Indoor and Outdoor Nanoparticle Concentrations in an Urban Background Area in Northern Sweden: The NanoOffice Study
Abstract
:1. Introduction
2. Materials and Methods
3. Results
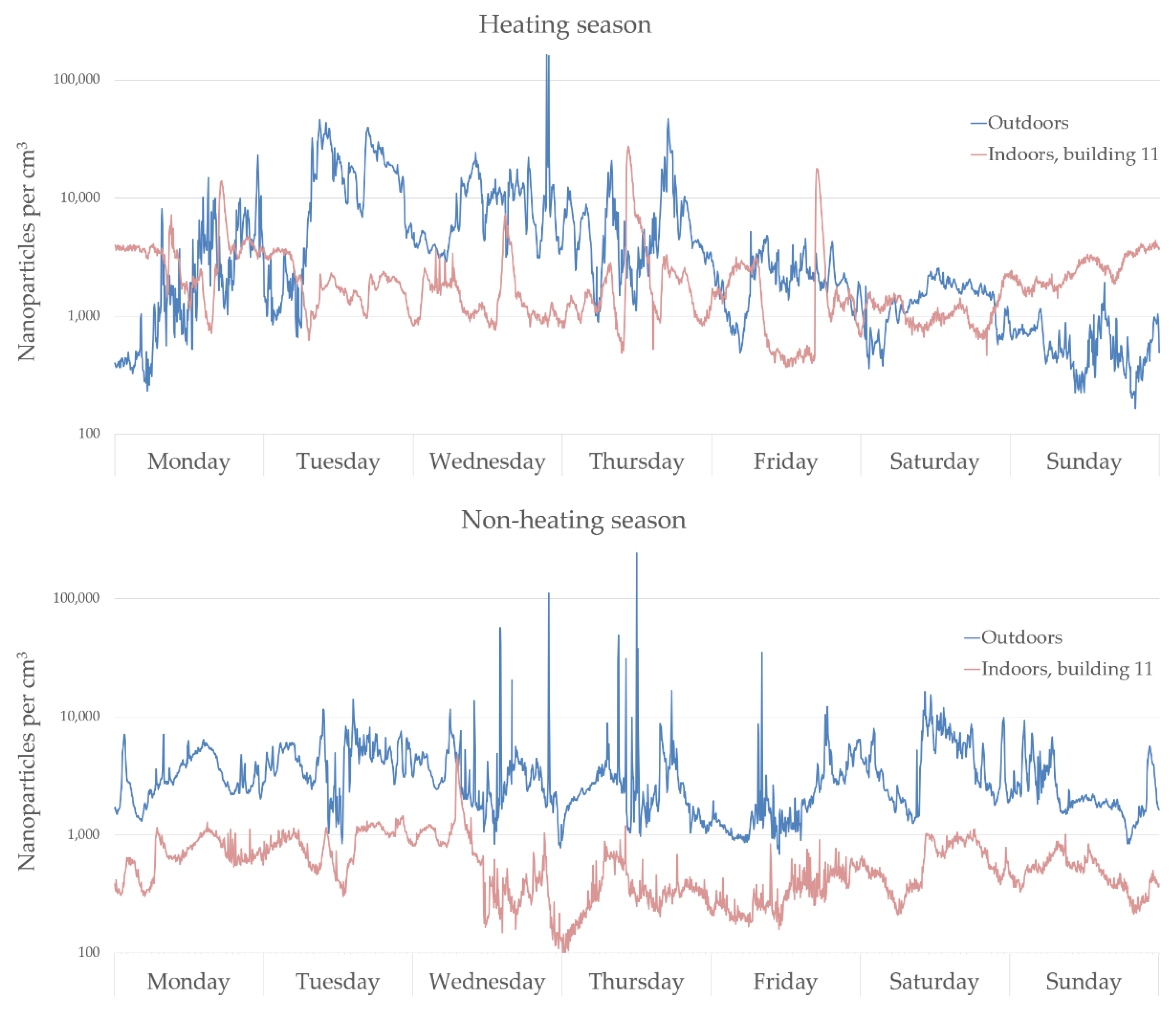
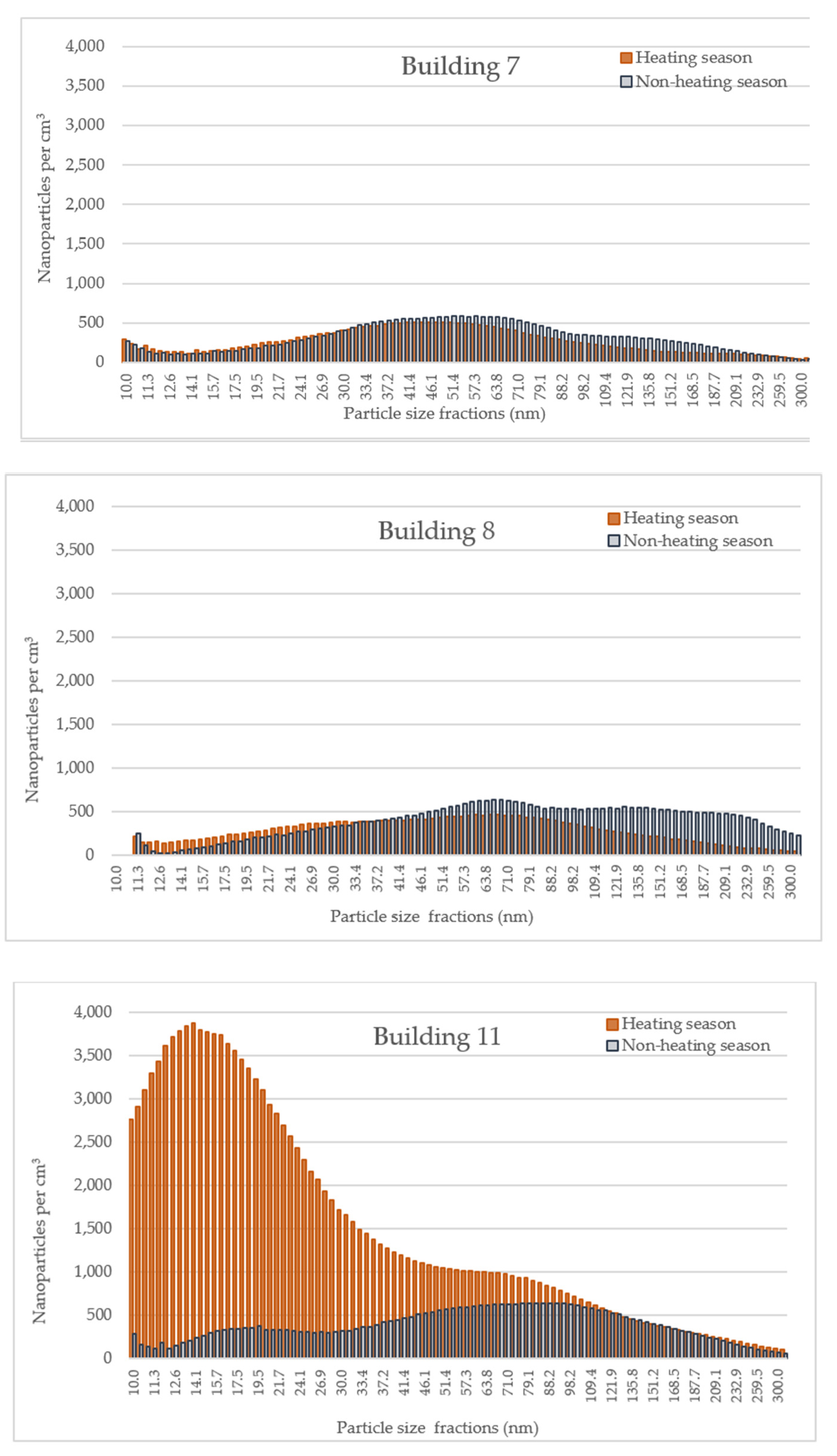
Measurement Results
4. Discussion
4.1. Outdoor Nanoparticles and Their Infiltration Indoors
4.2. Indoor Sources
4.3. Processes That Determine the Particle Properties Indoors and Outdoors
4.4. Strengths and Limitations of This Study
4.5. Regulatory Outlook
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Buildings 1–12 | Measurement Period, Heating Season | Temp, Average (Min, Max) (°C) | Wind Speed (m/s) | Relative Humidity (%) | Measurement Period, Non-Heating Season | Temp, Average (Min, Max) (°C) | Wind Speed (m/s) | Relative Humidity (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | 29 March 2018–5 April 2018 | −3.3 (−15.9, 6.1) | 2.1 | 77.5 | 31 May 2018–7 June 2018 | 13.0 (0, 25.7) | 4.6 | 52.7 |
| 2 | 4–11 April 2017 | 2.5 (−2.8, 10.3) | 2.7 | 78.2 | 29 June 2017–6 July 2017 | 14.0 (2.4, 22.0) | 3.5 | 68.6 |
| 3 | 13–20 March 2017 | 0.3 (−11.7, 7.6) | 3.4 | 83.0 | 24–31 May 2017 | 9.0 (−2.3, 18.5) | 3.6 | 65.2 |
| 4 | 21–28 March 2017 | 2.2 (−6.2, 11.1) | 5.1 | 69.1 | 6–14 July 2017 | 14.0 (2.4, 21.1) | 2.6 | 77.1 |
| 5 | 5–12 January 2018 | −6.5 (−18.5, 3.4) | 3.1 | 88.7 | 7–14 June 2018 | 10.7 (0, 22.4) | 2.9 | 69.5 |
