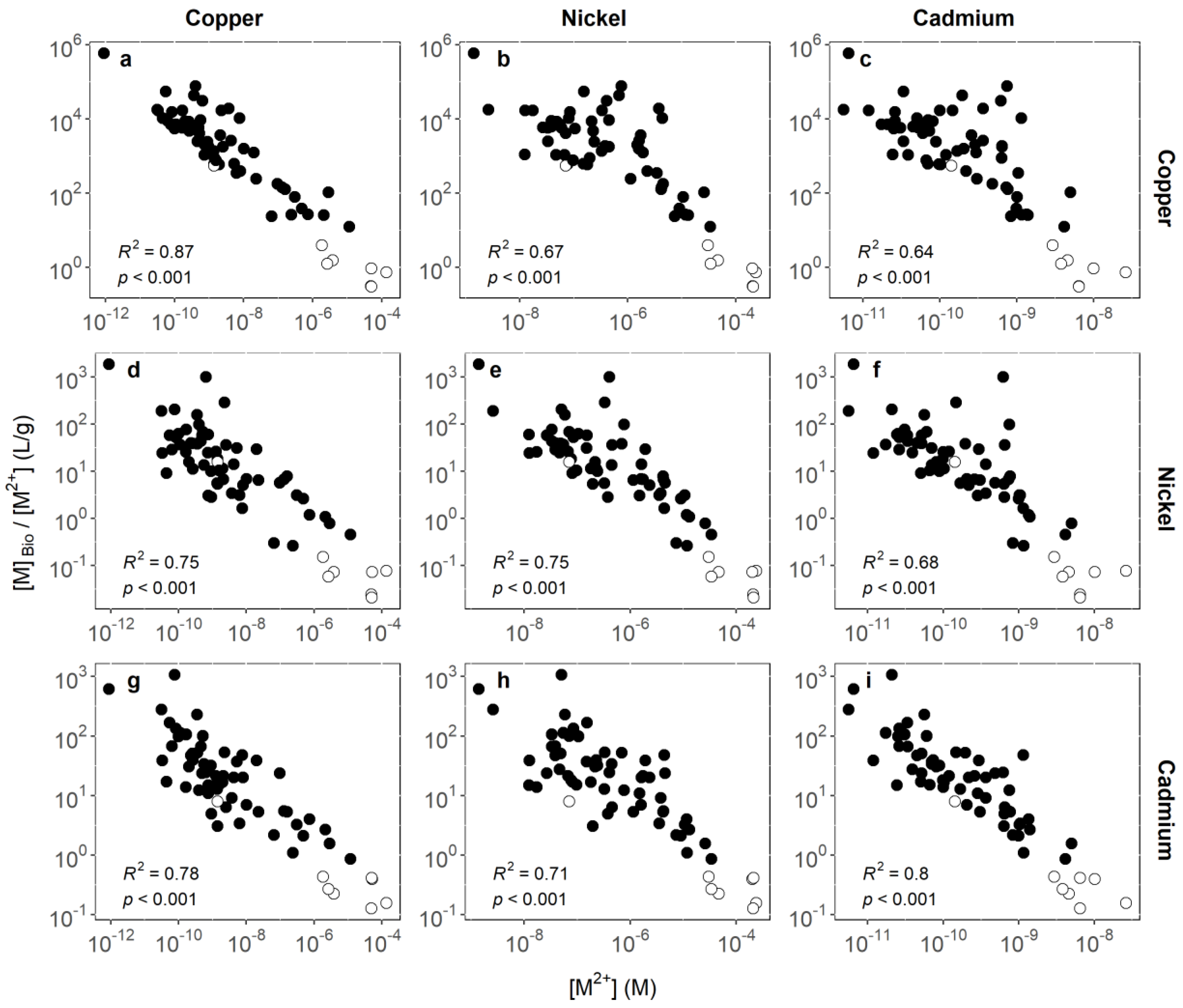
3.1. Identifying Key Modifying Variables for Metal Accumulation in Biofilms
3.2. Geographical Implications
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Data of the Materials
References
- Schaffartzik, A.; Mayer, A.; Eisenmenger, N.; Krausmann, F. Global patterns of metal extractivism, 1950–2010: Providing the bones for the industrial society’s skeleton. Ecol. Econ. 2016, 122, 101–110. [Google Scholar] [CrossRef]
- Pacyna, J.M.; Pacyna, E.G. An assessment of global and regional emissions of trace metals to the atmosphere from anthropogenic sources worldwide. Environ. Rev. 2001, 9, 269–298. [Google Scholar] [CrossRef]
- Sunda, W.G.; Guillard, R.R.L. The relationship between cupric ion activity and the toxicity of copper to phytoplankton. J. Mar. Res. 1976, 34, 511–529. [Google Scholar]
- Morel, F.M.M. Principles of Aquatic Chemistry; John Wiley & Sons: New York, NY, USA, 1983; p. 446. [Google Scholar]
- Campbell, P.G.C. Interactions between trace metals and aquatic organisms: A critique of the free-ion activity model. In Metal Speciation and Bioavailability in Aquatic Systems; Tessier, A., Turner, D.R., Eds.; John Wiley & Sons: New York, NY, USA, 1995; pp. 45–102. [Google Scholar]
- Di Toro, D.M.; Allen, H.E.; Bergman, H.L.; Meyer, J.S.; Paquin, P.R.; Santore, R.C. Biotic ligand model of the acute toxicity of metals. 1. Technical basis. Environ. Toxicol. Chem. 2001, 20, 2383–2396. [Google Scholar] [CrossRef]
- Mebane, C.A.; Chowdhury, M.J.; Vlaeminck, K.; Lofts, S.; Paquin, P.R.; Santore, R.C.; Wood, C.M. Metal Bioavailability models: Current status, lessons learned, considerations for regulatory use, and the path forward. Environ. Toxicol. Chem. 2020, 39, 60–84. [Google Scholar] [CrossRef] [Green Version]
- Brix, K.V.; Deforest, D.K.; Tear, L.; Peijnenburg, W.; Peters, A.; Middleton, E.T.; Erickson, R. Development of empirical bioavailability models for metals. Environ. Toxicol. Chem. 2020, 39, 85–100. [Google Scholar] [CrossRef] [Green Version]
- Campbell, P.G.C.; Fortin, C. Biotic Ligand Model. In Encyclopedia of Aquatic Ecotoxicology; Springer: Dordrecht, The Netherlands, 2013; pp. 237–246. [Google Scholar]
- Magalhães, D.D.P.; Marques, M.R.D.C.; Baptista, D.F.; Buss, D.F. Metal bioavailability and toxicity in freshwaters. Environ. Chem. Lett. 2015, 13, 69–87. [Google Scholar] [CrossRef]
- Morin, S.; Gómez, N.; Tornés, E.; Licursi, M.; Rosebery, J. Benthic Diatom Monitoring and Assessment of Freshwater Environments: Standard Methods and Future Challenges. In Aquatic Biofilms: Ecology, Water Quality and Wastewater Treatment; Caister Academic Press: Poole, UK, 2016; pp. 111–124. [Google Scholar]
- Hare, L. Sentinels (Biomonitors) of Trace Metals in Freshwaters. In Encyclopedia of Aquatic Ecotoxicology; Springer: Dordrecht, The Netherlands, 2013; pp. 1037–1046. [Google Scholar]
- Ponton, D.E.; Hare, L. Nickel dynamics in the lakewater metal biomonitor Chaoborus. Aquat. Toxicol. 2010, 96, 37–43. [Google Scholar] [CrossRef]
