A Way to Membrane-Based Environmental Remediation for Heavy Metal Removal
Abstract
:1. Introduction
2. Polymeric Membranes for Heavy Metal Removal
2.1. Ultrafiltration Process
2.2. Nanofiltration Process
2.3. Reverse Osmosis Process
2.4. Nanocomposite Membranes for Heavy Metal Removal
2.5. Electrospun Nanofiber Membranes for Heavy Metal Adsorption
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Shao, L.; Chen, G.Q. Water footprint assessment for wastewater treatment: Method, indicator, and application. Environ. Sci. Technol. 2013, 47, 7787–7794. [Google Scholar] [CrossRef]
- Jasper, J.T.; Yang, Y.; Hoffmann, M.R. Toxic Byproduct Formation during Electrochemical Treatment of Latrine Wastewater. Environ. Sci. Technol. 2017, 51, 7111–7119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, Z.; Yuan, R.; Wang, F.; Chen, Z.; Zhou, B.; Chen, H. Preparation of magnetic biochar and its application in catalytic degradation of organic pollutants: A review. Sci. Total Environ. 2020, 765, 142673. [Google Scholar] [CrossRef]
- Cantiello, A.; Candamano, S.; De Luca, P. Photocatalytic treatment of water contaminated by organic dye with ETS-10 titanium silicate. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1048, 012004. [Google Scholar] [CrossRef]
- Bernaudo, I.; Tagarelli, A.; Elliani, R.; Candamano, S.; Macario, A.; De Luca, P. Use of Geopolymers in the Treatment of Water Contaminated by Industrial Waste. IOP Conf. Ser. Mater. Sci. Eng. 2020, 739, 012053. [Google Scholar] [CrossRef]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60. [Google Scholar] [CrossRef] [Green Version]
- Khoso, W.A.; Haleem, N.; Baig, M.A.; Jamal, Y. Synthesis, characterization and heavy metal removal efficiency of nickel ferrite nanoparticles (NFN’s). Sci. Rep. 2021, 11, 3790–3800. [Google Scholar] [CrossRef] [PubMed]
- Sankaran, R.; Show, P.L.; Ooi, C.W.; Ling, T.C.; Shu-Jen, C.; Chen, S.Y.; Chang, Y.K. Feasibility assessment of removal of heavy metals and soluble microbialproducts from aqueous solutions using eggshell wastes. Clean Technol. Environ. Policy 2020, 22, 773–778. [Google Scholar] [CrossRef]
- Evariste, L.; Barret, A.M.; Mottier, F.; Mouchet, L.; Gauthier, E. Pinell Gut microbiota of aquatic organisms: A key endpoint for ecotoxicological studie. Environ. Pollut. 2019, 248, 989–999. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.; Ranjan, M.R. Heavy metal removal from wastewater using low cost adsorbents. J. Bioremed. Biodeg. 2015, 315, 1–6. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Chan, G.Y.S.; Lo, W.H.; Babel, S. Comparisons of low-cost adsorbents for treating wastewaters laden with heavy metals. Sci. Total Environ. 2006, 366, 409–426. [Google Scholar] [CrossRef]
- Kapahi, M.; Sachdeva, S. Bioremediation options for heavy metal pollution. JHP 2019, 9, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Macedonio, F.; Drioli, E. Membrane Engineering for Green Process Engineering. Engineering 2017, 3, 290–298. [Google Scholar] [CrossRef]
- Hamingerova, M.; Borunsky, L.; Beckmann, M. Membrane Technologies for Water and Wastewater Treatment on the European and Indian Market; Techview Report; Fraunhofer Center for International Management and Knowledge Economy: Leipzig, Germany, 2010; p. 37. [Google Scholar]
- Warsinger, D.M.; Chakraborty, S.; Tow, E.W.; Plumlee, M.H.; Bellona, C.; Loutatidou, S.; Karimi, L.; Mikelonis, A.M.; Achilli, A.; Ghassemi, A.; et al. A review of polymeric membranes and processes for potable water reuse. Prog. Polym. Sci. 2018, 81, 209–237. [Google Scholar] [CrossRef] [PubMed]
- Algieri, C.; Donato, L.; Bonacci, P.; Giorno, L. Tyrosinase immobilised on polyamide tubular membrane for the l-DOPA production: Total recycle and continuous reactor study. Biochem. Eng. J. 2012, 66, 14–19. [Google Scholar] [CrossRef]
- Iben Nasser, I.; Algieri, C.; Garofalo, A.; Drioli, E.; Ahmed, C.; Donato, L. Hybrid imprinted membranes for selective recognition of quercetin. Sep. Purif. Technol. 2016, 163, 331–340. [Google Scholar] [CrossRef]
- Minardi, E.R.; Chakraborty, S.; Calabrò, V.; Curcio, S.; Drioli, E. Membrane applications for biogas production and purification processes: An overview on a smart alternative for process intensification. RSC Adv. 2015, 5, 14156–14186. [Google Scholar] [CrossRef]
- Nasrollahi, N.; Ghalamchia, L.; Vatanpour, V.; Khataee, A. Photocatalytic-membrane technology: A critical review for membrane fouling mitigation. Ind. Eng. Chem. Res. 2021, 93, 101–116. [Google Scholar] [CrossRef]