| 6 | 16–23 January 2018 | −8.0 (−21.9, 0.6) | 3.2 | 90.3 | 26 June 2018–3 July 2018 | 14.7 (2.1, 23.4) | 3.4 | 57.6 |
| 7 | 21–28 March 2018 | −4.0 (−19.8, 4.7) | 2.9 | 75.5 | 18–25 June 2018 | 13.5 (3.6, 22.5) | 4.0 | 68.0 |
| 8 | 15–22 February 2017 | −2.9 (−20.2, 6.4) | 2.3 | 89.2 | 16–23 June 2017 | 13.4 (3.6, 22.7) | 3.3 | 71.1 |
| 9 | 28 March 2017–4 April 2017 | −0.2 (−8.8, 9.6) | 3.4 | 78.4 | 7–14 June 2017 | 13.3 (4.1, 22.4) | 2.9 | 76.9 |
| 10 | 23–30 January 2017 | −0.5 (−11.1, 8.0) | 3.2 | 87.3 | 25 July 2017–1 August 2017 | 16.8 (5.6, 22.2) | 2.7 | 81.9 |
| 11 | 2–9 February 2017 | −6.0 (−19.5, 0.1) | 1.7 | 90.4 | 31 May 2017–7 June 2017 | 8.5 (−2.1, 21.4) | 3.7 | 68.5 |
| 12 | 3–10 March 2017 | −6.4 (−19.3, 6.2) | 2.3 | 89.0 | 17–24 July 2017 | 14.1 (5.4, 22.5) | 2.9 | 76.0 |
References
- Barua, S.; Mitragotri, S. Challenges associated with penetration of nanoparticles across cell and tissue barriers: A review of current status and future prospects. Nano Today 2014, 9, 223–243. [Google Scholar] [CrossRef]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Muller, R.N. Magnetic Iron Oxide Nanoparticles: Synthesis, Stabilization, Vectorization, Physicochemical Characterizations, and Biological Applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef]
