A Case Study on Metal Contamination in Water and Sediment near a Coal Thermal Power Plant on the Eastern Coast of Bangladesh
Abstract
:1. Introduction
- (a)
- To assess the water quality parameters of the study area.
- (b)
- To investigate the elemental concentration in sub-surface water and bottom-surface sediment
- (c)
- To understand the sediment pollution level
- (d)
- To identify the possible environmental and human health risks
- (e)
- To identify the sources of the pollutants
2. Materials and Methods
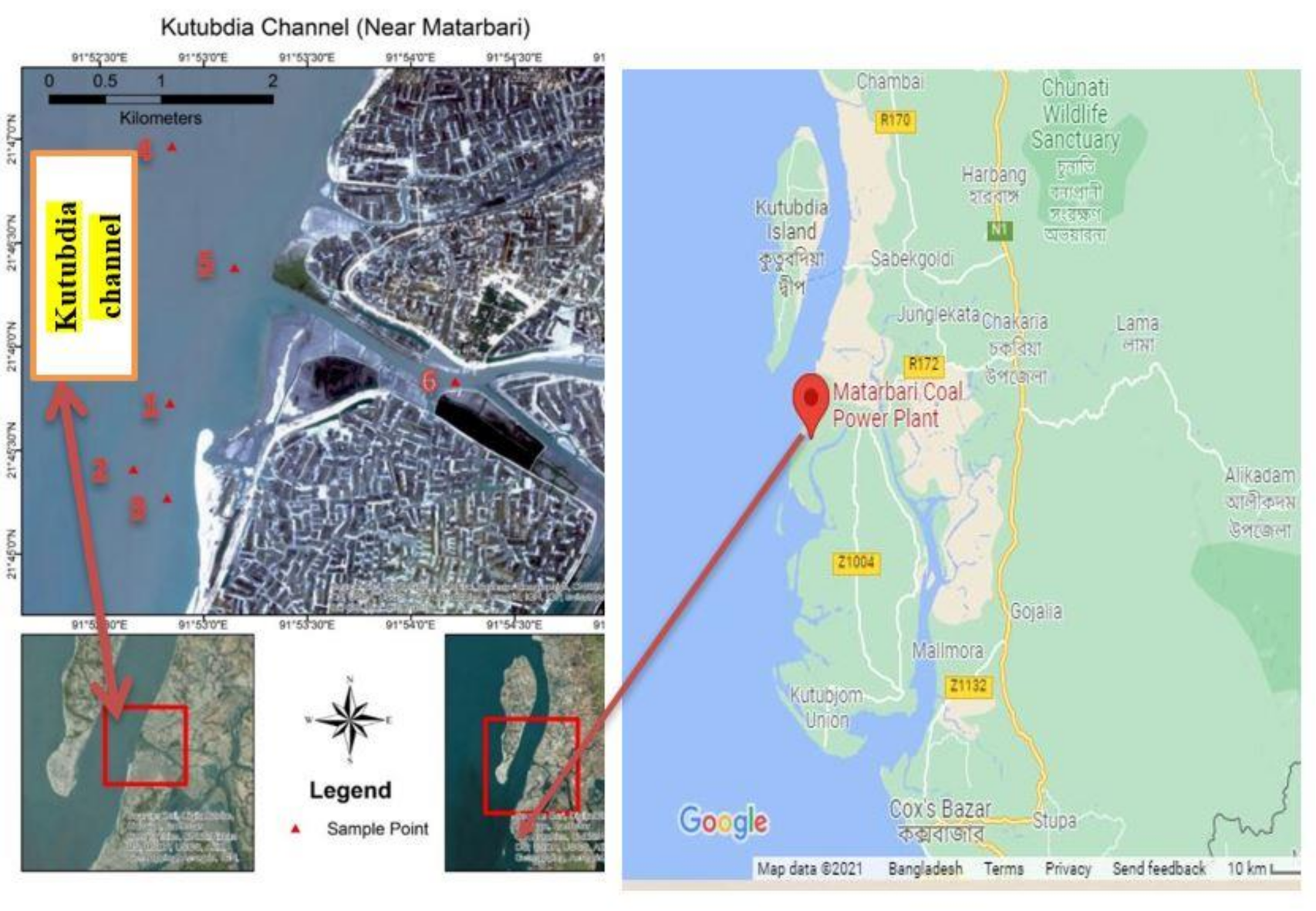
2.1. Study Area
2.2. Water Multi-Parameters Detection
2.3. Sample Collection
2.4. Sample Preparation and Analysis
2.5. Sediment Pollution Assessment
2.5.1. Contamination Factor (CF) and Degree of Contamination (CD)
2.5.2. Modified Degree of Contamination (MCD)
2.5.3. The Pollution Load Index (PLI)
2.5.4. Geoaccumulation Index (Igeo)
2.5.5. Enrichment Factors (EF)
2.6. Potential Ecological Risk (PER) Assessment
2.7. Human Health Risk Assessment Index
2.8. Statistical Analysis
2.9. Accuracy and Precision
3. Results and Discussion
3.1. Physicochemical Qualities of Water
3.2. Trace and Heavy Metal Contamination in Meghna Estuarine Water
3.3. Elemental Concentration in Sediment
3.4. Sediment Pollution Assessment
3.5. Potential Ecological Risk (PER) Assessment
3.6. Human Health Risk Assessment
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jayaprakash, M.; Urban, B.; Velmurugan, P.M.; Srinivasalu, S. Accumulation of total trace metals due to rapid urbanization in microtidal zone of Pallikaranai marsh, South of Chennai, India. Environ. Monit. Assess. 2010, 170, 609–629. [Google Scholar] [CrossRef] [PubMed]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef] [PubMed]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. Exp. Suppl. 2012, 101, 133–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, H.; Khan, E.; Ilahi, I. Environmental Chemistry and Ecotoxicology of Hazardous Heavy Metals: Environmental Persistence, Toxicity, and Bioaccumulation. J. Chem. 2019, 2019, 6730305. [Google Scholar] [CrossRef] [Green Version]
- Giri, S.; Singh, A.K. Human health risk and ecological risk assessment of metals in fishes, shrimps and sediment from a tropical river. Int. J. Environ. Sci. Technol. 2015, 12, 2349–2362. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.S.; Saha, N.; Molla, A.H. Potential ecological risk assessment of heavy metal contamination in sediment and water body around Dhaka export processing zone, Bangladesh. Environ. Earth Sci. 2014, 71, 2293–2308. [Google Scholar] [CrossRef]
- Ahmad, W.; Alharthy, R.D.; Zubair, M.; Ahmed, M.; Hameed, A.; Rafique, S. Toxic and heavy metals contamination assessment in soil and water to evaluate human health risk. Sci. Rep. 2021, 11, 17006. [Google Scholar] [CrossRef]
- Palansooriya, K.N.; Shaheen, S.M.; Chen, S.S.; Tsang, D.C.W.; Hashimoto, Y.; Hou, D.; Bolan, N.S.; Rinklebe, J.; Ok, Y.S. Soil amendments for immobilization of potentially toxic elements in contaminated soils: A critical review. Environ. Int. 2020, 134, 105046. [Google Scholar] [CrossRef]
