Depuration Performance of Aerated Tanks Simulating Lagoons to Treat Olive Oil Mill Wastewater under Different Airflow Rates, and Concentrations of Polyphenols and Nitrogen
Abstract
:1. Introduction
2. Materials and Methods
2.1. Description of the Experimental Pilot Plant
2.2. Experimental Design
2.3. Initial Characteristics of OMW and Tank Preparation
2.4. Sample Analysis
2.5. Statistical Analysis
3. Results and Discussion
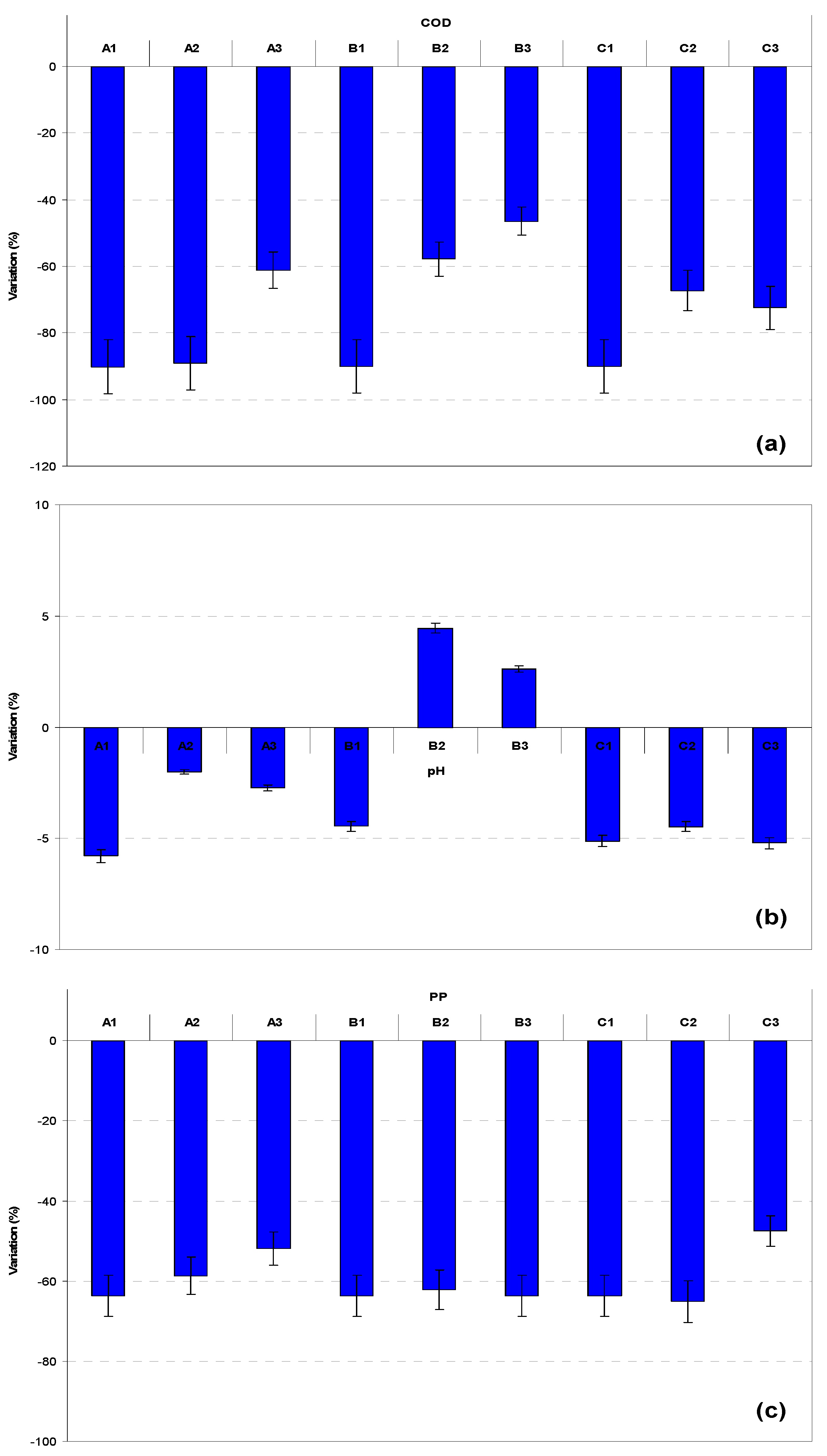
3.1. Tanks “A” (Effects of Aeration)
3.2. Tanks “B” (Effect of PP)
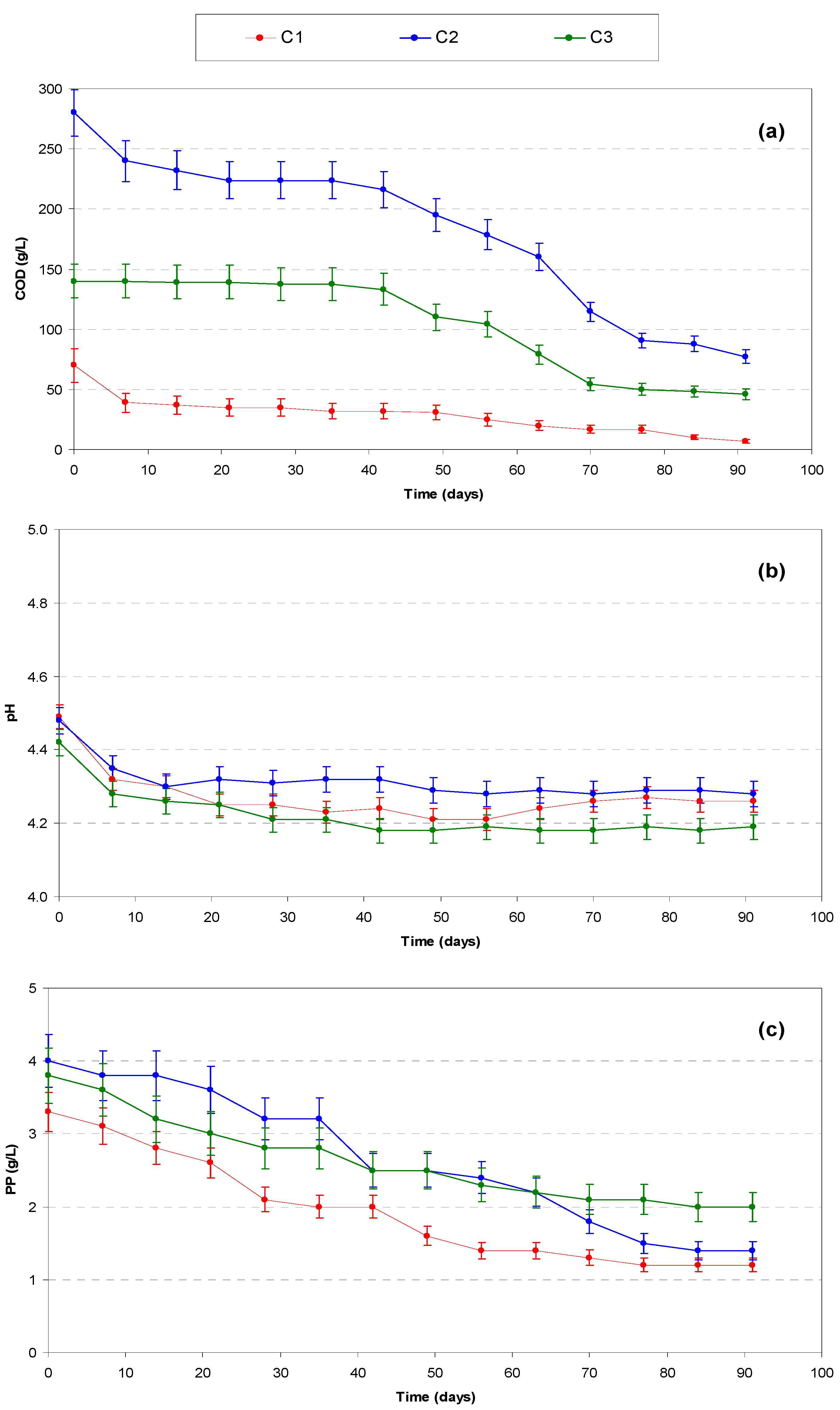
3.3. Tanks “C” (Effect of COD:N Ratio)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zema, D.A.; Esteban Lucas-Borja, M.; Andiloro, S.; Tamburino, V.; Zimbone, S.M. Short-Term Effects of Olive Mill Wastewater Application on the Hydrological and Physico-Chemical Properties of a Loamy Soil. Agric. Water Manag. 2019, 221, 312–321. [Google Scholar] [CrossRef]
- Bombino, G.; Andiloro, S.; Folino, A.; Lucas-Borja, M.E.; Zema, D.A. Short-Term Effects of Olive Oil Mill Wastewater Application on Soil Water Repellency. Agric. Water Manag. 2021, 244, 106563. [Google Scholar] [CrossRef]
- Komnitsas, K.; Zaharaki, D. Pre-Treatment of Olive Mill Wastewaters at Laboratory and Mill Scale and Subsequent Use in Agriculture: Legislative Framework and Proposed Soil Quality Indicators. Resour. Conserv. Recycl. 2012, 69, 82–89. [Google Scholar] [CrossRef]
- Moreno, L.; González, A.; Cuadros-Salcedo, F.; Cuadros-Blázquez, F. Feasibility of a Novel Use for Agroindustrial Biogas. J. Clean. Prod. 2017, 144, 48–56. [Google Scholar] [CrossRef]
- Di Serio, M.G.; Lanza, B.; Mucciarella, M.R.; Russi, F.; Iannucci, E.; Marfisi, P.; Madeo, A. Effects of Olive Mill Wastewater Spreading on the Physico-Chemical and Microbiological Characteristics of Soil. Int. Biodeterior. Biodegrad. 2008, 62, 403–407. [Google Scholar] [CrossRef]