- Höck, J.; Epprecht, T.; Hofmann, H.; Höhner, K.; Krug, H.; Lorenz, C.; Limbach, L.; Gehr, P.; Nowack, B.; Riediker, M. Guidelines on The Precautionary Matrix for Synthetic Nanomaterials; Federal Office for Public Health and Federal Office for the Environment: Bern, Switzerland, 2010.
- Commission, E. Commission recommendation of 18 October 2011 on the definition of nanomaterial. Off. J. Eur. Union 2011, 275, 38. [Google Scholar]
- Schuster, B.S.; Suk, J.S.; Woodworth, G.F.; Hanes, J. Nanoparticle diffusion in respiratory mucus from humans without lung disease. Biomaterials 2013, 34, 3439–3446. [Google Scholar] [CrossRef] [Green Version]
- Schneider, C.S.; Xu, Q.; Boylan, N.J.; Chisholm, J.; Tang, B.C.; Schuster, B.S.; Henning, A.; Ensign, L.M.; Lee, E.; Adstamongkonkul, P.; et al. Nanoparticles that do not adhere to mucus provide uniform and long-lasting drug delivery to airways following inhalation. Sci. Adv. 2017, 3, e1601556. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Qin, Z.; Zeng, W.; Yang, T.; Cao, Y.; Mei, C.; Kuang, Y. Toxicity assessment of nanoparticles in various systems and organs. Nanotechnol. Rev. 2017, 6, 279–289. [Google Scholar] [CrossRef]
- Rodríguez, F.; Bernard, Y.; Dornoff, J.; Mock, P. Recommendations for post-Euro 6 standards for light-duty vehicles in the European Union. Communications 2019, 49. Available online: https://theicct.org/publications/recommendations-post-euro-6-eu (accessed on 1 July 2021).