- Rinklebe, J.; Antoniadis, V.; Shaheen, S.M.; Rosche, O.; Altermann, M. Health risk assessment of potentially toxic elements in soils along the Central Elbe River, Germany. Environ. Int. 2019, 126, 76–88. [Google Scholar] [CrossRef]
- Hosono, T.; Su, C.-C.; Delinom, R.; Umezawa, Y.; Toyota, T.; Kaneko, S.; Taniguchi, M. Decline in heavy metal contamination in marine sediments in Jakarta Bay, Indonesia due to increasing environmental regulations. Estuar. Coast. Shelf Sci. 2011, 92, 297–306. [Google Scholar] [CrossRef]
- Safiur Rahman, M.; Solaiman Hossain, M.; Ahmed, M.K.; Akther, S.; Jolly, Y.N.; Akhter, S.; Jamiul Kabir, M.; Choudhury, T.R. Assessment of heavy metals contamination in selected tropical marine fish species in Bangladesh and their impact on human health. Environ. Nanotechnol. Monit. Manag. 2019, 11, 100210. [Google Scholar] [CrossRef]
- Hossain, M.S.; Ahmed, M.K.; Sarker, S.; Rahman, M.S. Seasonal variations of trace metals from water and sediment samples in the northern Bay of Bengal. Ecotoxicol. Environ. Saf. 2020, 193, 110347. [Google Scholar] [CrossRef]
- Filgueiras, A.; Lavilla, I.; Bendicho, C. Evaluation of distribution, mobility and binding behaviour of heavy metals in surficial sediments of Louro River (Galicia, Spain) using chemometric analysis: A case study. Sci. Total Environ. 2004, 330, 115–129. [Google Scholar] [CrossRef]
- Joksimovic, D.; Tomic, I.; Stankovic, A.R.; Jovic, M.; Stankovic, S. Trace metal concentrations in Mediterranean blue mussel and surface sediments and evaluation of the mussels quality and possible risks of high human consumption. Food Chem. 2011, 127, 632–637. [Google Scholar] [CrossRef] [PubMed]
- Luoma, S.N.; Bryan, G. A statistical assessment of the form of trace metals in oxidized estuarine sediments employing chemical extractants. Sci. Total Environ. 1981, 17, 165–196. [Google Scholar] [CrossRef]
- Rahman, M.S.; Saha, N.; Molla, A.H.; Al-Reza, S.M. Assessment of Anthropogenic Influence on Heavy Metals Contamination in the Aquatic Ecosystem Components: Water, Sediment, and Fish. Soil Sediment Contam. Int. J. 2014, 23, 353–373. [Google Scholar] [CrossRef]
- CPGCBL. The Current Project of CPGCBL (The Coal Power Generation Company Bangladesh Limited): Matarbari Coal Thermal Power Plant; Ministry of Power: Dhaka, Bangladesh, 2021. [Google Scholar]
- Oyedotun, T.D.T. X-ray fluorescence (XRF) in the investigation of the composition of earth materials: A review and an overview. Geol. Ecol. Landsc. 2018, 2, 148–154. [Google Scholar] [CrossRef]
- Hossain, M.B.; Habib, S.B.; Hossain, M.S.; Jolly, Y.N.; Kamal, A.H.M.; Idris, M.H.; Rakib, M.R.J. Data set on trace metals in surface sediment and water from a sub-tropical estuarine system, Bay of Bengal, Bangladesh. Data Brief 2020, 31, 105911. [Google Scholar] [CrossRef]