- Amaral, C.; Lucas, M.S.; Coutinho, J.; Crespí, A.L.; do Rosário Anjos, M.; Pais, C. Microbiological and Physicochemical Characterization of Olive Mill Wastewaters from a Continuous Olive Mill in Northeastern Portugal. Bioresour. Technol. 2008, 99, 7215–7223. [Google Scholar] [CrossRef] [PubMed]
- Tziotzios, G.; Michailakis, S.; Vayenas, D.V. Aerobic Biological Treatment of Olive Mill Wastewater by Olive Pulp Bacteria. Int. Biodeterior. Biodegrad. 2007, 60, 209–214. [Google Scholar] [CrossRef]
- Karaouzas, I.; Skoulikidis, N.T.; Giannakou, U.; Albanis, T.A. Spatial and Temporal Effects of Olive Mill Wastewaters to Stream Macroinvertebrates and Aquatic Ecosystems Status. Water Res. 2011, 45, 6334–6346. [Google Scholar] [CrossRef]
- Dermeche, S.; Nadour, M.; Larroche, C.; Moulti-Mati, F.; Michaud, P. Olive Mill Wastes: Biochemical Characterizations and Valorization Strategies. Process. Biochem. 2013, 48, 1532–1552. [Google Scholar] [CrossRef]
- Azbar, N.; Bayram, A.; Filibeli, A.; Muezzinoglu, A.; Sengul, F.; Ozer, A. A Review of Waste Management Options in Olive Oil Production. Crit. Rev. Environ. Sci. Technol. 2004, 34, 209–247. [Google Scholar] [CrossRef]
- Aggoun, M.; Arhab, R.; Cornu, A.; Portelli, J.; Barkat, M.; Graulet, B. Olive Mill Wastewater Microconstituents Composition According to Olive Variety and Extraction Process. Food Chem. 2016, 209, 72–80. [Google Scholar] [CrossRef]
- McNamara, C.J.; Anastasiou, C.C.; O’Flaherty, V.; Mitchell, R. Bioremediation of Olive Mill Wastewater. Int. Biodeterior. Biodegrad. 2008, 61, 127–134. [Google Scholar] [CrossRef]
- Jarboui, R.; Hadrich, B.; Gharsallah, N.; Ammar, E. Olive Mill Wastewater Disposal in Evaporation Ponds in Sfax (Tunisia): Moisture Content Effect on Microbiological and Physical Chemical Parameters. Biodegradation 2009, 20, 845–858. [Google Scholar] [CrossRef] [PubMed]
