Potential Influence of Sewage Phosphorus and Wet and Dry Deposition Detected in Fish Collected in the Athabasca River North of Fort McMurray
Abstract
:1. Introduction
2. Methods
2.1. Fish Collection
2.2. Regression Analyses: Attributing Variance
Bootstrapping Procedures: Detecting Potentially Relevant Differences
2.3. Data Interpretation
3. Results
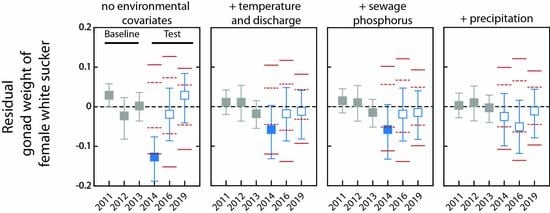
3.1. Analysis of Fish without Environmental Covariates
3.2. Do Discharge and Temperature Account for Changes in Fish?
3.3. Does Including Sewage Phosphorus Improve the Descriptive Models?
3.4. What Is the Status of Fish Health after Accounting for Sewage Phosphorus?
3.5. Does Summer Precipitation Influence the Health of Fish?
3.6. What Is the Status of Fish Health after Accounting for Summer Precipitation?
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Percy, K.E. Geoscience of Climate and Energy 11. Ambient Air Quality and Linkage to Ecosystems in the Athabasca Oil Sands, Alberta. Geosci. Can. 2013, 40, 182–201. [Google Scholar] [CrossRef] [Green Version]
- Schindler, D. Tar sands need solid science. Nat. Cell Biol. 2010, 468, 499–501. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Sharma, K.; Brassard, B.W.; Hazewinkel, R. Polycyclic Aromatic Hydrocarbon Deposition in the Snowpack of the Athabasca Oil Sands Region of Alberta, Canada. Water Air Soil Pollut. 2014, 225, 1–16. [Google Scholar] [CrossRef]
- Edgerton, E.S.; Hsu, Y.-M.; White, E.M.; Fenn, M.E.; Landis, M.S. Ambient concentrations and total deposition of inorganic sulfur, inorganic nitrogen and base cations in the Athabasca Oil Sands Region. Sci. Total Environ. 2020, 706, 134864. [Google Scholar] [CrossRef] [PubMed]
- Gopalapillai, Y.; Kirk, J.L.; Landis, M.S.; Muir, D.C.G.; Cooke, C.A.; Gleason, A.; Ho, A.; Kelly, E.N.; Schindler, D.W.; Wang, X.; et al. Source analysis of pollutant elements in winter air deposition in the Athabasca Oil Sands Region: A temporal and spatial study. ACS Earth Space Chem. 2019, 3, 1656–1668. [Google Scholar] [CrossRef]
- Landis, M.S.; Pancras, J.P.; Graney, J.; Stevens, R.K.; Percy, K.; Krupa, S.V. Receptor modeling of epiphytic lichens to elucidate the sources and spatial distribution of inorganic air pollution in the Athabasca Oil Sands Region. In Alberta Oil Sands, 1st ed.; Percy, K.E., Ed.; Elsevier Ltd.: Oxford, UK, 2012; Volume 11, pp. 427–467. [Google Scholar]
- Shotyk, W.; Belland, R.; Duke, J.; Kempter, H.; Krachler, M.; Noernberg, T.; Pelletier, R.; Vile, M.A.; Wieder, K.; Zaccone, C.; et al. Sphagnum mosses from 21 ombrotrophic bogs in the Athabasca Bituminous Sands Region show no significant atmospheric contamination of “Heavy Metals”. Environ. Sci. Technol. 2014, 48, 12603–12611. [Google Scholar] [CrossRef]
- Korosi, J.; Irvine, G.; Skierszkan, E.; Doyle, J.; Kimpe, L.; Janvier, J.; Blais, J. Localized enrichment of polycyclic aromatic hydrocarbons in soil, spruce needles, and lake sediments linked to in-situ bitumen extraction near Cold Lake, Alberta. Environ. Pollut. 2013, 182, 307–315. [Google Scholar] [CrossRef]
- Munkittrick, K.R.; Arciszewski, T.J. Using normal ranges for interpreting results of monitoring and tiering to guide future work: A case study of increasing polycyclic aromatic compounds in lake sediments from the Cold Lake oil sands (Alberta, Canada) described in Korosi et al. (2016). Environ. Pollut. 2017, 231, 1215–1222. [Google Scholar] [CrossRef]