- Tiwari, M.; Sahu, S.K.; Rathod, T.D.; Bhangare, R.C.; Ajmal, P.Y.; Vinod Kumar, A. Determination of trace elements in salt and seawater samples by energy dispersive X-ray fluorescence spectrometry. J. Radioanal. Nucl. Chem. 2020, 325, 751–756. [Google Scholar] [CrossRef]
- Tung, J.W. Determination of metal components in marine sediments using energy-dispersive X-ray fluorescence (ED-XRF) spectrometry. Ann. Chim. 2004, 94, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.; Taylor, A. On site determination of trace metals in estuarine sediments by field-portable-XRF. Talanta 2018, 190, 498–506. [Google Scholar] [CrossRef]
- Hakanson, L. An ecological risk index for aquatic pollution control. A sedimentological approach. Water Res. 1980, 14, 975–1001. [Google Scholar] [CrossRef]
- Turekian, K.K.; Wedepohl, K.H. Distribution of the elements in some major units of the earth’s crust. Geol. Soc. Am. Bull. 1961, 72, 175–192. [Google Scholar] [CrossRef]
- Abrahim, G.M.S.; Parker, R.J. Assessment of heavy metal enrichment factors and the degree of contamination in marine sediments from Tamaki Estuary, Auckland, New Zealand. Environ. Monit. Assess. 2008, 136, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, D.; Wilson, J.; Harris, C.; Jeffrey, D. Problems in the assessment of heavy-metal levels in estuaries and the formation of a pollution index. Helgoländer Meeresunters 1980, 33, 566. [Google Scholar] [CrossRef] [Green Version]
- Muller, G. Index of geoaccumulation in sediments of the Rhine River. Geojournal 1969, 2, 108–118. [Google Scholar]
- Rabee, A.M.; Al-Fatlawy, Y.F.; Nameer, M. Using Pollution Load Index (PLI) and geoaccumulation index (I-Geo) for the assessment of heavy metals pollution in Tigris river sediment in Baghdad Region. Al-Nahrain J. Sci. 2011, 14, 108–114. [Google Scholar]
- Abolfazl, N.; Ahmad, I. Risk assessment of mercury contamination in surface sediment of the Klang River, Malaysia. Aust. J. Basic Appl. Sci. 2011, 5, 215–221. [Google Scholar]
- Qiao, Y.; Yang, Y.; Zhao, J.; Tao, R.; Xu, R. Influence of urbanization and industrialization on metal enrichment of sediment cores from Shantou Bay, South China. Environ. Pollut. 2013, 182, 28–36. [Google Scholar] [CrossRef]
- Zhang, L.; Ye, X.; Feng, H.; Jing, Y.; Ouyang, T.; Yu, X.; Liang, R.; Gao, C.; Chen, W. Heavy metal contamination in western Xiamen Bay sediments and its vicinity, China. Mar. Pollut. Bull. 2007, 54, 974–982. [Google Scholar] [CrossRef]
- Neto, J.B.; Smith, B.; McAllister, J. Heavy metal concentrations in surface sediments in a nearshore environment, Jurujuba Sound, Southeast Brazil. Environ. Pollut. 2000, 109, 1–9. [Google Scholar] [CrossRef]
- Zoller, W.H.; Gladney, E.S.; Duce, R.A. Atmospheric concentrations and sources of trace metals at the South pole. Science 1974, 183, 198–200. [Google Scholar] [CrossRef]
- USEPA. Human Health Risk Assessment; Environmetal Protection Agency, Ed.; USEPA: Washington, DC, USA, 2020. [Google Scholar]