- Gómez, A.; Zubizarreta, J.; Rodrigues, M.; Dopazo, C.; Fueyo, N. An Estimation of the Energy Potential of Agro-Industrial Residues in Spain. Resour. Conserv. Recycl. 2010, 54, 972–984. [Google Scholar] [CrossRef]
- Dourou, M.; Kancelista, A.; Juszczyk, P.; Sarris, D.; Bellou, S.; Triantaphyllidou, I.E.; Rywinska, A.; Papanikolaou, S.; Aggelis, G. Bioconversion of Olive Mill Wastewater into High-Added Value Products. J. Clean. Prod. 2016, 139, 957–969. [Google Scholar] [CrossRef]
- Orive, M.; Cebrián, M.; Zufía, J. Techno-Economic Anaerobic Co-Digestion Feasibility Study for Two-Phase Olive Oil Mill Pomace and Pig Slurry. Renew. Energy 2016, 97, 532–540. [Google Scholar] [CrossRef]
- Calabrò, P.S.; Fòlino, A.; Tamburino, V.; Zappia, G.; Zema, D.A. Increasing the Tolerance to Polyphenols of the Anaerobic Digestion of Olive Wastewater through Microbial Adaptation. Biosyst. Eng. 2018, 172, 19–28. [Google Scholar] [CrossRef]
- Zema, D.A. Planning the Optimal Site, Size, and Feed of Biogas Plants in Agricultural Districts. Biofuels Bioprod. Bioref. 2017, 11, 454–471. [Google Scholar] [CrossRef]
- Barbera, A.C.; Maucieri, C.; Ioppolo, A.; Milani, M.; Cavallaro, V. Effects of Olive Mill Wastewater Physico-Chemical Treatments on Polyphenol Abatement and Italian Ryegrass (Lolium Multiflorum Lam.) Germinability. Water Res. 2014, 52, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Mekki, A.; Dhouib, A.; Sayadi, S. Changes in Microbial and Soil Properties Following Amendment with Treated and Untreated Olive Mill Wastewater. Microbiol. Res. 2006, 161, 93–101. [Google Scholar] [CrossRef]
- Chartzoulakis, K.; Psarras, G.; Moutsopoulou, M.; Stefanoudaki, E. Application of Olive Mill Wastewater to a Cretan Olive Orchard: Effects on Soil Properties, Plant Performance and the Environment. Agric. Ecosyst. Environ. 2010, 138, 293–298. [Google Scholar] [CrossRef]