- Myung, C.L.; Park, S. Exhaust nanoparticle emissions from internal combustion engines: A review. Int. J. Automot. Technol. 2012, 13, 9–22. [Google Scholar] [CrossRef]
- D’Anna, A. Combustion-formed nanoparticles. Proc. Combust. Inst. 2009, 32, 593–613. [Google Scholar] [CrossRef]
- Karner, A.A.; Eisinger, D.S.; Niemeier, D.A. Near-roadway air quality: Synthesizing the findings from real-world data. Environ. Sci. Technol. 2010, 44, 5334–5344. [Google Scholar] [CrossRef] [PubMed]
- Viitanen, A.-K.; Uuksulainen, S.; Koivisto, A.J.; Hämeri, K.; Kauppinen, T. Workplace Measurements of ultrafine particles—A literature review. Ann. Work. Expo. Health 2017, 61, 749–758. [Google Scholar] [CrossRef]
- Grigoratos, T.; Martini, G. Non-exhaust traffic related emissions. Brake and tyre wear PM. Rep. No. Rep. EUR 2014, 26648, 7–9. [Google Scholar]
- Maher, B.A.; Ahmed, I.A.M.; Karloukovski, V.; MacLaren, D.A.; Foulds, P.G.; Allsop, D.; Mann, D.M.A.; Torres-Jardón, R.; Calderon-Garciduenas, L. Magnetite pollution nanoparticles in the human brain. Proc. Natl. Acad. Sci. USA 2016, 113, 10797–10801. [Google Scholar] [CrossRef] [Green Version]
- Kazimirova, A.; Peikertova, P.; Barancokova, M.; Staruchova, M.; Tulinská, J.; Vaculik, M.; Vávra, I.; Kukutschova, J.; Filip, P.; Dusinska, M. Automotive airborne brake wear debris nanoparticles and cytokinesis-block micronucleus assay in peripheral blood lymphocytes: A pilot study. Environ. Res. 2016, 148, 443–449. [Google Scholar] [CrossRef]
- Walser, T.; Limbach, L.K.; Brogioli, R.; Erismann, E.; Flamigni, L.; Hattendorf, B.; Juchli, M.; Krumeich, F.; Ludwig, C.; Prikopsky, K.; et al. Persistence of engineered nanoparticles in a municipal solid-waste incineration plant. Nat. Nanotechnol. 2012, 7, 520–524. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.A.M.; Fogarin, H.M.; Costa, A.F.M.; Pires, L.O.; Silva, D.D.V.; Lima-Souza, M.; Dussán, K.J. Nanoparticles emitted by biomass burning: Characterization and monitoring of risks. In Nanomaterials: Ecotoxicity, Safety, and Public Perception; Springer Science and Business Media LLC.: Cham, Switzerland, 2018; pp. 253–279. [Google Scholar]
- Shukhnova, A.; Bozrova, S.; Sokolov, P.; Berestovoy, M.; Karaulov, A.; Nabiev, I. Dependence of nanoparticle toxicity on their physical and chemical properties. Nanoscale Res. Lett. 2018, 13, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Wallace, L.A.; Ott, W.R.; Weschler, C.J. Ultrafine particles from electric appliances and cooking pans: Experiments suggesting desorption/nucleation of sorbed organics as the primary source. Indoor Air 2014, 25, 536–546. [Google Scholar] [CrossRef] [PubMed]
- Kaluza, S.; Balderhaar, J.K.; Orthen, B.; Honnert, B.; Jankowska, E.; Pietrowski, P.; Rosell, M.; Tanarro, C.; Tejedor, J.; Zugasti, A. Literature Review—Workplace Exposure to Nanoparticles; EU-OSHA-European Agency for Safety and Health at Work: Bilbao, Spain, 2009.