- Bonnineau, C.; Artigas, J.; Chaumet, B.; Dabrin, A.; Faburé, J.; Ferrari, B.J.D.; Lebrun, J.D.; Margoum, C.; Mazzella, N.; Miège, C.; et al. Role of biofilms in contaminant bioaccumulation and trophic transfer in aquatic ecosystems: Current state of knowledge and future challenges. In Reviews of Environmental Contamination and Toxicology (Continuation of Residue Reviews); Springer: New York, NY, USA, 2020. [Google Scholar]
- Morin, S.; Cordonier, A.; Lavoie, I.; Arini, A.; Blanco, S.; Duong, T.T.; Tornés, E.; Bonet, B.; Corcoll, N.; Faggiano, L.; et al. Consistency in Diatom Response to Metal-Contaminated Environments. In ICT for Smart Water Systems: Measurements and Data Science; Springer Nature: Basel, Switzerland, 2012; pp. 117–146. [Google Scholar]
- Newman, M.C.; McIntosh, A.W. Appropriateness of Aufwuchs as a monitor of bioaccumulation. Environ. Pollut. 1989, 60, 83–100. [Google Scholar] [CrossRef]
- Lavoie, I.; Campeau, S.; Zugic-Drakulic, N.; Winter, J.G.; Fortin, C. Using diatoms to monitor stream biological integrity in Eastern Canada: An overview of 10 years of index development and ongoing challenges. Sci. Total Environ. 2014, 475, 187–200. [Google Scholar] [CrossRef]
- Flemming, H.-C.; Wingender, J. The biofilm matrix. Nat. Rev. Genet. 2010, 8, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Lavoie, I.; Lavoie, M.; Fortin, C. A mine of information: Benthic algal communities as biomonitors of metal contamination from abandoned tailings. Sci. Total Environ. 2012, 425, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, W.O.; Collyard, S.A.; Larson, C.; Carey, A.J.; O’Neill, S.M. Toxic burdens of freshwater biofilms and use as a source tracking tool in rivers and streams. Environ. Sci. Technol. 2019, 53, 11102–11111. [Google Scholar] [CrossRef] [PubMed]
- Dranguet, P.; Le Faucheur, S.; Slaveykova, V.I. Mercury bioavailability, transformations, and effects on freshwater biofilms. Environ. Toxicol. Chem. 2017, 36, 3194–3205. [Google Scholar] [CrossRef]
- Markwart, B.; Liber, K.; Xie, Y.; Raes, K.; Hecker, M.; Janz, D.M.; Doig, L.E. Selenium oxyanion bioconcentration in natural freshwater periphyton. Ecotoxicol. Environ. Saf. 2019, 180, 693–704. [Google Scholar] [CrossRef]
- Lopez, A.R.; Funk, D.H.; Buchwalter, D.B. Arsenic (V) bioconcentration kinetics in freshwater macroinvertebrates and periphyton is influenced by pH. Environ. Pollut. 2017, 224, 82–88. [Google Scholar] [CrossRef]
- Bradac, P.; Wagner, B.; Kistler, D.; Traber, J.; Behra, R.; Sigg, L. Cadmium speciation and accumulation in periphyton in a small stream with dynamic concentration variations. Environ. Pollut. 2010, 158, 641–648. [Google Scholar] [CrossRef]
- Le Faucheur, S.; Behra, R.; Sigg, L. Thiol and metal contents in periphyton exposed to elevated copper and zinc concentrations: A field and microcosm study. Environ. Sci. Technol. 2005, 39, 8099–8107. [Google Scholar] [CrossRef]