- Juanicó, M.; Milstein, A. Semi-Intensive Treatment Plants for Wastewater Reuse in Irrigation. Water Sci. Technol. 2004, 50, 55–60. [Google Scholar] [CrossRef] [Green Version]
- Salgot, M.; Huertas, E.; Weber, S.; Dott, W.; Hollender, J. Wastewater Reuse and Risk: Definition of Key Objectives. Desalination 2006, 187, 29–40. [Google Scholar] [CrossRef]
- Zema, D.A.; Calabro, P.S.; Folino, A.; Tamburino, V.; Zappia, G.; Zimbone, S.M. Wastewater Management in Citrus Processing Industries: An Overview of Advantages and Limits. Water (Switz.) 2019, 11, 2481. [Google Scholar] [CrossRef] [Green Version]
- Hamza, R.A.; Iorhemen, O.T.; Tay, J.H. Anaerobic-Aerobic Granular System for High-Strength Wastewater Treatment in Lagoons. Adv. Environ. Res. 2016, 5, 169–178. [Google Scholar] [CrossRef]
- Isosaari, P.; Hermanowicz, S.W.; Rubin, Y. Sustainable Natural Systems for Treatment and Disposal of Food Processing Wastewater. Crit. Rev. Environ. Sci. Technol. 2010, 40, 662–697. [Google Scholar] [CrossRef]
- Gikas, G.D.; Tsakmakis, I.D.; Tsihrintzis, V.A. Hybrid Natural Systems for Treatment of Olive Mill Wastewater. J. Chem. Technol. Biotechnol. 2018, 93, 800–809. [Google Scholar] [CrossRef]
- Pishgar, R.; Hamza, R.A.; Tay, J.H. Augmenting Lagoon Process Using Reactivated Freeze-Dried Biogranules. Appl. Biochem. Biotechnol. 2017, 183, 137–154. [Google Scholar] [CrossRef]
- Rozzi, A.; Malpei, F. Treatment and Disposal of Olive Mill Effluents. Int. Biodeterior. Biodegrad. 1996, 38, 135–144. [Google Scholar] [CrossRef]
- Rajbhandari, B.K.; Annachhatre, A.P. Anaerobic Ponds Treatment of Starch Wastewater: Case Study in Thailand. Bioresour. Technol. 2004, 95, 135–143. [Google Scholar] [CrossRef]
- Andiloro, S.; Bombino, G.; Tamburino, V.; Zema, D.A.; Zimbone, S.M. Aerated Lagooning of Agro-Industrial Wastewater: Depuration Performance and Energy Requirements. J. Agric. Eng. 2013, 7, 44. [Google Scholar] [CrossRef]