- Zhang, W.; Ding, L.; Luo, J.; Jaffrin, M.Y.; Tang, B. Membrane fouling in photocatalytic membrane reactors (PMRs) for water and wastewater treatment: A critical review. Chem. Eng. Technol. 2016, 302, 446–458. [Google Scholar] [CrossRef]
- Castro-Munoz, R.; Rodríguez-Romero, V.; Yanez-Fernandez, Y. Water production from food processing wastewaters by integrated membrane systems: Sustainable approach. Water Technol. Sci. 2017, 8, 129–136. [Google Scholar] [CrossRef]
- Santos, A.; Judd, S. The fate of metals in wastewater treated by the activated sludge process and membrane bioreactors: A brief review. J. Environ. Monit. 2010, 12, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Criscuoli, A.; Carnevale, M.C. Membrane distillation for the treatment of waters contaminated by arsenic, fluoride and uranium. In Membrane Technologies for Water Treatment (Bll 237–255); Figoli, A., Hoinkis, J., Bundschuh, J., Eds.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2015. [Google Scholar]
- Bey, S.; Criscuoli, A.; Figoli, A.; Leopold, A.; Simone, S.; Benamor, M.; Drioli, E. Removal of As(V) by PVDF hollow fibers membrane contactors using Aliquat-336 as extractant. Desalination 2010, 264, 193–200. [Google Scholar] [CrossRef]
- Donato, L.; Algieri, C.; Miriello, V.; Mazzei, R.; Clarizia, G.; Giorno, L. Biocatalytic zeolite membrane for the production of l-DOPA. J. Membr. Sci. 2012, 407–408, 86–92. [Google Scholar] [CrossRef]
- Ezugbe, E.O.; Rathilal, S. Membrane Technologies in Wastewater Treatment: A Review. Membranes 2020, 10, 89. [Google Scholar] [CrossRef]
- Ghaffour, N.; Missimer, T.M.; Amy, G.L. Technical review and evaluation of the economics of water desalination: Current and future challenges for better water supply sustainability. Desalination 2013, 309, 197–207. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.P.; Arnot, T.C.; Mattia, D. A review of reverse osmosis membrane materials for desalination development to date and future potential. J. Membr. Sci. 2011, 370, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Seah, M.Q.; Lau, W.J.; Goh, P.S.; Tseng, H.H.; Wahab, R.; Ismail, A.F. Progress of interfacial polymerization techniques for polyamide Thin Film (nano) composite membrane fabrication: A comprehensive review. Polymers 2020, 12, 2817. [Google Scholar] [CrossRef]
- Abdullah, N.; Yusof, N.; Lau, W.J.; Jaafar, J.; Ismail, A.F. Recent trends of heavy metal removal from water/wastewater by membrane technologies. J. Ind. Eng. Chem. 2019, 76, 17–38. [Google Scholar] [CrossRef]
- Bryjak, M.; Duraj, I.; Pozniak, G. Colloid-enhanced ultrafiltration in removal of traces amounts of borates from water. Environ. Geochem. Health 2010, 32, 275–277. [Google Scholar] [CrossRef]
- Molinaro, R.; Lavorato, C.; Argurio, P. Application of Hybrid Membrane Processes Coupling Separation and Biological or Chemical Reaction in Advanced Wastewater Treatment. Membranes 2020, 10, 281. [Google Scholar] [CrossRef]
- Garba, M.D.; Usman, M.; Mazumder, M.A.; Al-Ahmed, A. Complexing agents for metal removal using ultrafiltration membranes: A review. Environ. Chem. Lett. 2019, 17, 1195–1208. [Google Scholar] [CrossRef]
- Bodzek, M.; Korus, I.; Loska, K. Application of the hybrid complexation ultrafiltration process for removal of metal ions from galvanic wastewater. Desalination 1999, 121, 117–121. [Google Scholar] [CrossRef]
- Cañizares, P.; Pérez, A.; Camarillo, R.; Linares, J.J. A semi-continuous laboratory-scale polymer enhanced ultrafiltration process for the recovery of cadmium and lead from aqueous effluents. J. Membr. Sci. 2004, 240, 197–209. [Google Scholar] [CrossRef]
- Borbély, G.; Nagy, E. Removal of zinc and nickel ions by complexation membrane-filtration process from industrial wastewater. Desalination 2009, 240, 218–226. [Google Scholar] [CrossRef]
- Huang, Y.; Du, J.R.; Zhang, Y.; Lawless, D.; Feng, X. Removal of mercury (II) from wastewater by polyvinylamine-enhanced ultrafiltration. Sep. Purif. Technol. 2015, 154, 1–10. [Google Scholar] [CrossRef]
- Giacobbo, A.; Bernardes, A.M.; Rosa, M.J.F.; de Pinho, M.N. Concentration Polarization in Ultrafiltration/Nanofiltration for the Recovery of Polyphenols from Winery Wastewaters. Membranes 2018, 8, 46. [Google Scholar] [CrossRef] [Green Version]