- Allen, H.E. Importance of clean techniques and speciation in assessing water quality for metals. Hum. Ecol. Risk Assess. Int. J. 2000, 6, 989–1002. [Google Scholar] [CrossRef]
- Conley, J.M.; Funk, D.H.; Buchwalter, D.B. Selenium bioaccumulation and maternal transfer in the mayfly Centroptilum triangulifer in a life-cycle, periphyton-biofilm trophic assay. Environ. Sci. Technol. 2009, 43, 7952–7957. [Google Scholar] [CrossRef]
- Xie, L.; Funk, D.H.; Buchwalter, D.B. Trophic transfer of Cd from natural periphyton to the grazing mayfly Centroptilum triangulifer in a life cycle test. Environ. Pollut. 2010, 158, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Funk, D.H.; Buchwalter, D.B. Dietary (periphyton) and aqueous Zn bioaccumulation dynamics in the mayfly Centroptilum triangulifer. Ecotoxicology 2012, 21, 2288–2296. [Google Scholar] [CrossRef] [PubMed]
- Leguay, S.; Lavoie, I.; Levy, J.L.; Fortin, C. Using biofilms for monitoring metal contamination in lotic ecosystems: The protective effects of hardness and pH on metal bioaccumulation. Environ. Toxicol. Chem. 2016, 35, 1489–1501. [Google Scholar] [CrossRef]
- Lavoie, I.; Morin, S.; Laderriere, V.; Paris, L.-E.; Fortin, C. Assessment of diatom assemblages in close proximity to mining activities in Nunavik, Northern Quebec (Canada). Environments 2019, 6, 74. [Google Scholar] [CrossRef] [Green Version]
- Perdue, E.; Ritchie, J. Dissolved Organic Matter in Freshwaters; Elsevier BV: Amsterdam, The Netherlands, 2003; Volume 5, pp. 273–318. [Google Scholar]
- Campbel, P.G.C.; Stokes, P.M. Acidification and toxicity of metals to aquatic biota. Can. J. Fish. Aquat. Sci. 1985, 42, 2034–2049. [Google Scholar] [CrossRef]
- Wilde, K.L.; Stauber, J.L.; Markich, S.J.; Franklin, N.M.; Brown, P.L. The effect of pH on the uptake and toxicity of copper and zinc in a tropical freshwater alga (Chlorella sp.). Arch. Environ. Contam. Toxicol. 2006, 51, 174–185. [Google Scholar] [CrossRef] [PubMed]
- François, L.; Fortin, C.; Campbell, P.G.C.; François, L. pH modulates transport rates of manganese and cadmium in the green alga Chlamydomonas reinhardtii through non-competitive interactions: Implications for an algal BLM. Aquat. Toxicol. 2007, 84, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Playle, R.C. Modelling metal interactions at fish gills. Sci. Total. Environ. 1998, 219, 147–163. [Google Scholar] [CrossRef]
- Brown, P. Evaluation of the free ion activity model of metal-organism interaction: Extension of the conceptual model. Aquat. Toxicol. 2000, 51, 177–194. [Google Scholar] [CrossRef]
- Hare, L.; Tessier, A. Predicting animal cadmium concentrations in lakes. Nat. Cell Biol. 1996, 380, 430–432. [Google Scholar] [CrossRef]
- Croteau, M.-N.; Hare, L.; Tessier, A. Refining and testing a trace metal biomonitor (Chaoborus) in highly acidic lakes. Environ. Sci. Technol. 1998, 32, 1348–1353. [Google Scholar] [CrossRef]
- Ponton, D.E.; Hare, L. Assessment of nickel contamination in lakes using the phantom midge Chaoborus as a biomonitor. Environ. Sci. Technol. 2009, 43, 6529–6534. [Google Scholar] [CrossRef] [PubMed]