- Ihedioha, J.; Ukoha, P.; Ekere, N. Ecological and human health risk assessment of heavy metal contamination in soil of a municipal solid waste dump in Uyo, Nigeria. Environ. Geochem. Health 2017, 39, 497–515. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Chen, J. Risk assessment of potentially toxic elements (PTEs) pollution at a rural industrial wasteland in an abandoned metallurgy factory in North China. Int. J. Environ. Res. Public Health 2018, 15, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onyele, O.G.; Anyanwu, E.D. Human health risk assessment of some heavy metals in a Rural Spring, Southeastern Nigeria. Afr. J. Environ. Nat. Sci. Res 2018, 1, 15–23. [Google Scholar]
- Sojka, M.; Siepak, M.; Zioła, A.; Frankowski, M.; Murat-Błażejewska, S.; Siepak, J. Application of multivariate statistical techniques to evaluation of water quality in the Mała Wełna River (Western Poland). Environ. Monit. Assess. 2008, 147, 159–170. [Google Scholar] [CrossRef]
- Siepak, M.; Sojka, M. Application of multivariate statistical approach to identify trace elements sources in surface waters: A case study of Kowalskie and Stare Miasto reservoirs, Poland. Environ. Monit. Assess. 2017, 189, 364. [Google Scholar] [CrossRef] [Green Version]
- Sojka, M.; Siepak, M.; Jaskuła, J.; Wicher-Dysarz, J. Heavy Metal Transport in a River-Reservoir System: A Case Study from Central Poland. Pol. J. Environ. Stud. 2018, 27, 1725–1734. [Google Scholar] [CrossRef]
- Patil, P.; Sawant, D.; Deshmukh, R. Physico-chemical parameters for testing of water–A review. Int. J. Environ. Sci. 2012, 3, 1194–1207. [Google Scholar]
- DPHE. Water Quality Parameters Bangladesh Standards & WHO Guide Lines; Rahman, M.S., Ed.; DPHE: Dhaka, Bangladesh, 2019. [Google Scholar]
- Patel, H.; Vashi, R.T. (Eds.) Chapter 2—Characterization of Textile Wastewater. In Characterization and Treatment of Textile Wastewater; Elsevier: Boston, MA, USA, 2015; pp. 21–71. [Google Scholar] [CrossRef]
- Ahmed, M.F.; Rahman, M. Water Supply and Sanitation: Rural and low income urban communities. ITN-Bangladesh; Center for Water Supply and Waste Management, BUET: Dhaka, Bangladesh, 2000; pp. 191–195. [Google Scholar]
- Aoi, W.; Marunaka, Y. Importance of pH homeostasis in metabolic health and diseases: Crucial role of membrane proton transport. BioMed Res. Int. 2014, 2014, 598986. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.; Chowdhury, S. Physical and chemical limnology of lake Kaptai, Bangladesh. Trop. Ecol. 1994, 35, 35–51. [Google Scholar]
- WHO. Guidelines for drinking-water quality, [Chapter 12] Chemical fact sheets. Fluoride 2011, 38, 370–373. [Google Scholar]
- Omer, N.H. Water quality parameters. In Water Quality-Science, Assessments and Policy; Book Chapter; IntechOpen: London, UK, 2019; p. 18. [Google Scholar]
- Kumar, N.; Kumar, D.; Kumar, S.; Shukla, V.; Shukla, P.; Raj, B. Spatio-temporal variations in hydro-geochemistry of groundwater at rural, urban and industrial areas of Kanpur, India. Environ. Sustain. 2018, 1, 197–208. [Google Scholar] [CrossRef]