- Gul, A.; Hruza, J.; Yalcinkaya, F. Fouling and Chemical Cleaning of Microfiltration Membranes: A Mini-Review. Polymers 2021, 13, 846. [Google Scholar] [CrossRef]
- Lam, B.; Déon, S.; Morin-Crini, N.; Crini, G.; Fievet, P. Polymer enhanced ultrafiltration for heavy metal removal: Influence of chitosan and carboxymethyl cellulose on filtration performances. J. Clean. Prod. 2018, 171, 927–933. [Google Scholar] [CrossRef]
- Aroua, M.K.; Zuki, F.M.; Sulaiman, N.M. Removal of chromium ions from aqueous solutions by polymer-enhanced ultrafltration. J. Hazard. Mater. 2007, 147, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Barakat, M.A.; Schmidt, E. Polymer-enhanced ultrafiltration process for heavy metals removal from industrial wastewater. Desalination 2010, 256, 90–93. [Google Scholar] [CrossRef]
- Huang, Y.; Wu, D.; Wang, X.; Huang, W.; Lawless, D.; Feng, X. Removal of heavy metals from water using polyvinylamine by polymer-enhanced ultrafiltration and flocculation. Sep. Purif. Technol. 2016, 158, 124–136. [Google Scholar] [CrossRef]
- Jellouli, E.D.; Gzara, L.; Ramzi Ben Romdhane, M.; Dhahbi, M. Cadmium removal from aqueous solutions by polyelectrolyte enhanced ultrafiltration. Desalination 2009, 246, 363–369. [Google Scholar] [CrossRef]
- Huang, Y.; Du, J.; Zhang, Y.; Lawless, D.; Feng, X. Batch process of polymer-enhanced ultrafiltration to recover mercury (II) from wastewater. J. Membr. Sci. 2016, 514, 229–240. [Google Scholar] [CrossRef]
- Chou, Y.H.; Choo, K.H.; Chen, S.S.; Yu, J.H.; Peng, C.Y.; Li, C.W. Copper recovery via polyelectrolyte enhanced ultrafiltration followed by dithionite based chemical reduction: Effects of solution pH and polyelectrolyte type. Sep. Purif. Technol. 2018, 198, 113–120. [Google Scholar] [CrossRef]
- Abbasi-Garravand, E.; Mulligan, C.N. Using micellar enhanced ultrafiltration and reduction techniques for removal of Cr (VI) and Cr (III) from water. Separ. Purif. Technol. 2014, 132, 505–512. [Google Scholar] [CrossRef]
- Tortora, F.; Innocenzi, V.; Prisciandaro, M.; Veglio, F.; Di Celso, G.M. Heavy metal removal from liquid wastes by using micellar-enhanced ultrafiltration. Water Air Soil Pollut. 2016, 227, 1–11. [Google Scholar] [CrossRef]
- Lin, W.; Jing, L.; Zhu, Z.; Cai, Q.; Zhang, B. Removal of heavy metals from mining wastewater by Micellar-Enhanced Ultrafiltration (MEUF): Experimental investigation and Monte Carlo-based artificial neural network modeling. Water Air Soil Pollut. 2017, 228, 206. [Google Scholar] [CrossRef]
- Jung, J.; Yang, J.S.; Kim, S.H.; Yang, J.W. Feasibility of micellar-enhanced ultrafiltration (MEUF) or the heavy metal removal in soil washing effluent. Desalination. 2008, 222, 202–211. [Google Scholar] [CrossRef]
- Kim, H.J.; Baek, K.; Kim, B.K.; Yang, J.W. Humic substance-enhanced ultrafiltration for removal of cobalt. J. Hazard. Mater. 2005, 122, 31–36. [Google Scholar] [CrossRef]
- Gao, J.; Qiu, Y.; Hou, B.; Zhang, Q.; Zhang, X. Treatment of wastewater containing nickel by complexation- ultrafiltration using sodium polyacrylate and the stability of PAA-Ni complex in the shear field. Chem. Eng. J. 2018, 334, 1878–1885. [Google Scholar] [CrossRef]
- Zhao, S.; Liao, Z.; Fane, A.; Li, J.; Tang, C.; Zheng, C.; Lin, J.; Kong, L. Engineering antifouling reverse osmosis membranes: A review. Desalination 2021, 499, 114857. [Google Scholar] [CrossRef]
- Fujioka, T.; Oshima, N.; Suzuki, R.; Price, W.E. Nghiem, L.D. Probing the internal structure of reverse osmosis membranes by positron annihilation spectroscopy: Gaining more insight into the transport of water and small solute. J. Membr. Sci. 2015, 486, 105–116. [Google Scholar] [CrossRef] [Green Version]
- Siddique, T.A.; Dutta, N.K.; Choudhury, N.R. Nanofiltration for arsenic removal: Challenges, recent developments, and perspectives. Nanomaterials 2020, 10, 1323. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, A.W.; Teow, Y.H.; Ang, W.L.; Chung, Y.T.; Oatley-Radcliffe, D.L.; Hilal, N. Nanofiltrationmembranes review: Recent advances and future prospects. Desalination 2015, 356, 226–254. [Google Scholar] [CrossRef]
- Sumisha, A.; Arthanareeswaran, G.; Lukka, T.Y.; Ismail, A.F.; Chakraborty, S. Treatment of laundry wastewater using polyethersulfone/polyvinylpyrollidone ultrafiltration membranes. Ecotoxicol. Environ. Saf. 2015, 121, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Ahn, K.H.; Song, K.G.; Cha, H.Y.; Yeom, I.T. Removal of ions in nickel electroplating rinse water using low-pressure nanofiltration. Desalination 1999, 2, 77–84. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, G.; Fan, Z.; Yang, X.; Wang, J.; Wang, S. Experimental study on treatment of electroplating wastewater by nanofiltration. J. Membr. Sci. 2007, 305, 185–195. [Google Scholar] [CrossRef]