- Schindler, D.W. Geoscience of climate and energy 12. Water quality issues in the Oil Sands Region of the Lower Athabasca River, Alberta. Geosci. Can. 2013, 40, 202–214. [Google Scholar] [CrossRef]
- Birks, S.; Cho, S.; Taylor, E.; Yi, Y.; Gibson, J. Characterizing the PAHs in surface waters and snow in the Athabasca region: Implications for identifying hydrological pathways of atmospheric deposition. Sci. Total Environ. 2017, 603, 570–583. [Google Scholar] [CrossRef]
- Conly, F.M.; Crosley, R.W.; Headley, J.V.; Quagraine, E.K. Assessment of metals in bed and suspended sediments in tributaries of the Lower Athabasca River. J. Environ. Sci. Health Part A 2007, 42, 1021–1028. [Google Scholar] [CrossRef]
- Dolgova, S.; Popp, B.N.; Courtoreille, K.; Espie, R.H.; MacLean, B.; McMaster, M.; Straka, J.R.; Tetreault, G.R.; Wilkie, S.; Hebert, C.E.; et al. Spatial trends in a biomagnifying contaminant: Application of amino acid compound-specific stable nitrogen isotope analysis to the interpretation of bird mercury levels. Environ. Toxicol. Chem. 2018, 37, 1466–1475. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.; McMaster, M.; Muir, D.; Parrott, J.; Tetreault, G.; Keating, J. Forage fish and polycyclic aromatic compounds in the Fort McMurray oil sands area: Body burden comparisons with environmental distributions and consumption guidelines. Environ. Pollut. 2019, 255, 113135. [Google Scholar] [CrossRef]
- Evans, M.S.; Talbot, A. Investigations of mercury concentrations in walleye and other fish in the Athabasca River ecosystem with increasing oil sands developments. J. Environ. Monit. 2012, 14, 1989–2003. [Google Scholar] [CrossRef] [PubMed]
- Glozier, N.E.; Pippy, K.; Levesque, L.; Ritcey, A.; Armstrong, B.; Tobin, O.; Cooke, C.A.; Conly, M.; Dirk, L.; Epp, C.; et al. Surface Water Quality of the Athabasca, Peace and Slave Rivers and Riverine Waterbodies within the Peace-Athabasca Delta; Oil Sands Monitoring Program Technical Report Series No. 1.4; Government of Alberta: Edmonton, AB, Canada, 2018; ISBN 9781460140284. [Google Scholar]
- Jautzy, J.J.; Ahad, J.M.E.; Hall, R.I.; Wiklund, J.A.; Wolfe, B.B.; Gobeil, C.; Savard, M.M. Source apportionment of background PAHs in the Peace-Athabasca Delta (Alberta, Canada) using molecular level radiocarbon analysis. Environ. Sci. Technol. 2015, 49, 9056–9063. [Google Scholar] [CrossRef] [PubMed]
- Klemt, W.H.; Kay, M.L.; Wiklund, J.A.; Wolfe, B.B.; Hall, R.I. Assessment of vanadium and nickel enrichment in Lower Athabasca River floodplain lake sediment within the Athabasca Oil Sands Region (Canada). Environ. Pollut. 2020, 265, 114920. [Google Scholar] [CrossRef]
- Libera, N.; Summers, J.C.; Rühland, K.M.; Kurek, J.; Smol, J.P. Diatom assemblage changes in shallow lakes of the Athabasca Oil Sands Region are not tracking aerially deposited contaminants. J. Paleolimnol. 2020, 64, 257–272. [Google Scholar] [CrossRef]
- Pilote, M.; André, C.; Turcotte, P.; Gagné, F.; Gagnon, C. Metal bioaccumulation and biomarkers of effects in caged mussels exposed in the Athabasca oil sands area. Sci. Total Environ. 2018, 610–611, 377–390. [Google Scholar] [CrossRef]
- Roy, J.; Bickerton, G.; Frank, R.; Grapentine, L.; Hewitt, L. Assessing risks of shallow riparian groundwater quality near an oil sands tailings pond. Ground Water 2016, 54, 545–558. [Google Scholar] [CrossRef]