- Kola, H.; Wilkinson, K.J. Cadmium uptake by a green alga can be predicted by equilibrium modelling. Environ. Sci. Technol. 2005, 39, 3040–3047. [Google Scholar] [CrossRef] [PubMed]
- Paquin, P.R.; Gorsuch, J.W.; Apte, S.; Batley, G.E.; Bowles, K.C.; Campbell, P.G.; Delos, C.G.; Di Toro, D.M.; Dwyer, R.L.; Galvez, F.; et al. The biotic ligand model: A historical overview. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2002, 133, 3–35. [Google Scholar] [CrossRef]
- Pagenkopf, G.K. Gill surface interaction model for trace-metal toxicity to fishes: Role of complexation, pH, and water hardness. Environ. Sci. Technol. 1983, 17, 342–347. [Google Scholar] [CrossRef]
- Ardestani, M.M.; Van Straalen, N.M.; Van Gestel, C.A.M. Biotic ligand modeling approach: Synthesis of the effect of major cations on the toxicity of metals to soil and aquatic organisms. Environ. Toxicol. Chem. 2015, 34, 2194–2204. [Google Scholar] [CrossRef] [Green Version]
- Crémazy, A.; Brix, K.V.; Wood, C.M. Using the Biotic Ligand Model framework to investigate binary metal interactions on the uptake of Ag, Cd, Cu, Ni, Pb and Zn in the freshwater snail Lymnaea stagnalis. Sci. Total Environ. 2019, 647, 1611–1625. [Google Scholar] [CrossRef]
- Lavoie, M.; Campbell, P.G.C.; Fortin, C. Predicting cadmium accumulation and toxicity in a green alga in the presence of varying essential element concentrations using a biotic ligand model. Environ. Sci. Technol. 2014, 48, 1222–1229. [Google Scholar] [CrossRef]
- Mebane, C.A.; Schmidt, T.S.; Miller, J.L.; Balistrieri, L.S. Bioaccumulation and toxicity of cadmium, copper, nickel and zinc and their mixtures to aquatic insect communities. Environ. Toxicol. Chem. 2020, 39, 812–833. [Google Scholar] [CrossRef] [Green Version]
- McGeer, J.C.; Brix, K.V.; Skeaff, J.M.; Deforest, D.K.; Brigham, S.I.; Adams, W.J.; Green, A. Inverse relationship between bioconcentration factor and exposure concentration for metals: Implications for hazard assessment of metals in the aquatic environment. Environ. Toxicol. Chem. 2003, 22, 1017–1037. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laderriere, V.; Paris, L.-E.; Fortin, C. Proton Competition and Free Ion Activities Drive Cadmium, Copper, and Nickel Accumulation in River Biofilms in a Nordic Ecosystem. Environments 2020, 7, 112. https://0-doi-org.brum.beds.ac.uk/10.3390/environments7120112
Laderriere V, Paris L-E, Fortin C. Proton Competition and Free Ion Activities Drive Cadmium, Copper, and Nickel Accumulation in River Biofilms in a Nordic Ecosystem. Environments. 2020; 7(12):112. https://0-doi-org.brum.beds.ac.uk/10.3390/environments7120112
Chicago/Turabian StyleLaderriere, Vincent, Louise-Emmanuelle Paris, and Claude Fortin. 2020. "Proton Competition and Free Ion Activities Drive Cadmium, Copper, and Nickel Accumulation in River Biofilms in a Nordic Ecosystem" Environments 7, no. 12: 112. https://0-doi-org.brum.beds.ac.uk/10.3390/environments7120112