- Murthy, Z.V.P.; Chaudhar, L.B. Application of nanofiltration for the rejection of nickel ions from aqueous solutions and estimation of membrane transport parameters. J. Hazard. Mater. 2008, 160, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Alfano, M.D. Surface charge on loose nanofiltration membranes. Desalination 2008, 191, 262–272. [Google Scholar]
- Freger, V.; Arnot, T.C.; Howell, J.A. Separation of concentrated organic/inorganic salt mixtures by nanofiltration. J. Membr. Sci. 2000, 78, 185–193. [Google Scholar] [CrossRef]
- Figoli, A.; Cassano, A.; Criscuoli, A.; Mozumder, M.S.I.; Uddin, T.; Islam, M.A.; Drioli, E. Influence of operating parameters on the arsenic removal by nanofiltration. Water Res. 2010, 4, 97–104. [Google Scholar] [CrossRef]
- Wei, X.; Kong, X.; Wang, S.; Xiang, H.; Wang, J.; Chen, J. Removal of heavy metals from electroplating Wastewater by Thin-Film Composite Nanofiltration Hollow-Fiber Membranes. Ind. Eng. Chem. Res. 2013, 52, 17583–17590. [Google Scholar] [CrossRef]
- Yawei, Q.; Lifang, Z.; Shena, X.; Sotto, A.; Gao, C.; Jiangnan, S. Polythyleneimine-modified original positive charged nanofiltration membrane: Removal of heavy metal ions and dyes. Sep. Purif. Technol. 2019, 222, 117–124. [Google Scholar]
- Zhao, Y.; Gao, C.; Bruggen, B.V. Technology-driven layer-by-layer assembly of a membrane for selective separation of monovalent anions and antifouling. Nanoscale 2019, 11, 2264–2274. [Google Scholar] [CrossRef] [PubMed]
- Lavanya, C.; Balakrishna, R.G.; Soontarapa, K.; Padaki, M.S. Fouling resistant functional blend membrane for removal of organic matter and heavy metal. Environ. Manag. 2019, 232, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.; Ghaleni, M.M.; Isloor, A.M.; Bavarian, M.; Nejati, S. Poly(homopiperazine−amide) thin-film composite membrane for nanofiltration of heavy metal ions. ACS Omega 2020, 5, 28749–28759. [Google Scholar] [CrossRef]
- Wang, J.; Yu, W.; Graham, N.J.D.; Jiang, L. Evaluation of a novel polyamide-polyethylenimine nanofiltration membrane for wastewater treatment: Removal of Cu2+ ions. Chem. Eng. J. 2020, 392, 123769. [Google Scholar] [CrossRef]
- Gao, J.; Wang, K.Y.; Chung, T.S. Design of nanofiltration (NF) hollow fiber membranes made from functionalized bore fluids containing polyethyleneimine (PEI) for heavy metal removal. J. Membr. Sci. 2020, 603, 118022. [Google Scholar] [CrossRef]
- Al-Rashdi, B.A.M.; Johnson, D.J.; Hilal, N. Removal of heavy metal ions by nanofiltration. Desalination 2013, 315, 2–17. [Google Scholar] [CrossRef]
- Zhang, S.; Hui Peh, M.; Thong, Z.; Chung, T.S. Thin film interfacial cross-linking approach to fabricate a chitosan rejecting layer over poly(ether sulfone) support for heavy metal removal. Ind. Eng. Chem. Res. 2014, 54, 472–479. [Google Scholar] [CrossRef]
- Sangeetha, K.; Sudha, P.N.; Faleh, A.A.; Sukumaran, A. Novel chitosan based thin sheet nanofiltration membrane for rejection of heavy metal chromium. Int. J. Biol. Macromol. 2019, 132, 939–953. [Google Scholar]
- Jiang, S.; Li, Y.; Ladewig, B.P. A review of reverse osmosis membrane fouling and control strategies. Sci. Total Environ. 2017, 595, 567–583. [Google Scholar] [CrossRef]
- Nath, A.; Mondal, S.; Chakraborty, S.; Bhattacharjee, C.; Chowdhury, R. Production, purification, characterization, immobilization, and application of β-galactosidase: A review. Asia Pac. J. Chem. Eng. 2014, 9, 330–348. [Google Scholar] [CrossRef]
- Dasgupta, J.; Mondal, D.; Chakraborty, S.; Sikder, J.; Curcio, S.; Arafat, H.A. Nanofiltration based water reclamation from tannery effluent following coagulation pretreatment. Ecotoxicol. Environ. Saf. 2015, 121, 22–30. [Google Scholar] [CrossRef]
- Kremen, S.S.; Hayes, C.; Dubos, M. Large-scale reverse osmosis processing of metal finishing rinse waters. Desalination 1977, 20, 71–80. [Google Scholar] [CrossRef]
- Ujang, Z.; Anderson, G.K. Effect of the operating parameters on the separation of metal chelates using low pressure reverse osmosis membrane (LPROM). Water Sci. Technol. 1996, 34, 247–253. [Google Scholar] [CrossRef]