- Paraskeva, P.; Diamadopoulos, E. Technologies for Olive Mill Wastewater (OMW) Treatment: A Review. J. Chem. Technol. Biotechnol. 2006, 81, 1475–1485. [Google Scholar] [CrossRef]
- S’habou, R.; Zairi, M.; Kallel, A.; Aydi, A.; Ben Dhia, H. Assesing the Effect of an Olive Mill Wastewater Evaporation Pond in Sousse, Tunisia. Environ. Geol. 2009, 58, 679. [Google Scholar] [CrossRef]
- Benitez, F.J.; Beltran-Heredia, J.; Torregrosa, J.; Acero, J.L. Treatment of Olive Mill Wastewaters by Ozonation, Aerobic Degradation and the Combination of Both Treatments. J. Chem. Technol. Biotechnol. 1999, 74, 639–646. [Google Scholar] [CrossRef]
- Zema, D.A.; Andiloro, S.; Bombino, G.; Caridi, A.; Sidari, R.; Tamburino, V. Comparing Different Schemes of Agricultural Wastewater Lagooning: Depuration Performance and Microbiological Characteristics. Water Air Soil Pollut. 2016, 227. [Google Scholar] [CrossRef]
- Mantzavinos, D.; Kalogerakis, N. Treatment of Olive Mill Effluents. Environ. Int. 2005, 31, 289–295. [Google Scholar] [CrossRef]
- Jail, A.; Boukhoubza, F.; Nejmeddine, A.; Sayadi, S.; Hassani, L. Co-Treatment of Olive-Mill and Urban Wastewaters by Experimental Stabilization Ponds. J. Hazard. Mater. 2010, 176, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Jail, A.; Boukhoubza, F.; Nejmeddine, A.; Duarte, J.C.; Sayadi, S.; Hassani, L. Traitement des effluents d’huileries par un procédé combinant un traitement intensif (Jet-Loop Reactor) suivi d’un traitement extensif (bassins de stabilisation): Treatment of olive mill wastewater by a process combining an intensive treatment (Jet-Loop Reactor) followed by an extensive treatment (stabilization ponds). Environ. Technol. 2010, 31, 533–543. [Google Scholar] [CrossRef]