- Mondal, N.; Singh, V.; Singh, V.; Saxena, V. Determining the interaction between groundwater and saline water through groundwater major ions chemistry. J. Hydrol. 2010, 388, 100–111. [Google Scholar] [CrossRef]
- Lawson, E. Physico-chemical parameters and heavy metal contents of water from the Mangrove Swamps of Lagos Lagoon, Lagos, Nigeria. Adv. Biol. Res. 2011, 5, 8–21. [Google Scholar]
- Lotliker, A.A.; Baliarsingh, S.K.; Sahu, K.C.; Kumar, T.S. Long-term chlorophyll-a dynamics in tropical coastal waters of the western Bay of Bengal. Environ. Sci. Pollut. Res. 2020, 27, 6411–6419. [Google Scholar] [CrossRef]
- Murty, V.; Subrahmanyam, B.; Gangadhara Rao, L.; Reddy, G. Seasonal variation of sea surface temperature in the Bay of Bengal during 1992 as derived from NOAA-AVHRR SST data. Int. J. Remote Sens. 1998, 19, 2361–2372. [Google Scholar] [CrossRef]
- EWG. EWG’s Guide to Safe Drinking Water: Reduce Your Exposures to Common Drinking Water Pollutants with EWG’s Handy Tipsheet; Environmental Working Group (EWG): Washington, DC, USA, 2019; p. 20009. [Google Scholar]
- Melnyk, L.J.; Donohue, M.J.; Pham, M.; Donohue, J. Absorption of strontium by foods prepared in drinking water. J. Trace Elem. Med. Biol. 2019, 53, 22–26. [Google Scholar] [CrossRef]
- Nordstrom, D.K. Public health. Worldwide occurrences of arsenic in ground water. Science 2002, 296, 2143–2145. [Google Scholar] [CrossRef]
- Linos, A.; Petralias, A.; Christophi, C.A.; Christoforidou, E.; Kouroutou, P.; Stoltidis, M.; Veloudaki, A.; Tzala, E.; Makris, K.C.; Karagas, M.R. Oral ingestion of hexavalent chromium through drinking water and cancer mortality in an industrial area of Greece--an ecological study. Environ Health 2011, 10, 5057Liu. [Google Scholar] [CrossRef] [Green Version]
- Ward, M.H.; Jones, R.R.; Brender, J.D.; de Kok, T.M.; Weyer, P.J.; Nolan, B.T.; Villanueva, C.M.; van Breda, S.G. Drinking Water Nitrate and Human Health: An Updated Review. Int. J. Environ. Res. Public Health 2018, 15, 1557. [Google Scholar] [CrossRef] [Green Version]
- Asante, K.A.; Agusa, T.; Subramanian, A.; Ansa-Asare, O.D.; Biney, C.A.; Tanabe, S. Contamination status of arsenic and other trace elements in drinking water and residents from Tarkwa, a historic mining township in Ghana. Chemosphere 2007, 66, 1513–1522. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, D.D.; Ingersoll, C.G.; Berger, T.A. Development and evaluation of consensus-based sediment quality guidelines for freshwater ecosystems. Arch. Environ. Contam. Toxicol. 2000, 39, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Woszczyk, M.; Spychalski, W.; Boluspaeva, L. Trace metal (Cd, Cu, Pb, Zn) fractionation in urban-industrial soils of Ust-Kamenogorsk (Oskemen), Kazakhstan-implications for the assessment of environmental quality. Environ. Monit. Assess. 2018, 190, 362. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Lu, H.; Lei, K.; Wang, W.; Guan, Y. Trace metal element pollution of soil and water resources caused by small-scale metallic ore mining activities: A case study from a sphalerite mine in North China. Environ. Sci. Pollut. Res. Int. 2019, 26, 24630–24644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reimann, C.; de Caritat, P. Distinguishing between natural and anthropogenic sources for elements in the environment: Regional geochemical surveys versus enrichment factors. Sci. Total Environ. 2005, 337, 91–107. [Google Scholar] [CrossRef] [PubMed]
- Shotyk, W.; Krachler, M.; Aeschbach-Hertig, W.; Hillier, S.; Zheng, J.J. Trace elements in recent groundwater of an artesian flow system and comparison with snow: Enrichments, depletions, and chemical evolution of the water. J. Environ. Monit. 2010, 12, 208–217. [Google Scholar] [CrossRef]
- Wuana, R.A.; Okieimen, F.E. Heavy Metals in Contaminated Soils: A Review of Sources, Chemistry, Risks and Best Available Strategies for Remediation. ISRN Ecol. 2011, 2011, 402647. [Google Scholar] [CrossRef] [Green Version]
- Hou, S.; Zheng, N.; Tang, L.; Ji, X.; Li, Y.; Hua, X. Pollution characteristics, sources, and health risk assessment of human exposure to Cu, Zn, Cd and Pb pollution in urban street dust across China between 2009 and 2018. Environ. Int. 2019, 128, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Said, I.; Salman, S.A.; Elnazer, A.A. Multivariate statistics and contamination factor to identify trace elements pollution in soil around Gerga City, Egypt. Bull. Natl. Res. Cent. 2019, 43, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Devi, N.L.; Yadav, I.C.; Shihua, Q.; Dan, Y.; Zhang, G.; Raha, P. Environmental carcinogenic polycyclic aromatic hydrocarbons in soil from Himalayas, India: Implications for spatial distribution, sources apportionment and risk assessment. Chemosphere 2016, 144, 493–502. [Google Scholar] [CrossRef] [PubMed]


| Sampling Station No. | Latitude | Longitude | Temp (°C) | DO (ppm) | Salinity (PSU) | pH | TD S(g/L) | EC (mS/cm) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre M | Post M | Pre M | Post M | Pre M | Post M | Pre M | Post M | Pre M | Post M | Pre M | Post M | |||
| 01 | 21°45.269′ N | 91°52.824′ E | 28.1 | 29.7 | 7.51 | 7.11 | 31.0 | 22.10 | 8.35 | 7.89 | 29.7 | 21.30 | 52.8 | 37.8 |
| 02 | 21°45.409′ N | 91°52.661′ E | 28.3 | 29.3 | 7.58 | 7.16 | 30.9 | 21.87 | 8.52 | 7.95 | 29.6 | 20.96 | 51.7 | 37.2 |
| 03 | 21°45.728′ N | 91°52.838′ E | 29.0 | 29.8 | 7.89 | 7.20 | 30.9 | 21.94 | 8.52 | 8.06 | 29.8 | 21.11 | 52.7 | 37.3 |
| 04 | 21°46.381′ N | 91°53.149′ E | 28.5 | 29.7 | 7.77 | 7.20 | 30.8 | 21.53 | 8.51 | 8.05 | 29.6 | 20.21 | 51.7 | 36.0 |
| 05 | 21°46.966′ N | 91°52.847′ E | 28.1 | 29.8 | 7.87 | 6.99 | 30.5 | 21.95 | 8.37 | 8.02 | 29.4 | 20.77 | 51.0 | 36.9 |
| 06 | 21°45.832′ N | 91°54.213′ E | 28.7 | 30.3 | 7.43 | 7.25 | 31.3 | 21.68 | 8.46 | 8.04 | 30.1 | 20.90 | 52.4 | 37.1 |
| Average | 28.45 | 29.77 | 7.68 | 7.15 | 30.9 | 21.8 | 8.46 | 8 | 29.7 | 20.88 | 52.05 | 37.05 | ||