- Qdais, H.A.; Moussa, H. Removal of heavy metals from wastewater by membrane processes: A comparative study. Desalination 2004, 164, 105–110. [Google Scholar] [CrossRef]
- Petrinic, I.P.; Korenak, J.; Povodnik, D.; Hélix-Nielsen, C. A feasibility study of ultrafiltration/reverse osmosis (UF/RO)-based wastewater treatment and reuse in the metal finishing industry. J. Clean. Prod. 2015, 101, 292–300. [Google Scholar] [CrossRef]
- Mnif, A.; Bejaoui, I.; Mouelhi, M.; Hamrouni, B. Hexavalent chromium removal from model water and car shock absorber factory effluent by nanofiltration and reverse osmosis membrane. Int. J. Anal. Chem. 2017, 2017, 1–10. [Google Scholar] [CrossRef]
- Mohsen-Nia, M.; Montazeri, P.; Modarress, H. Removal of Cu2+ from wastewater with a chelating agent and reverse osmosis processes. Desalination 2007, 217, 276–281. [Google Scholar] [CrossRef]
- Ipek, U. Removal of Ni (ii) and Zn (ii) from an aqueous solution by reverse osmosis. Desalination 2005, 174, 161–169. [Google Scholar] [CrossRef]
- Chan, B.; Dudeney, A. Reverse osmosis removal of arsenic residues from bioleaching of refractory gold concentrates. Miner. Eng. 2008, 21, 272–278. [Google Scholar] [CrossRef]
- Ozaki, H.; Sharma, K.; Saktaywin, W. Performance of an ultra-low-pressure reverse osmosis membrane (ulprom) for separating heavy metal: Effects of interference parameters. Desalination 2002, 144, 287–294. [Google Scholar] [CrossRef]
- Chang, F.F.; Liu, W.J.; Wang, X.M. Comparison of polyamide nanofiltration and low-pressure reverse osmosis membranes on As (III) rejection under various operational conditions. Desalination 2014, 334, 10–16. [Google Scholar] [CrossRef]
- Abejón, A.; Garea, A.; Irabien, A. Arsenic removal from drinking water by reverse osmosis: Minimization of costs and energy consumption. Sep. Purif. Technol. 2015, 144, 46–53. [Google Scholar] [CrossRef]
- Yang, Z.; Guo, H.; Tang, C.Y. The upper bound of thin-film composite (TFC) polyamide membranes for desalination. J. Membr. Sci. 2019, 590, 117297. [Google Scholar] [CrossRef]
- Agboola, O.; Fayomi, O.S.I.; Ayodeji, A.; Ayeni, A.O.; Alagbe, E.E.; Sanni, S.E.; Okoro, E.E.; Moropeng, L.; Sadiku, R.; Kupolati, K.W.; et al. A Review on polymer nanocomposites and their effective applications in membranes and adsorbents for water treatment and gas separation. Membranes 2021, 11, 139. [Google Scholar] [CrossRef]
- Jeon, S.; Park, C.H.; Park, S.H.; Shin, M.G.; Kim, H.J.; Baek, K.Y.; Chan, E.P.; Bang, J.; Lee, J.H. Star polymer-assembled thin film composite membranes with high separation performance and low fouling. J. Membr. Sci. 2018, 555, 369–378. [Google Scholar] [CrossRef]
- Zheng, J.; Li, M.; Yu, K.; Hu, J.; Zhang, X.; Wang, L. Sulfonated multiwall carbon nanotubes assisted thin-film nanocomposite membrane with enhanced water flux and anti-fouling property. J. Membr. Sci. 2017, 524, 344–353. [Google Scholar] [CrossRef]
- Bi, R.; Zhang, Q.; Zhang, R.; Su, Y.; Jiang, Z. Thin film nanocomposite membranes incorporated with graphene quantum dots for high flux and antifouling property. J. Membr. Sci. 2018, 553, 17–24. [Google Scholar] [CrossRef]
- Castro-Muňoz, R.; Gonzalez-Melgoza, L.L.; García-Depraect, O. Ongoing progress on novel nanocomposite membranes for the separation of heavy metals from contaminated water. Chemosphere 2021, 270, 129421. [Google Scholar] [CrossRef]
- Nasir, A.M.; Goh, P.S.; Abdullah, M.S.; Ng, B.C.; Ismail, A.F. Adsorptive nanocomposite membranes for heavy metal remediation: Recent progresses and challenges. Chemosphere 2019, 232, 96–112. [Google Scholar] [CrossRef]
- Zareei, F.; Hosseini, S.M. A new type of polyethersulfone based composite nanofiltration decorated by cobalt ferrite-copper oxide nanoparticles with enhanced performance and antifouling property. Sep. Purif. Technol. 2019, 226, 48–58. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Jashni, E.; Jafari, M.R.; van der Bruggen, B.; Shahedi, Z. Nanocomposite polyvinyl chloride-based heterogeneous cation exchange mem brane prepared by synthesized ZnQ nanoparticles: Ionic behaviour and morpho logical characterization. J. Membr. Sci. 2018, 560, 1–10. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Liu, Y.; Xu, J.; Han, Y.; Xu, X. Preparation, performances of PVDF/ZnO hybrid membranes and their applications in the removal of copper ions. Appl. Surf. Sci. 2014, 316, 333–334. [Google Scholar] [CrossRef]