- Zema, D.A.; Andiloro, S.; Bombino, G.; Tamburino, V.; Sidari, R.; Caridi, A. Depuration in Aerated Ponds of Citrus Processing Wastewater with a High Concentration of Essential Oils. Environ. Technol. 2012, 33. [Google Scholar] [CrossRef] [PubMed]
- Zema, D.A.; Zappia, G.; Benalia, S.; Zimbalatti, G.; Perri, E.; Urso, E.; Tamburino, V.; Bernardi, B. Limiting Factors to Anaerobic Digestion of Olive Mill Wastewater Blends under Mesophilic and Thermophilic Conditions. J. Agric. Eng. 2018, 49, 130–137. [Google Scholar] [CrossRef]
- Borja, R.; Banks, C.J.; Maestro-Durán, R.; Alba, J. The Effects of the Most Important Phenolic Constituents of Olive Mill Wastewater on Batch Anaerobic Methanogenesis. Environ. Technol. 1996, 17, 167–174. [Google Scholar] [CrossRef]
- Khoufi, S.; Aloui, F.; Sayadi, S. Treatment of Olive Oil Mill Wastewater by Combined Process Electro-Fenton Reaction and Anaerobic Digestion. Water Res. 2006, 40, 2007–2016. [Google Scholar] [CrossRef]
- Elimination of Polyphenols Toxicity from Olive Mill Wastewater Sludge by Its Co-Composting with Sesame Bark-ScienceDirect. Available online: https://0-www-sciencedirect-com.brum.beds.ac.uk/science/article/pii/S0304389408006134 (accessed on 8 June 2021).
- Zema, D.A.; Calabrò, P.S.; Folino, A.; Tamburino, V.; Zappia, G.; Zimbone, S.M. Valorisation of Citrus Processing Waste: A Review. Waste Manag. 2018, 80, 252–273. [Google Scholar] [CrossRef] [PubMed]
- Mielczarek, A.T.; Kragelund, C.; Eriksen, P.S.; Nielsen, P.H. Population Dynamics of Filamentous Bacteria in Danish Wastewater Treatment Plants with Nutrient Removal. Water Res. 2012, 46, 3781–3795. [Google Scholar] [CrossRef]
- Bashaar, Y.A. Nutrients Requirements in Biological Industrial Wastewater Treatment. Afr. J. Biotechnol. 2004, 3, 236–238. [Google Scholar] [CrossRef] [Green Version]