- Shotyk, W.; Appleby, P.G.; Bicalho, B.; Davies, L.; Froese, D.; Grant-Weaver, I.; Krachler, M.; Magnan, G.; Mullan-Boudreau, G.; Noernberg, T.; et al. Peat bogs in northern Alberta, Canada reveal decades of declining atmospheric Pb contamination. Geophys. Res. Lett. 2016, 43, 9964–9974. [Google Scholar] [CrossRef]
- Summers, J.C.; Kurek, J.; Rühland, K.M.; Neville, E.E.; Smol, J.P. Assessment of multi-trophic changes in a shallow boreal lake simultaneously exposed to climate change and aerial deposition of contaminants from the Athabasca Oil Sands Region, Canada. Sci. Total. Environ. 2017, 592, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Shotyk, W.; Cuss, C.W.; Donner, M.W.; Fennell, J.; Javed, M.; Noernberg, T.; Poesch, M.; Pelletier, R.; Sinnatamby, N.; et al. Characterization of naphthenic acids and other dissolved organics in natural water from the Athabasca Oil Sands Region, Canada. Environ. Sci. Technol. 2017, 51, 9524–9532. [Google Scholar] [CrossRef]
- Timoney, K.P.; Lee, P. Does the Alberta tar sands industry pollute? The scientific evidence. Open Conserv. Biol. J. 2009, 3, 65–81. [Google Scholar] [CrossRef]
- Alexander, A.; Chambers, P. Assessment of seven Canadian rivers in relation to stages in oil sands industrial development, 1972–2010. Environ. Rev. 2016, 24, 484–494. [Google Scholar] [CrossRef] [Green Version]
- Parrott, J.; Marentette, J.; Hewitt, L.; McMaster, M.; Gillis, P.; Norwood, W.; Kirk, J.; Peru, K.; Headley, J.; Wang, Z.; et al. Meltwater from snow contaminated by oil sands emissions is toxic to larval fish, but not spring river water. Sci. Total. Environ. 2018, 625, 264–274. [Google Scholar] [CrossRef]
- Tetreault, G.R.; McMaster, M.E.; Dixon, D.G.; Parrott, J.L. Using reproductive endpoints in small forage fish species to evaluate the effects of athabasca oil sands activities. Environ. Toxicol. Chem. 2003, 22, 2775–2782. [Google Scholar] [CrossRef]
- Arens, C.J.; Arens, J.C.; Hogan, N.S.; Kavanagh, R.J.; Berrue, F.; Van Der Kraak, G.J.; Heuvel, M.R.V.D. Population impacts in white sucker (Catostomus commersonii) exposed to oil sands-derived contaminants in the Athabasca River. Environ. Toxicol. Chem. 2017, 36, 2058–2067. [Google Scholar] [CrossRef]
- Schwalb, A.N.; Alexander, A.C.; Paul, A.J.; Cottenie, K.; Rasmussen, J.B. Changes in migratory fish communities and their health, hydrology, and water chemistry in rivers of the Athabasca oil sands region: A review of historical and current data. Environ. Rev. 2015, 23, 133–150. [Google Scholar] [CrossRef] [Green Version]
- Wasiuta, V.; Kirk, J.L.; Chambers, P.A.; Alexander, A.C.; Wyatt, F.R.; Rooney, R.C.; Cooke, C.A. Accumulating mercury and methylmercury burdens in watersheds impacted by oil sands pollution. Environ. Sci. Technol. 2019, 53, 12856–12864. [Google Scholar] [CrossRef]
- Hazewinkel, R.; Westcott, K. In Response: A provincial government perspective on the release of oil sands process-affected water. Environ. Toxicol. Chem. 2015, 34, 2684–2685. [Google Scholar] [CrossRef] [PubMed]
- McMaster, M.E.; Tetreault, G.R.; Clark, T.; Bennett, J.; Cunningham, J.; Ussery, E.J.; Evans, M. Baseline white sucker health and reproductive endpoints for use in assessment of further development in the alberta oil sands. Int. J. Environ. Impacts Manag. Mitig. Recover. 2020, 3, 219–237. [Google Scholar] [CrossRef]
- Fennell, J.; Arciszewski, T.J. Current knowledge of seepage from oil sands tailings ponds and its environmental influence in northeastern Alberta. Sci. Total Environ. 2019, 686, 968–985. [Google Scholar] [CrossRef] [PubMed]