| Elements | Pre-Monsoon | Post Monsoon | WHO Standard [47] (ppm = mg/L) | Bangladesh Standard [42] (mg/L) | U.S. EPA Standard [34] (mg/L) |
|---|---|---|---|---|---|
| Cr | 0.060 ± 0.004 | 0.060 ± 0.003 | 0.05 | 0.05 | 0.01 |
| Mn | 0.084 ± 0.009 | 0.090 ± 0.009 | 0.5 | 0.1 | 0.05 |
| Cu | 0.030 ± 0.003 | 0.026 ± 0.004 | 2 | 1 | 1.3 |
| Zn | 0.087 ± 0.016 | 0.073 ± 0.006 | NG | 5 | 5 |
| As | BDL | BDL | 0.01 | 0.05 | 0.01 |
| Pb | 0.026 ± 0.002 | 0.026 ± 0.003 | 0.01 | 0.05 | 0.015 |
| Co | BDL | BDL | NG | NG | 0.7 * |
| Fe | 0.080 ± 0.012 | 0.055 ± 0.003 | 2 | 0.3–1.0 | 0.3 |
| Sr | 0.511 ± 0.011 | 0.413 ± 0.012 | NG | NG | 4 |
| Sediment Quality Guidelines [60] | ||||||
|---|---|---|---|---|---|---|
| Metals | Pre-Monsoon | Post-Monsoon | World Average Shale [24] | TEL | PEL | SEL |
| Cr | 10.69 ± 1.46 | 12.22 ± 0.82 | 90 | 37.3 | 90 | 110 |
| Mn | 570.67 ± 31.64 | 606.25 ± 43.83 | 850 | NA | NA | 1100 |
| Cu | 145.61 ± 21.87 | 135.41 ± 9.38 | 45 | 35.7 | 197 | 110 |
| Zn | 149.83 ± 12.59 | 146.92 ± 6.43 | 95 | 123 | 315 | 820 |
| As | 7.93 ± 0.44 | 10.12 ± 1.49 | 13 | 5.9 | 17 | 33 |
| Pb | 21.55 ± 1.96 | 23.90 ± 2.46 | 20 | 35 | 91.3 | 250 |
| Co | 3.95 ± 0.15 | 4.04 ± 0.37 | 19 | NA | NA | NA |
| Fe | 2317.08 ± 116.22 | 2434.67 ± 165.66 | 47,200 | NA | NA | NA |
| Sr | 119.08 ± 2.91 | 128.17 ± 11.59 | 300 | NA | NA | NA |
| Stations | Contamination Factor, Cf | Cd | mCd | PLI | Contamination Level | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cr | Mn | Cu | Zn | As | Pb | Co | Fe | Sr | |||||
| Pre Monsoon | 0.12 | 0.67 | 3.24 a | 1.58 | 0.61 | 1.08 | 0.21 | 0.05 | 0.4 | 7.94 | 0.88 | 0 | Low risk/baseline pollution/perfection |
| Post Monsoon | 0.14 | 0.71 | 3.01 a | 1.55 | 0.78 | 1.2 | 0.21 | 0.05 | 0.43 | 8.07 | 0.9 | 0 | |
| Average | 0.13 | 0.69 | 3.13 a | 1.57 | 0.7 | 1.14 | 0.21 | 0.05 | 0.42 | 8.01 | 0.89 | 0 | |
| Element | Pollution Degree | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Season | Cu | Zn | Pb | Co | As | Mn | Sr | Cr | ||
| Geo-accumulation index, Igeo | Pre M | 1.11 | 0.07 | −0.48 | −2.85 | −1.3 | −1.16 | −1.92 | −3.66 | Unpolluted |
| Post M | 1 | 0.04 | −0.33 | −2.82 | −0.95 | −1.07 | −1.81 | −3.47 | ||
| Average | 1.06 | 0.06 | −0.41 | −2.84 | −1.13 | −1.12 | −1.87 | −3.57 | ||
| Enrichment factor, EF | Pre M | 65.9 b | 32.1 | 21.9 | 4.23 a | 12.4 | 13.7 | 8.09 a | 2.42 a | Severe enrichment |
| Post M | 58.3 b | 30 | 23.2 | 4.12 a | 15.1 | 13.8 | 8.28 a | 2.63 a | ||
| Average | 62.1 b | 31.05 | 22.6 | 4.18 a | 13.8 | 13.8 | 8.19 a | 2.53 a | ||
| Stations | Risk Index (RI) | Pollution Degree | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Cr | Mn | Cu | Zn | As | Pb | Co | |||
| Pre Monsoon | 0.06 | 0.67 | 0.65 | 1.58 | 0.06 | 0.22 | 0.04 | 3.27 | Low risk |
| Post Monsoon | 0.07 | 0.71 | 0.6 | 1.55 | 0.08 | 0.24 | 0.04 | 3.29 | Low risk |
| Average | 0.07 | 0.69 | 0.63 | 1.57 | 0.07 | 0.23 | 0.04 | 3.28 | Low risk |