- Elkacmi, R.; Bennajah, M. Advanced Oxidation Technologies for the Treatment and Detoxification of Olive Mill Wastewater: A General Review. J. Water Reuse Desalination 2019, 9, 463–505. [Google Scholar] [CrossRef] [Green Version]
- Frascari, D.; Rubertelli, G.; Arous, F.; Ragini, A.; Bresciani, L.; Arzu, A.; Pinelli, D. Valorisation of Olive Mill Wastewater by Phenolic Compounds Adsorption: Development and Application of a Procedure for Adsorbent Selection. Chem. Eng. J. 2019, 360, 124–138. [Google Scholar] [CrossRef]
- Ben Sassi, A.; Boularbah, A.; Jaouad, A.; Walker, G.; Boussaid, A. A Comparison of Olive Oil Mill Wastewaters (OMW) from Three Different Processes in Morocco. Process. Biochem. 2006, 41, 74–78. [Google Scholar] [CrossRef]
- Rahmanian, N.; Jafari, S.M.; Galanakis, C.M. Recovery and Removal of Phenolic Compounds from Olive Mill Wastewater. J. Am. Oil Chem. Soc. 2014, 91, 1–18. [Google Scholar] [CrossRef]
- Borja, R.; Martín, A.; Alonso, V.; García, I.; Banks, C.J. Influence of Different Aerobic Pretreatments on the Kinetics of Anaerobic Digestion of Olive Mill Wastewater. Water Res. 1995, 29, 489–495. [Google Scholar] [CrossRef]
- Folin, O.; Ciocalteu, V. On tyrosine and tryptophane determinations in Proteins. J. Biol. Chem. 1927, 73, 627–650. [Google Scholar] [CrossRef]
- Bawab, A.A.; Ghannam, N.; Abu-Mallouh, S.; Bozeya, A.; Abu-Zurayk, R.A.; Al-Ajlouni, Y.A.; Alshawawreh, F.; Odeh, F.; Abu-Dalo, M.A. Olive mill wastewater treatment in Jordan: A review. IOP Conf. Ser. Mater. Sci. Eng. 2018, 305, 012002. [Google Scholar] [CrossRef]
- Zenjari, B.; Hafidi, M.; Hadrami, I.; Bailly, J.R.; Nejmeddine, A. Traitment Aerobie Des Effluents d’huilerie Par Les Micro-Organismes Du Sol. Agrochim. PISA 1999, 43, 277–286. [Google Scholar]
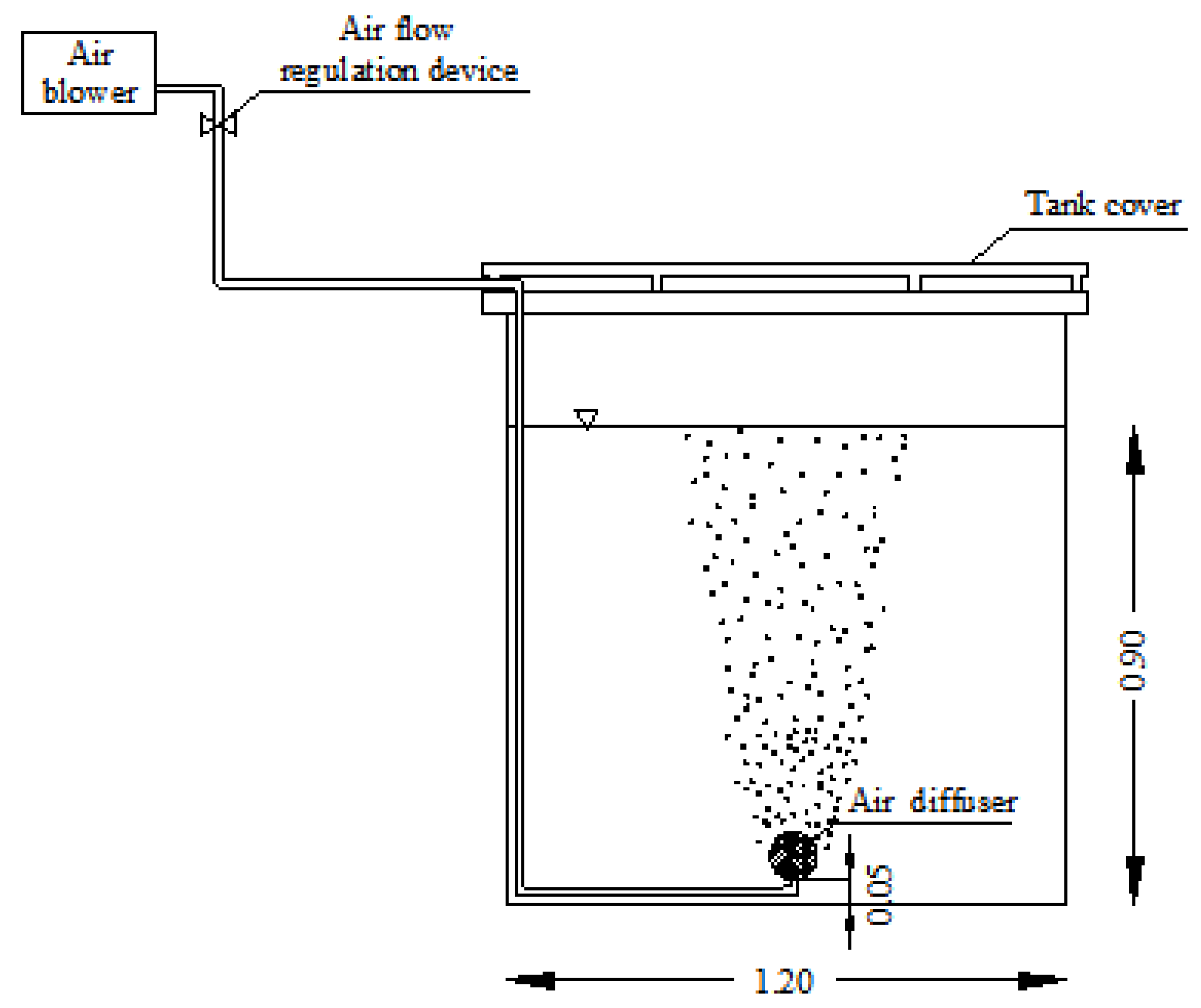


| Test Series | Tank | Airflow Rate and Aeration Time | PP Concentration | COD:N Ratio |
|---|---|---|---|---|
| A | A1 | Variable | Same | Same |