- Alexander, A.; Levenstein, B.; Sanderson, L.; Blukacz-Richards, E.; Chambers, P. How does climate variability affect water quality dynamics in Canada’s oil sands region? Sci. Total Environ. 2020, 732, 139062. [Google Scholar] [CrossRef] [PubMed]
- Emmerton, C.A.; Cooke, C.A.; Hustins, S.; Silins, U.; Emelko, M.B.; Lewis, T.; Kruk, M.K.; Taube, N.; Zhu, D.; Jackson, B.; et al. Severe western Canadian wildfire affects water quality even at large basin scales. Water Res. 2020, 183, 116071. [Google Scholar] [CrossRef] [PubMed]
- Ohiozebau, E.; Tendler, B.J.; Hill, A.; Codling, G.; Kelly, E.N.; Giesy, J.P.; Jones, P.D. Products of biotransformation of polycyclic aromatic hydrocarbons in fishes of the Athabasca/Slave river system, Canada. Environ. Geochem. Health 2015, 38, 577–591. [Google Scholar] [CrossRef]
- Lynam, M.M.; Dvonch, J.T.; Barres, J.A.; Morishita, M.; Legge, A.; Percy, K. Oil sands development and its impact on atmospheric wet deposition of air pollutants to the Athabasca Oil Sands Region, Alberta, Canada. Environ. Pollut. 2015, 206, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Droppo, I.G.; Di Cenzo, P.; Power, J.; Jaskot, C.; Chambers, P.A.; Alexander, A.C.; Kirk, J.; Muir, D. Temporal and spatial trends in riverine suspended sediment and associated polycyclic aromatic compounds (PAC) within the Athabasca oil sands region. Sci. Total. Environ. 2018, 626, 1382–1393. [Google Scholar] [CrossRef] [PubMed]
- McMaster, M.E.; Tetreatult, G.R.; Clark, T.; Bennett, J.; Cunningham, J.; Evans, M. Aquatic ecosystem health assessment of the athabasca river mainstem oil sands area using white sucker health. Environ. Impact IV 2018, 215, 411–420. [Google Scholar] [CrossRef] [Green Version]
- Donelson, J.; Munday, P.; McCormick, M.; Pankhurst, N.; Pankhurst, P. Effects of elevated water temperature and food availability on the reproductive performance of a coral reef fish. Mar. Ecol. Prog. Ser. 2010, 401, 233–243. [Google Scholar] [CrossRef] [Green Version]
- Donelson, J.M.; McCormick, M.I.; Booth, D.J.; Munday, P.L. Reproductive acclimation to increased water temperature in a tropical reef fish. PLoS ONE 2014, 9, e97223. [Google Scholar] [CrossRef]
- Jonsson, N. Influence of water flow, water temperature and light on fish migration in rivers. Nord. J. Freshw. Res. 1991, 66, 20–35. [Google Scholar]
- Tetreault, G.R.; Bennett, C.J.; Clark, T.W.; Keith, H.; Parrott, J.L.; McMaster, M.E. Fish performance indicators adjacent to oil sands activity: Response in performance indicators of Slimy Sculpin in the Steepbank River, Alberta, Adjacent to Oil Sands Mining Activity. Environ. Toxicol. Chem. 2020, 39, 396–409. [Google Scholar] [CrossRef]
- Simmons, D.B.; Sherry, J.P. Plasma proteome profiles of White Sucker (Catostomus commersonii) from the Athabasca River within the oil sands deposit. Comp. Biochem. Physiol. Part D Genom. Proteom. 2016, 19, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Arciszewski, T.J.; Munkittrick, K.R.; Kilgour, B.W.; Keith, H.M.; Linehan, J.E.; McMaster, M.E. Increased size and relative abundance of migratory fishes observed near the Athabasca oil sands. Facets 2017, 2, 833–858. [Google Scholar] [CrossRef] [Green Version]
- Shakibaeinia, A.; Dibike, Y.B.; Kashyap, S.; Prowse, T.D.; Droppo, I.G. A numerical framework for modelling sediment and chemical constituents transport in the Lower Athabasca River. J. Soils Sediments 2016, 17, 1140–1159. [Google Scholar] [CrossRef]