| Hazard Quotients (HQ.) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Metal | Children | Adult Male | Adult Female | Risk Level | ||||||
| ING | INH | DER | ING | INH | DER | ING | INH | DER | ||
| Mn | 5.37 × 10−2 | 5.37 × 102 | 4.18 × 102 | 4.23 × 10−3 | 4.23 × 10 | 3.29 × 10−1 | 4.96 × 10−3 | 4.96 × 10 | 3.86 × 10−1 | No risk |
| Fe | 4.34 × 10−2 | 0 | 0 | 3.42 × 10−3 | 0 | 0 | 4.01 × 10−3 | 0 | 0 | No risk |
| Cu | 4.49 × 10−2 | 4.47 × 10−2 | 1.5 × 10−1 | 3.54 × 10−3 | 3.52 × 10−3 | 1.18 × 10−2 | 4.15 × 10−3 | 4.13 × 10−3 | 1.38 × 10−2 | No risk |
| Zn | 6.32 × 10−3 | 6.32 × 10−3 | 3.16 × 10−2 | 4.98 × 10−4 | 4.98 × 10−4 | 2.49 × 10−3 | 5.84 × 10−4 | 5.84 × 10−4 | 2.92 × 10−3 | No risk |
| Sr | 2.63 × 10−6 | 0 | 0 | 2.08 × 10−7 | 0 | 0 | 2.43 × 10−7 | 0 | 0 | No risk |
| Pb | 8.3 × 10−2 | 8.25 × 10−2 | 5.53 × 10−1 | 6.54 × 10−3 | 6.5 × 10−3 | 4.36 × 10−2 | 7.67 × 10−3 | 7.62 × 10−3 | 5.11 × 10−2 | No risk |
| As | 3.85 × 10−1 | 7.69 × 10 | 9.38 × 10−1 | 3.03 × 10−2 | 6.06 × 10−1 | 7.39 × 10−2 | 3.55 × 10−2 | 7.11 × 10−1 | 8.66 × 10−2 | No risk |
| Cr | 4.88 × 10−2 | 5.12 × 10 | 2.44 × 10 | 3.85 × 10−3 | 4.03 × 10−1 | 1.92 × 10−1 | 4.51 × 10−3 | 4.73 × 10−1 | 2.25 × 10−1 | No risk |
| Co | NA | NA | NA | NA | NA | NA | NA | NA | NA | No risk |
| HI | 6.65 × 10−1 | 5.5 × 102 | 8.29 × 10 | 5.24 × 10−2 | 4.34 × 10 | 6.53 × 10−1 | 6.14 × 10−2 | 5.08 × 10 | 7.66 × 10−1 | No risk |
| Elements | PC 1 | PC 2 | ||
|---|---|---|---|---|
| Water | Sediment | Water | Sediment | |
| 1. Cr | −0.29 | −0.68 | 0.04 | 0.002 |
| 2. Mn | −0.02 | 0.38 | 0.10 | 0.01 |
| 3. Cu | −0.61 | −0.44 | −0.01 | −0.014 |
| 4. Zn | −0.10 | −0.43 | −0.01 | −0.01 |
| 5. As | −0.89 | −0.68 | −0.02 | 0.003 |
| 6. Pb | −0.63 | −0.66 | 0.01 | 0.003 |
| 7. Co | −0.89 | −0.69 | −0.02 | 0.002 |
| 8. Fe | −0.23 | 3.66 | −0.07 | −0.001 |
| 9. Sr | 3.67 | −0.47 | −0.01 | 0.004 |
| Eigenvalue | 2.00 | 2.00 | 0.002 | 4.5 × 10−5 |
| % Variance | 99.88 | 99.998 | 0.12 | 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hossain, M.S.; Ahmed, M.K.; Liyana, E.; Hossain, M.S.; Jolly, Y.N.; Kabir, M.J.; Akter, S.; Rahman, M.S. A Case Study on Metal Contamination in Water and Sediment near a Coal Thermal Power Plant on the Eastern Coast of Bangladesh. Environments 2021, 8, 108. https://0-doi-org.brum.beds.ac.uk/10.3390/environments8100108
Hossain MS, Ahmed MK, Liyana E, Hossain MS, Jolly YN, Kabir MJ, Akter S, Rahman MS. A Case Study on Metal Contamination in Water and Sediment near a Coal Thermal Power Plant on the Eastern Coast of Bangladesh. Environments. 2021; 8(10):108. https://0-doi-org.brum.beds.ac.uk/10.3390/environments8100108
Chicago/Turabian StyleHossain, Md. Solaiman, Md. Kawser Ahmed, Eurida Liyana, Md. Shahadat Hossain, Yeasmin Nahar Jolly, M. Jamiul Kabir, Shirin Akter, and M. Safiur Rahman. 2021. "A Case Study on Metal Contamination in Water and Sediment near a Coal Thermal Power Plant on the Eastern Coast of Bangladesh" Environments 8, no. 10: 108. https://0-doi-org.brum.beds.ac.uk/10.3390/environments8100108