| A2 | ||||
| A3 | ||||
| B | B1 | Same | Variable | Same |
| B2 | ||||
| B3 | ||||
| C | C1 | Same | Same | Variable |
| C2 | ||||
| C3 |
| Test Series | Tank | Condition | Air Flow | PP Concentration (g/L) | COD:N Ratio | |
|---|---|---|---|---|---|---|
| Rate (L/h-m3) | Time (Hours) | |||||
| A | A1 | Aerated | 7 | 24 | 3.3 ± 0.26 | 103:5 ± 1.81 |
| A2 | 14 | 12 | 2.9 ± 0.20 | 101:5 ± 1.65 | ||
| A3 | Non-aerated | 0 | 0 | 2.7 ± 0.21 | 105:5 ± 1.44 | |
| B | B1 | Aerated | 7 | 24 | 3.3 ± 0.25 | 395:5 ± 8.69 |
| B2 | 6.6 ± 0.62 | 401:5 ± 8.67 | ||||
| B3 | 13.2 ± 1.29 | 408:5 ± 8.99 | ||||
| C | C1 | Aerated | 7 | 24 | 3.4 ± 0.24 | 100:5 ± 1.01 |
| C2 | 4.0 ± 0.39 | 200:5 ± 2.25 | ||||
| C3 | 3.7 ± 0.27 | 400:5 ± 3.98 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andiloro, S.; Bombino, G.; Denisi, P.; Folino, A.; Zema, D.A.; Zimbone, S.M. Depuration Performance of Aerated Tanks Simulating Lagoons to Treat Olive Oil Mill Wastewater under Different Airflow Rates, and Concentrations of Polyphenols and Nitrogen. Environments 2021, 8, 70. https://0-doi-org.brum.beds.ac.uk/10.3390/environments8080070
Andiloro S, Bombino G, Denisi P, Folino A, Zema DA, Zimbone SM. Depuration Performance of Aerated Tanks Simulating Lagoons to Treat Olive Oil Mill Wastewater under Different Airflow Rates, and Concentrations of Polyphenols and Nitrogen. Environments. 2021; 8(8):70. https://0-doi-org.brum.beds.ac.uk/10.3390/environments8080070
Chicago/Turabian StyleAndiloro, Serafina, Giuseppe Bombino, Pietro Denisi, Adele Folino, Demetrio Antonio Zema, and Santo Marcello Zimbone. 2021. "Depuration Performance of Aerated Tanks Simulating Lagoons to Treat Olive Oil Mill Wastewater under Different Airflow Rates, and Concentrations of Polyphenols and Nitrogen" Environments 8, no. 8: 70. https://0-doi-org.brum.beds.ac.uk/10.3390/environments8080070