- Abdi, G.; Alizadeh, A.; Zinadini, S.; Moradi, G. Removal of dye and heavy metal ion using a novel synthetic polyethersulfone nanofiltration membrane modified by magnetic graphene oxide/metformin hybrid. J. Membr. Sci. 2018, 552, 326–335. [Google Scholar] [CrossRef]
- Dana, E.; Sayar, A. Adsorption of copper on amine-functionalized SBA-15 prepared by co-condensation: Equilibrium properties. Chem. Eng. J. 2011, 166, 445–453. [Google Scholar] [CrossRef]
- Yin, J.; Guocheng, Z.; Deng, B. Multi-walled carbon nanotubes (MWNTs)/polysulfone (PSU) mixed matrix hollow fiber membranes for enhanced water treatment. J. Membr. Sci. 2013, 437, 237–248. [Google Scholar] [CrossRef]
- Liu, L.; Son, M.; Chakraborty, S.; Bhattacharjee, C.; Choi, H. Fabrication of ultra-thin polyelectrolyte/carbon nanotube membrane by spray-assisted layer-by-layer technique: Characterization and its anti-protein fouling properties for water treatment. Desalin. Water Treat. 2013, 51, 6194–6200. [Google Scholar] [CrossRef]
- Ali, S.; Rehman, S.A.U.; Shaha, I.A.; Farid, M.U.; An, A.K.; Huang, H. Efficient removal of zinc from water and wastewater effluents by hydroxylated and carboxylated carbon nanotube membranes: Behaviors and mechanisms of dynamic filtration. J. Hazard. Mater. 2019, 365, 64–73. [Google Scholar] [CrossRef]
- Kumar, S.P.; Venkatesh, K.; Ling, E.; Sundaramurthy, J.; Singh, G.; Arthanareeswaran, G. Electrospun carbon nanofibers/TiO2 -PAN hybrid membranes for effective removal of metal ions and cationic dye. Environ. Nanotechnol. Monit. Manag. 2018, 10, 366–376. [Google Scholar] [CrossRef]
- Yurekli, Y. Removal of heavy metals in wastewater by using zeolite nano-particles impregnated polysulfone membranes. J. Hazard. Mater. 2016, 309, 53–64. [Google Scholar] [CrossRef]
- Yuan, J.; Hung, W.; Zhu, H.; Guan, K.; Ji, Y.; Mao, Y.; Jin, W. Fabrication of ZIF-300 membrane and its application for efficient removal of heavy metal ions from wastewater. J. Membr. Sci. 2019, 572, 20–27. [Google Scholar] [CrossRef]
- Wu, J.; Xue, S.; Bridges, D.; Yu, Y.; Zhang, L.; Pooran, J.; Hill, C.; Wu, J.; Hu, A. E-based ceramic nanocomposite membranes fabricated via e-spinning and vacuum filtration for Cd2+ ions removal. Chemosphere 2019, 230, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Liu, Y.; Hu, S.; Yu, F.; He, Z.; Zeng, G.; Feng, Z.; Sengupta, A. Construction of Fe3O4@MXene composite nanofiltration membrane for heavy metal ions removal from wastewater. Polym. Adv. Technol. 2021, 32, 1000–1010. [Google Scholar] [CrossRef]
- Naguib, M.; Mochalin, V.N.; Barsoum, M.W.; Gogotsi, Y. MXenes: A new family of two-dimensional materials. Adv. Mater. 2014, 26, 992–1005. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Zheng, Y.M.; Zhang, B.G.; Dai, Y.R. A critical review on the electrospun nanofibrous membranes for the adsorption of heavy metals in water treatment. J. Hazard. Mater. 2021, 401, 123608. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Gómez, C.P.; Cecilia, J.A. Chitosan: A natural biopolymer with a wide and varied range of applications. Molecules 2020, 25, 3981. [Google Scholar] [CrossRef] [PubMed]
- Habiba, U.; Afifi, A.M.; Salleh, A.; Ang, B.C. Chitosan/(polyvinyl alcohol)/zeolite electrospun composite nanofibrous membrane for adsorption of Cr6+, Fe3+ and Ni2+. J. Hazard. Mater. 2017, 322, 182–194. [Google Scholar] [CrossRef] [PubMed]
- Pala, P.; Pala, A.; Nakashimad, K.; Yadav, B.K. Applications of chitosan in environmental remediation: A review. Chemosphere 2021, 266, 128934. [Google Scholar] [CrossRef]
- Min, L.L.; Yuan, Z.H.; Zhong, L.B.; Liu, Q.; Wu, R.X.; Zheng, Y.M. Preparation of chitosan based electrospun nanofber membrane and its adsorptive removal of arsenate from aqueous solution. Chem. Eng. J. 2015, 267, 132–141. [Google Scholar] [CrossRef]
- Chauhan, D.; Dwivedi, J.; Sankararamakrishnan, N. Novel chitosan/PVA/zerovalent iron biopolymeric nanofibers with enhanced arsenic removal applications. Environ. Sci. Pollut. 2014, 21, 9430–9442. [Google Scholar] [CrossRef]
- Yang, D.; Li, L.; Chen, B.; Shi, S.; Nie, J.; Ma, G. Functionalized chitosan electrospun nanofiber membranes for heavy-metal removal. Polymers 2019, 163, 74–85. [Google Scholar] [CrossRef]
- Peer, F.E.; Bahramifar, N.; Younesi, H. Removal of Cd (II), Pb (II) and Cu (II) ions from aqueous solution by polyamidoamine dendrimer grafted magnetic graphene oxide nanosheets. J. Taiwan Inst. Chem. Eng. 2018, 87, 225–240. [Google Scholar] [CrossRef]