- Arciszewski, T.J.; Munkittrick, K.R. Development of an adaptive monitoring framework for long-term programs: An example using indicators of fish health. Integr. Environ. Assess. Manag. 2015, 11, 701–718. [Google Scholar] [CrossRef]
- Kilgour, B.W.; Munkittrick, K.R.; Hamilton, L.; Proulx, C.L.; Somers, K.M.; Arciszewski, T.; McMaster, M. Developing triggers for environmental effects monitoring programs for Trout-Perch in the Lower Athabasca River (Canada). Environ. Toxicol. Chem. 2019, 38, 1890–1901. [Google Scholar] [CrossRef]
- Bond, W.A.; Berry, D.K. Fishery Resources of the Athabasca River Downstream of Fort McMurray, Alberta; Alberta Oil Sands Environmental Research Program, Government of Alberta: Edmonton, AB, Canada, 1980; Volume II. [Google Scholar]
- Walker, S.; Ribey, S.; Trudel, L.; Porter, E. Canadian Environmental effects monitoring: Experiences with pulp and paper and metal mining regulatory programs. Environ. Monit. Assess. 2003, 88, 311–326. [Google Scholar] [CrossRef] [PubMed]
- Cooke, C.A.; Schwindt, C.; Davies, M.; Donahue, W.F.; Azim, E. Initial environmental impacts of the Obed Mountain coal mine process water spill into the Athabasca River (Alberta, Canada). Sci. Total Environ. 2016, 557, 502–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hicks, K.; Scrimgeour, G. A Study Design for Enhanced Environmental Monitoring of the Lower Athabasca River; Government of Alberta, Ministry of Environment and Parks: Edmonton, AB, Canada, 1 November 2019; ISBN 1460145364. [Google Scholar]
- Bissell, D.; Montgomery, D.C. Introduction to statistical quality control. J. R. Stat. Soc. Ser. D Stat. 1986, 35, 81. [Google Scholar] [CrossRef]
- Koenker, R.; Portnoy, S.; Ng, P.T.; Zeileis, A.; Grosjean, P.; Ripley, B.D. Package ‘Quantreg’. 2020. Available online: https://cran.r-project.org/web/packages/quantreg/index.html (accessed on 5 November 2020).
- Culp, J.M.; Brua, R.B.; Luiker, E.; Glozier, N.E. Ecological causal assessment of benthic condition in the oil sands region, Athabasca River, Canada. Sci. Total Environ. 2020, 749, 141393. [Google Scholar] [CrossRef]
- Lynam, M.; Dvonch, J.T.; Barres, J.; Percy, K. Atmospheric wet deposition of mercury to the Athabasca Oil Sands Region, Alberta, Canada. Air Qual. Atmos. Health 2017, 11, 83–93. [Google Scholar] [CrossRef]
- Cheng, I.; Wen, D.; Zhang, L.; Wu, Z.; Qiu, X.; Yang, F.; Harner, T. Deposition mapping of polycyclic aromatic compounds in the Oil Sands Region of Alberta, Canada and linkages to ecosystem impacts. Environ. Sci. Technol. 2018, 52, 12456–12464. [Google Scholar] [CrossRef] [PubMed]
- Beran, R. Prepivoting test statistics: A bootstrap view of asymptotic refinements. J. Am. Stat. Assoc. 1988, 83, 687–697. [Google Scholar] [CrossRef]
- Martin, M.A. On bootstrap iteration for coverage correction in confidence intervals. J. Am. Stat. Assoc. 1990, 85, 1105–1118. [Google Scholar] [CrossRef]
- Arciszewski, T.; McMaster, M.; Munkittrick, K. Long-term studies of fish health before and after the closure of a bleached kraft pulp mill in Northern Ontario, Canada. Environ. Toxicol. Chem. 2021, 40, 162–176. [Google Scholar] [CrossRef]
- Brown, B.M.; Hall, P.; Young, G.A. The smoothed median and the bootstrap. Biometrika 2001, 88, 519–534. [Google Scholar] [CrossRef]
- Wand, M.; Ripley, B.; Ripley, M.B. Package ‘KernSmooth’. 2020. Available online: https://cran.r-project.org/web/packages/KernSmooth/index.html (accessed on 5 November 2020).
- Arciszewski, T.J.; Munkittrick, K.R.; Scrimgeour, G.J.; Dubé, M.G.; Wrona, F.J.; Hazewinkel, R.R. Using adaptive processes and adverse outcome pathways to develop meaningful, robust, and actionable environmental monitoring programs. Integr. Environ. Assess. Manag. 2017, 13, 877–891. [Google Scholar] [CrossRef] [Green Version]
- Chambers, P.A.; Allard, M.; Walker, S.L.; Marsalek, J.; Lawrence, J.; Servos, M.; Busnarda, J.; Munger, K.S.; Adare, K.; Jefferson, C.; et al. Impacts of municipal wastewater effluents on Canadian waters: A Review. Water Qual. Res. J. 1997, 32, 659–714. [Google Scholar] [CrossRef]
- Kilgour, B.; Mahaffey, A.; Brown, C.; Hughes, S.; Hatry, C.; Hamilton, L. Variation in toxicity and ecological risks associated with some oil sands groundwaters. Sci. Total Environ. 2019, 659, 1224–1233. [Google Scholar] [CrossRef]
- Landis, M.S.; Berryman, S.D.; White, E.M.; Graney, J.R.; Edgerton, E.S.; Studabaker, W.B. Use of an epiphytic lichen and a novel geostatistical approach to evaluate spatial and temporal changes in atmospheric deposition in the Athabasca Oil Sands Region, Alberta, Canada. Sci. Total Environ. 2019, 692, 1005–1021. [Google Scholar] [CrossRef]
- Bahamonde, P.A.; Fuzzen, M.L.; Bennett, C.J.; Tetreault, G.R.; McMaster, M.E.; Servos, M.R.; Martyniuk, C.J.; Munkittrick, K.R. Whole organism responses and intersex severity in rainbow darter (Etheostoma caeruleum) following exposures to municipal wastewater in the Grand River basin, ON, Canada. Part A. Aquat. Toxicol. 2015, 159, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Kidd, K.A.; Blanchfield, P.J.; Mills, K.H.; Palace, V.P.; Evans, R.E.; Lazorchak, J.M.; Flick, R.W. Collapse of a fish population after exposure to a synthetic estrogen. Proc. Natl. Acad. Sci. USA 2007, 104, 8897–8901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiseman, S.; Anderson, J.; Liber, K.; Giesy, J. Endocrine disruption and oxidative stress in larvae of Chironomus dilutus following short-term exposure to fresh or aged oil sands process-affected water. Aquat. Toxicol. 2013, 2013, 414–421. [Google Scholar] [CrossRef]
- Frank, R.A.; Roy, J.W.; Bickerton, G.; Rowland, S.J.; Headley, J.V.; Scarlett, A.G.; West, C.E.; Peru, K.M.; Parrott, J.L.; Conly, F.M.; et al. Profiling oil sands mixtures from industrial developments and natural groundwaters for source identification. Environ. Sci. Technol. 2014, 48, 2660–2670. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, L.M.; Roy, J.W.; Rowland, S.J.; Bickerton, G.; De Silva, A.O.; Headley, J.V.; Milestone, C.B.; Scarlett, A.G.; Brown, S.; Spencer, C.; et al. Advances in distinguishing groundwater influenced by Oil Sands Process-Affected Water (OSPW) from natural bitumen-influenced groundwaters. Environ. Sci. Technol. 2020, 54, 1522–1532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Cheng, I.; Muir, D.; Charland, J.-P. Scavenging ratios of polycyclic aromatic compounds in rain and snow in the Athabasca oil sands region. Atmos. Chem. Phys. Discuss. 2015, 15, 1421–1434. [Google Scholar] [CrossRef] [Green Version]
- Fenn, M.; Bytnerowicz, A.; Schilling, S.; Ross, C. Atmospheric deposition of nitrogen, sulfur and base cations in jack pine stands in the Athabasca Oil Sands Region, Alberta, Canada. Environ. Pollut. 2015, 196, 497–510. [Google Scholar] [CrossRef]