- Ren, J.; Yan, C.; Liu, Q.; Yang, Q.; Lu, G.; Song, Y.; Li, Y. Preparation of amidoxime-modifed polyacrylonitrile nanofbrous adsorbents for the extraction of copper(II) and lead(II) ions and dye from aqueous media. J. Appl. Polym. Sci. 2018, 135, 45697. [Google Scholar] [CrossRef]
- Fang, Y.; Liu, X.; Wu, X.; Tao, X.; Fei, W. Electrospun polyurethane/phytic acid nanofibrous membrane for high efficient removal of heavy metal ions. Environ. Technol. 2021, 42, 1053–1060. [Google Scholar] [CrossRef]
- Brandes, R.; Belosinschi, D.; Brouillette, F.; Chabot, B. A new electrospun chitosan/phosphorylated nanocellulose biosorbent for the removal of cadmium ions from aqueous solutions. J. Environ. Chem. Eng. 2019, 7, 103477. [Google Scholar] [CrossRef]
- Alharbi, H.F.; Haddad, M.Y.; Aijaz, M.O.; Assaifan, A.K.; Karim, M.R. Electrospun Bilayer PAN/Chitosan Nanofiber Membranes Incorporated with Metal Oxide Nanoparticles for Heavy Metal Ion Adsorption. Coatings 2020, 10, 285. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Xue, C.H.; Ma, H.R.; Ding, Y.R.; Jia, S.T. Fabrication of PAN electrospun nanofibers modified by tannin for effective removal of trace Cr(III) in organic complex from wastewater. Polymers 2020, 12, 210. [Google Scholar] [CrossRef] [PubMed] [Green Version]


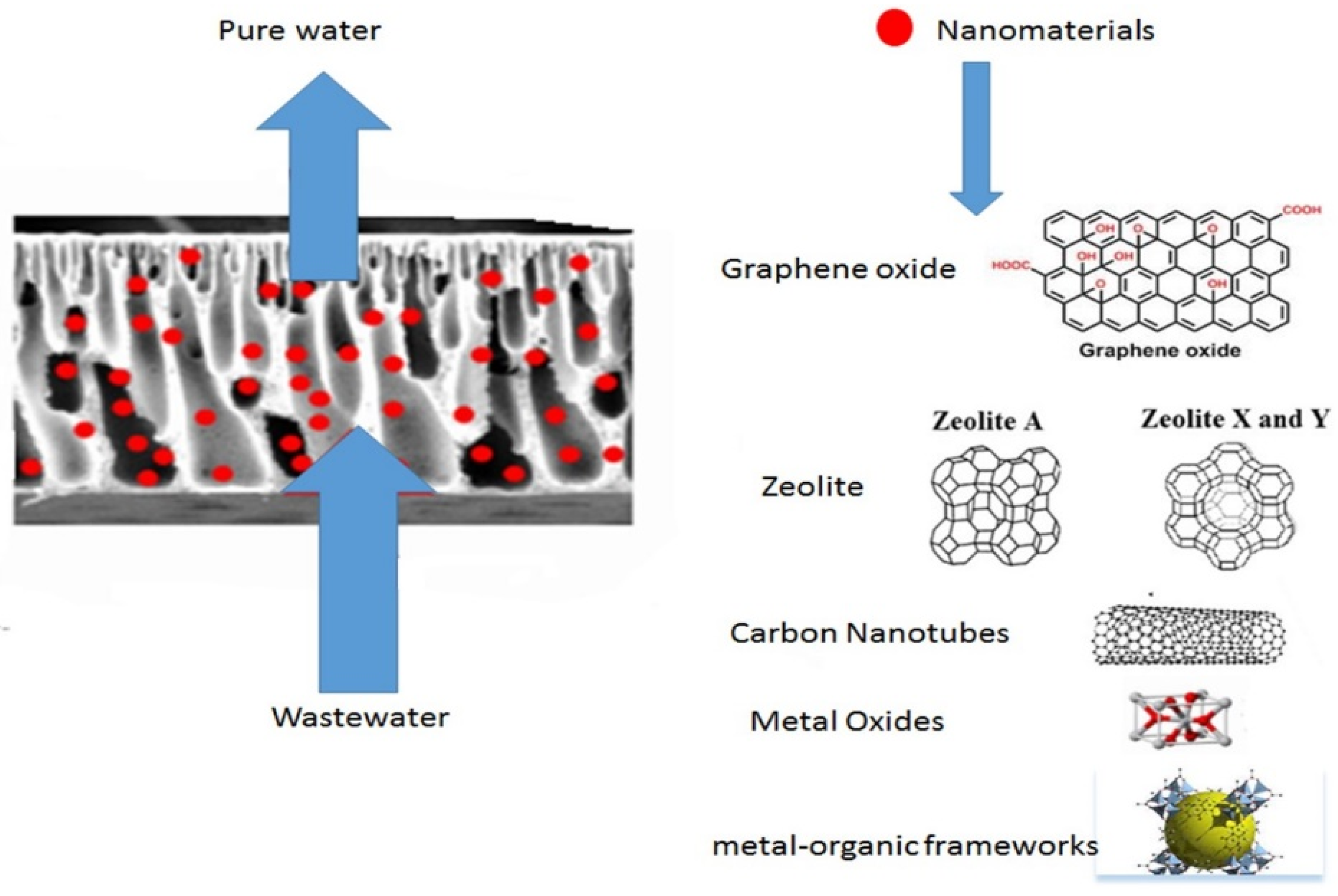
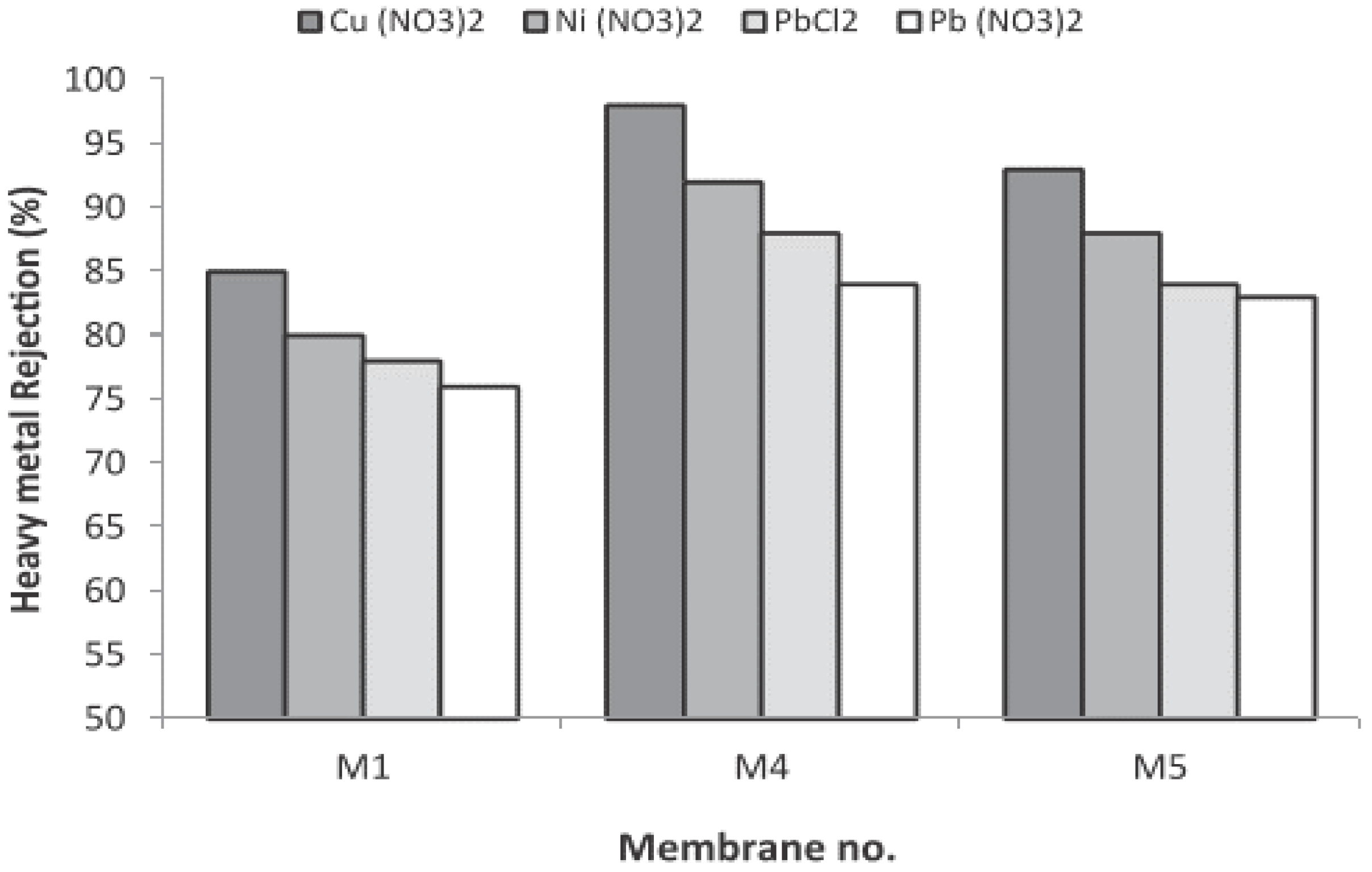
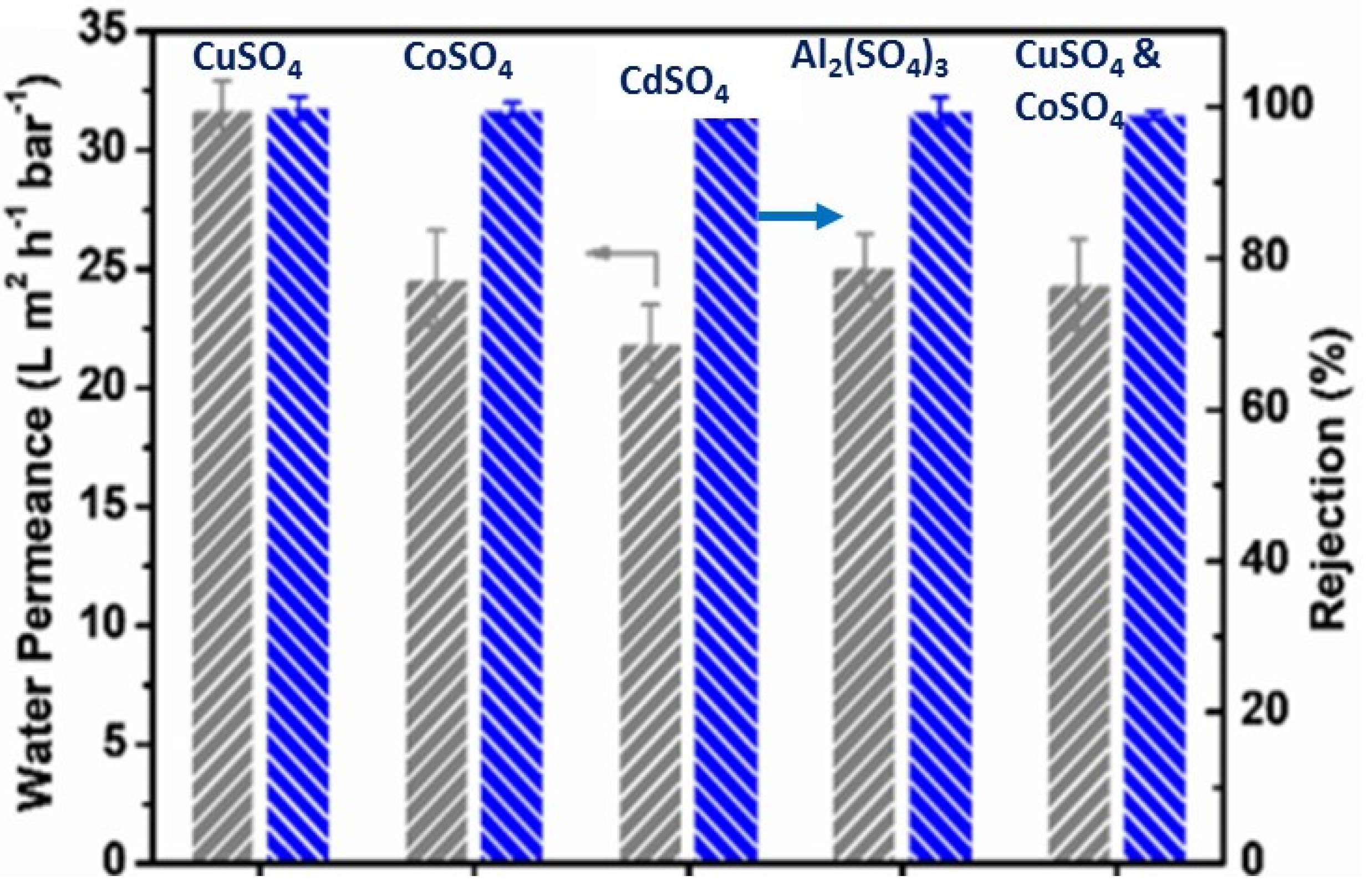

| Heavy Metal | MCL (mg/L) | Potential Health Effects from Long-Term Exposure above the MCL | Source of Contaminant |
|---|---|---|---|
| Cadmium | 0.005 | Kidney damage | Discharge from metal refineries; runoff from waste batteries and paints |
| Chromium | 0.1 | Headache, nausea, diarrhea, vomiting, carcinogenic to human | Discharge from steel mills; erosion of natural deposits |
| Lead | 0.015 | Babies and children: Delays in physical or mental development;Adults: Kidney problems; high pressure of blood | Corrosion of household plumbing systems; erosion of natural deposits |
| Mercury | 0.002 | Kidney disease | Discharge from refineries and factories; runoff from landfills and croplands |
| Nickel | 0.20 | Dermatitis, nausea, cough, Cancer | - |
| Arsenic | 0.010 | Risk of developing cancer | Erosion of the rocks; industries for manufacturing ceramic, pesticides, semiconductors |
| Membrane Process | Applied Pressure (bar) | Molecula Weight Cut-Off * (kDa) | Membrane Characteristics | Permeability (Lm−2 h−1 bar−1) | Species Removed |
|---|---|---|---|---|---|
| MF | 1–3 | >500 | Porous; Asymmetric or symmetric | 500 | Suspended particles (bacteria, fat, oil, colloids, organics, microparticles) |
| UF | 2–5 | 5–500 | Microporous; Asymmetric | 150 | Macro and micromolecules (proteins, pigments, oils, sugar, organics, microplastics) |
| NF | 5–15 | 0.1–5 | Finely porous Asymmetric and thin-film composite | 10–20 | Divalent cations and anions, lactose, sucrose |
| RO | 15–75 | <100 Da | Non-porous Asymmetric and thin-film composite | 5–10 | Monovalent ions and all contaminants |
| Membrane Characteristics | |||
| Type | Material of Membrane | MWCO (KDa) | Manufacturer |
| PES-10 | Polyether sulphone | 10 | Alfa-Laval |
| PES-20 | Polyether sulphone | 20 | AlfA-Laval |
| CAc-40 | Cellulose acetate | 40 | Celgard |
| PES-100 | Polyether sulphone | 100 | Celgard |
| Complexing Agent Characteristics | |||
| Sign | Material | Molecular Weight (g/mol) | Manufacturer |
| PEI-25 | Poly(ethylenimine) | 25,000 | Aldrich |
| PEI-70 | Poly(ethylenimine) | 70,000 | |
| PAA | Poly(acrylic acid) | - | |
| Membrane Characteristics | |||
|---|---|---|---|
| Type | PEI-25 | PEI-70 | PAA |
| PES-10 | 92.9 97.8 * | 98.2 | 31.5 78.1 * |
| PES-20 | 98.1 | 97.0 | 64.1 |
| CAc-40 | 84.1 88.8 * | 90.2 90.3 * | 79.8 |
| PES-100 | 72.6 | 89.3 | 62.1 |
| Ions | ||||||
|---|---|---|---|---|---|---|
| Ni | Sr | Zn | Fe | Co | Mg | |
| Concentrations in effluent (mg/L) | 0.20 | 0.26 | 0.72 | 0.59 | 1.52 | 3.46 |
| Concentrations after UF (mg/L) | 0.10 | 0.23 | 0.42 | 0.14 | 1.08 | 3.19 |
| Concentrations after PEUF (mg/L) | 0.06 | 0.22 | 0.28 | 0.09 | 0.76 | 9.27 |
| Rejections after UF(%) | 50 | 12 | 41 | 76 | 29 | 9 |
| Rejections after PEUF (%) | 60 | 24 | 64 | 87 | 45 | 29 |
| Process | Membrane | MWCO * kDa | Complexing Agent | Metal Ion | Rejection (%) | References |
|---|---|---|---|---|---|---|