- Shotyk, W.; Bicalho, B.; Cuss, C.W.; Duke, M.J.M.; Noernberg, T.; Pelletier, R.; Steinnes, E.; Zaccone, C. Dust is the dominant source of “heavy metals” to peat moss (Sphagnum fuscum) in the bogs of the Athabasca Bituminous Sands region of northern Alberta. Environ. Int. 2016, 494–506. [Google Scholar] [CrossRef]
- Zhang, Y.; Shotyk, W.; Zaccone, C.; Noernberg, T.; Pelletier, R.; Bicalho, B.; Froese, D.G.; Davies, L.; Martin, J.W. Airborne petcoke dust is a major source of polycyclic aromatic hydrocarbons in the Athabasca Oil Sands Region. Environ. Sci. Technol. 2016, 50, 1711–1720. [Google Scholar] [CrossRef]
- Alexander, A.; Chambers, P.; Jeffries, D. Episodic acidification of 5 rivers in Canada’s oil sands during snowmelt: A 25-year record. Sci. Total Environ. 2017, 599–600, 739–749. [Google Scholar] [CrossRef]
- Ahad, J.M.; Jautzy, J.J.; Cumming, B.F.; Das, B.; Laird, K.R.; Sanei, H. Sources of polycyclic aromatic hydrocarbons (PAHs) to northwestern Saskatchewan lakes east of the Athabasca oil sands. Org. Geochem. 2015, 80, 35–45. [Google Scholar] [CrossRef]
- Landis, M.S.; Edgerton, E.S.; White, E.M.; Wentworth, G.R.; Sullivan, A.P.; Dillner, A.M. The impact of the 2016 Fort McMurray Horse River wildfire on ambient air pollution levels in the Athabasca Oil Sands Region, Alberta, Canada. Sci. Total. Environ. 2018, 618, 1665–1676. [Google Scholar] [CrossRef]
- Liu, J.C.; Pereira, G.; Uhl, S.A.; Bravo, M.A.; Bell, M.L. A systematic review of the physical health impacts from non-occupational exposure to wildfire smoke. Environ. Res. 2015, 136, 120–132. [Google Scholar] [CrossRef] [Green Version]
- Ohiozebau, E.; Tendler, B.; Codling, G.; Kelly, E.; Giesy, J.P.; Jones, P.D. Potential health risks posed by polycyclic aromatic hydrocarbons in muscle tissues of fishes from the Athabasca and Slave Rivers, Canada. Environ. Geochem. Health 2017, 39, 139–160. [Google Scholar] [CrossRef]
- Tendler, B.; Ohiozebau, E.; Codling, G.; Giesy, J.P.; Jones, P.D. Concentrations of metals in fishes from the Athabasca and Slave Rivers of Northern Canada. Environ. Toxicol. Chem. 2020, 39, 2180–2195. [Google Scholar] [CrossRef] [PubMed]
- Gibson, J.; Yi, Y.; Birks, S. Isotope-based partitioning of streamflow in the oil sands region, northern Alberta: Towards a monitoring strategy for assessing flow sources and water quality controls. J. Hydrol. Reg. Stud. 2016, 5, 131–148. [Google Scholar] [CrossRef] [Green Version]
- Arciszewski, T.J.; Hazewinkel, R.R.; Munkittrick, K.R.; Kilgour, B.W. Developing and applying control charts to detect changes in water chemistry parameters measured in the Athabasca River near the oil sands: A tool for surveillance monitoring. Environ. Toxicol. Chem. 2018, 37, 2296–2311. [Google Scholar] [CrossRef] [PubMed]
- Gerner, N.V.; Koné, M.; Ross, M.S.; Pereira, A.; Ulrich, A.C.; Martin, J.W.; Liess, M. Stream invertebrate community structure at Canadian oil sands development is linked to concentration of bitumen-derived contaminants. Sci. Total. Environ. 2017, 575, 1005–1013. [Google Scholar] [CrossRef] [PubMed]
- Schlosser, I.J. Stream fish ecology: A landscape perspective. BioScience 1991, 41, 704–712. [Google Scholar] [CrossRef]
- Van Der Kraak, G.; Pankhurst, N.W. Temperature effects on the reproductive performance of fish. Glob. Warm. 2011, 61, 159–176. [Google Scholar] [CrossRef]
- Englander, J.G.; Bharadwaj, S.; Brandt, A.R. Historical trends in greenhouse gas emissions of the Alberta oil sands (1970–2010). Environ. Res. Lett. 2013, 8, 044036. [Google Scholar] [CrossRef] [Green Version]
- Gray, M.A.; Curry, A.R.; Munkittrick, K.R. Non-Lethal Sampling Methods for assessing environmental impacts using a small-bodied sentinel fish species. Water Qual. Res. J. 2002, 37, 195–211. [Google Scholar] [CrossRef]







| Model Number | Model Form |
|---|---|
| 1 | De = I + T + D |
| 2 | De = I + T + D + T2 + D2 |
| 3 | De = I + A + T + D |
| 4 | De = I + A + T + D + T2 + D2 |
| 5 | De = I + A + T + D + D2 |
| 6 | De = I + T + D + D2 |
| 7 | De = I + A + D + T + T2 |
| 8 | De = I + D + T + T2 |
| Sex | Site | DV | AICs | Coefficients | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| T + D | +P | +P + P2 | +Pr | +Pr + Pr2 | P | P2 | Pr | Pr2 | |||
| F | DSM3 | GW | −124.65 | −126.62 | −130.41 | −133.04 | −131.34 | 2.26 | 1.89 | −2.42 | |
| LW | −179.87 | −177.9 | −180.35 | −179.21 | −177.49 | 1.83 | 1.62 | ||||
| BW | −306.63 | −306.1 | −329.13 | −327.66 | −326 | 1.13 | 0.98 | ||||
| USM4 | GW | −71.92 | −71.18 | −74.18 | −73.92 | −71.93 | −2.11 | −1.78 | |||
| LW | −124.14 | −123.95 | −122.03 | −123.77 | −122.03 | ||||||
| BW | −208.99 | −207.68 | −205.78 | −209.64 | −207.77 | −0.05 | |||||
| DSM4 | GW | −115.53 | −114.77 | −112.8 | −114.82 | −112.85 | |||||
| LW | −124.69 | −127.85 | −126.84 | −127.61 | −125.62 | −0.09 | |||||
| BW | −236.00 | −236.95 | −244.1 | −249.06 | −247.09 | 0.59 | 0.47 | −0.64 | |||
| allOSR | GW | −302.88 | −301.64 | −301.47 | −302.22 | −305.71 | 0.71 | −1.94 | |||
| LW | −447.3 | −448.59 | −446.61 | −447.88 | −458.94 | −0.07 | 2.62 | −5.86 | |||
| BW | −776.94 | −776.9 | −787.08 | −802.03 | −804.64 | 0.55 | 0.45 | 0.07 | −0.64 | ||
| M | DSM3 | GW | −132.67 | −131.59 | −129.67 | −131.6 | −130.94 | ||||
| LW | −104.96 | −110.22 | −112.28 | −110.69 | −110.49 | −9.99 | −9.07 | ||||
| BW | −258.31 | −256.9 | −254.93 | −256.9 | −254.99 | ||||||
| USM4 | GW | −101.75 | −99.93 | −98.04 | −99.97 | −98.02 | |||||
| LW | −120.5 | −119.41 | −117.56 | −119.51 | −120.36 | ||||||
| BW | −204.03 | −204.42 | −204.02 | −202.63 | −204.73 | −0.04 | 0.56 | −1.42 | |||
| DSM4 | GW | −184 | −182.24 | −180.25 | −182.26 | −180.61 | |||||
| LW | −149.44 | −147.5 | −146.36 | −147.47 | −145.61 | ||||||
| BW | −303.15 | −302.18 | −301.17 | −302.42 | −301.77 | ||||||
| allOSR | GW | −436.02 | −434.08 | −433.31 | −434.38 | −432.42 | |||||
| LW | −390.82 | −389 | −387.42 | −388.84 | −387.02 | ||||||
| BW | −795.28 | −793.58 | −792.18 | −793.41 | −796.82 | −0.19 | −0.51 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arciszewski, T.J.; McMaster, M.E. Potential Influence of Sewage Phosphorus and Wet and Dry Deposition Detected in Fish Collected in the Athabasca River North of Fort McMurray. Environments 2021, 8, 14. https://0-doi-org.brum.beds.ac.uk/10.3390/environments8020014
Arciszewski TJ, McMaster ME. Potential Influence of Sewage Phosphorus and Wet and Dry Deposition Detected in Fish Collected in the Athabasca River North of Fort McMurray. Environments. 2021; 8(2):14. https://0-doi-org.brum.beds.ac.uk/10.3390/environments8020014
Chicago/Turabian StyleArciszewski, Tim J., and Mark E. McMaster. 2021. "Potential Influence of Sewage Phosphorus and Wet and Dry Deposition Detected in Fish Collected in the Athabasca River North of Fort McMurray" Environments 8, no. 2: 14. https://0-doi-org.brum.beds.ac.uk/10.3390/environments8020014