| PEUF ** | Polysulfone | 50 | Polyethyleneimine | Cr | 100 | [40] |
| PEUF | Polyether sulfone | 10 | Carboxyl methylcellulose | Ni | 99 | [42] |
| PEUF | Polyether sulfone | 10 | Polyvinylamine | Pb | 99 | [43] |
| PEUF | Polyether sulfone | 10 | Poly (ammonium acrylate) | Cd | 99 | [44] |
| PEUF | Polyether sulfone | 10 | Polyvinylamine | Hg | >90 | [45] |
| PEUF | Polyether sulfone | 60 | Polyethylenimine | Cu | 94 | [46] |
| MEUF ° | - | 10 | Rhamnolipid | Ni | 99.9 | [47] |
| MEUF | Alumina | 200 | Sodium dodecyl sulfate | Ni | 87 | [48] |
| MEUF | Alumina | 200 | Sodium dodecyl sulfate | Co | 88 | [48] |
| MEUF | Cellulosa | 10 | Cetylpyridinium chloride | Cd | 92 | [49] |
| MEUF | Cellulosa | 10 | Cetylpyridinium chloride | Pb | 92 | [49] |
| MEUF | Cellulosa | 3 | Humic acid | Co | 95 | [50] |
| MEUF | Cellulosa | 10 | Humic acid | Co | 90 | [50] |
| NF Membranes | MWCO | Max Operating Temperature (°C) | pH Tolerance | Manufacturer | Cr Rejection (%) | Cu Rejection (%) |
|---|---|---|---|---|---|---|
| DL * | 150–300 | 50 | 2–11 | Osmonics | 96.6 | 90 |
| DK * | 94.7 | 82 | ||||
| NTR-7450 ° | 200 | 40 | 2–14 | Hydranautics | <70 | <70 |
| Membrane Module | ||
|---|---|---|
| NF90–2540 | NF30F-2440 | |
| MWCO (Da) | 200 | 400 |
| Membrane Material | Polyamide thin film composite membranes | Hydrophilized polyethersulfone |
| Maximum operating temperature (°C) | 40 | 50 |
| pH range | 2–11 | 2–11 |
| Maximun feed flow rate (m3/h) | 1.4 | - |
| MgSO4 rejction (%) | >97 | - |
| NaCl rejction (%) | 85–95 | 25–35 |
| Manufacturer | Dow Chemical | Microdyn-Nadir |
| Operating conditions used during the experiments | ||
| Trans-membrane pressure (bar) | 2–12 | |
| pH | 3.5–10 | |
| Temperature (°C) | 15–40 | |
| As feed concentration (ppb) | 100–1000 | |
| Membrane Material | MWCO (Da) | Ion Rejection (%) | References |
|---|---|---|---|
| CA/PMVEMA * | - | Cd2+ (72) Pb2+ (85) | [67] |
| PHMA ** | 300 | Cd2+ (96) Pb2+ (98) | [68] |
| PA °/PEI ● | - | Cu2+ (>90) | [69] |
| SPSf/PES °° | 157 | Ni2+ (>90) Zn2+ (>90) Cu2+ (>90) | [70] |
| PA ° | - | Cd2+ (99) | [71] |
| CS ●● | - | Cd2+ (96.3) Pb2+ (93) | [72] |
| CS/PVA/MMT ●° | - | Cr6+ (88) | [73] |
| Process | Membrane Material | Configuration | Ion Rejection (%) | References |
|---|---|---|---|---|
| RO | Polyamide (TFC) | Spiral Wound | Cu2+ (99.5) Ni2+ (99.5) | [82] |
| RO | AG4021FF (Osmonics) | - | Ni2+ (99.3) Zn2+ (98.9) | [83] |
| RO | - | - | As(V) (91–99%) As(III) (20–55%) | [84] |
| RO | - | |||
| RO | Polyamide | - | Ni2+ (99.3) | [85] |
| RO | Polyamide | - | As(III) (90) | [86] |
| RO | Polyamide | - | As(V)+ (99.8) | [87] |
| Membrane | Filler (%) | Water Contact Angle (°) | Pure Water Flux (Lm−2 h−1) |
|---|---|---|---|
| M1 | 0.00 | 70 | 12.0 |
| M2 | 0.05 | 62 | 15.0 |
| M3 | 0.10 | 56 | 24.8 |
| M4 | 0.50 | 35 | 34.2 |
| M5 | 1.00 | 48 | 28.0 |
| Membrane | Fe3O4 (mg) | MXene (mg) | Water Flux (Lm−2 h−1) | Cu2+ Removal (%) | Cd2+ Removal (%) | Cr6+ Removal (%) |
|---|---|---|---|---|---|---|
| M1 * | 0 | 8 | 80 | 29.7 | 30.7 | 32.8 |
| M4 * | 4 | 8 | 125 | 63.2 | 64.1 | 70.2 |
| M4 * | 4 | 8 | 105 * | 48.0 * | - | - |
| Material | Chemical Modifier | Concentration * (mg/L) | T (°C) | Metal Ion | qmax (mg/g) | Ref. |
|---|---|---|---|---|---|---|
| Polyacrylonitrile | Amodoxime | 100 | 30 | Cu(II) Pb(II) | 143.47 178.57 | [117] |
| Polyurethane | Phytic acid | 400 | - | Pb(II) | 136.52 | [118] |
| Chitosa/Poly(ethy-lene oxide | Phosphorylated Nanocellulos | - | 25 | Cd(II) | 232.5 | [119] |
| Polyacrylonitrile/Chitosan | ZnO TiO2 | - | - | Pb(II) Cd(II) | 390 461 | [120] |
| Polyacrylonitrile | Tannic Acid | 200 | Cr(III) | 79.48 | [121] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Algieri, C.; Chakraborty, S.; Candamano, S. A Way to Membrane-Based Environmental Remediation for Heavy Metal Removal. Environments 2021, 8, 52. https://0-doi-org.brum.beds.ac.uk/10.3390/environments8060052
Algieri C, Chakraborty S, Candamano S. A Way to Membrane-Based Environmental Remediation for Heavy Metal Removal. Environments. 2021; 8(6):52. https://0-doi-org.brum.beds.ac.uk/10.3390/environments8060052
Chicago/Turabian StyleAlgieri, Catia, Sudip Chakraborty, and Sebastiano Candamano. 2021. "A Way to Membrane-Based Environmental Remediation for Heavy Metal Removal" Environments 8, no. 6: 52. https://0-doi-org.brum.beds.ac.uk/10.3390/environments8060052