- Gu, J.; Karrasch, S.; Salthammer, T. Review of the characteristics and possible health effects of particles emitted from laser printing devices. Indoor Air 2020, 30, 396–421. [Google Scholar] [CrossRef] [PubMed]
- Spiru, P.; Simona, P.L. A review on interactions between energy performance of the buildings, outdoor air pollution and the indoor air quality. Energy Procedia 2017, 128, 179–186. [Google Scholar] [CrossRef]
- Bone, A.; Murray, V.; Myers, I.; Dengel, A.; Crump, D. Will drivers for home energy efficiency harm occupant health? Perspect. Public Health 2010, 130, 233–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreno-Rangel, A.; Sharpe, T.; McGill, G.; Musau, F. Indoor air quality in passivhaus dwellings: A literature review. Int. J. Environ. Res. Public Health 2020, 17, 4749. [Google Scholar] [CrossRef]
- Lespes, G.; Faucher, S.; Slaveykova, V.I. Natural nanoparticles, anthropogenic nanoparticles, where is the frontier? Front. Environ. Sci. 2020, 8, 71. [Google Scholar] [CrossRef]
- Zhang, R.; Khalizov, A.; Wang, L.; Hu, M.; Xu, W. Nucleation and growth of nanoparticles in the atmosphere. Chem. Rev. 2011, 112, 1957–2011. [Google Scholar] [CrossRef] [PubMed]
- Zoroddu, M.; Medici, S.; Ledda, A.; Nurchi, V.M.; Lachowicz, J.I.; Peana, M. Toxicity of nanoparticles. Curr. Med. Chem. 2014, 21, 3837–3853. [Google Scholar] [CrossRef] [PubMed]
- Knol, A.B.; De Hartog, J.J.; Boogaard, H.; Slottje, P.; Van Der Sluijs, J.P.; Lebret, E.; Cassee, F.R.; Wardekker, J.A.; Ayres, J.G.; Borm, P.J.; et al. Expert elicitation on ultrafine particles: Likelihood of health effects and causal pathways. Part. Fibre Toxicol. 2009, 6, 19. [Google Scholar] [CrossRef] [PubMed]
- de Prado-Bert, P.; Mercader, E.M.H.; Pujol, J.; Sunyer, J.; Mortamais, M. The effects of air pollution on the brain: A review of studies interfacing environmental epidemiology and neuroimaging. Curr. Environ. Health Rep. 2018, 5, 351–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kittelson, D.B. Engines and nanoparticles: A review. J. Aerosol Sci. 1998, 29, 575–588. [Google Scholar] [CrossRef]
- Diaz, E.; Mariën, K.; Manahan, L.; Fox, J. Summary of Health Research on Ultrafine Particles. DOH 334-454; Washington State Department of Health: Tumwater, WA, USA, 2019.
- Ohlwein, S.; Kappeler, R.; Joss, M.K.; Künzli, N.; Hoffmann, B. Health effects of ultrafine particles: A systematic literature review update of epidemiological evidence. Int. J. Public Health 2019, 64, 547–559. [Google Scholar] [CrossRef]
- Downward, G.S.; Van Nunen, E.J.; Kerckhoffs, J.; Vineis, P.; Brunekreef, B.; Boer, J.M.; Messier, K.P.; Roy, A.; Verschuren, W.M.M.; Van Der Schouw, Y.T.; et al. Long-term exposure to ultrafine particles and incidence of cardiovascular and cerebrovascular disease in a prospective study of a dutch cohort. Environ. Health Perspect. 2018, 126, 127007. [Google Scholar] [CrossRef] [Green Version]
- Atkinson, R.W.; Mills, I.C.; Walton, H.; Anderson, H.R. Fine particle components and health—A systematic review and meta-analysis of epidemiological time series studies of daily mortality and hospital admissions. J. Expo. Sci. Environ. Epidemiol. 2015, 25, 208–214. [Google Scholar] [CrossRef] [Green Version]
- HEI. Review Panel on Ultrafine Particles. Understanding the Health Effects of Ambient Ultrafine Particles. HEI Perspectives 3; Health Effects Institute: Boston, MA, USA, 2013. [Google Scholar]
- Ibald-Mulli, A.; Wichmann, H.-E.; Kreyling, W.; Peters, A. Epidemiological evidence on health effects of ultrafine particles. J. Aerosol Med. 2002, 15, 189–201. [Google Scholar] [CrossRef]
- Reche, C.; Viana, M.; Rivas, I.; Bouso, L.; Àlvarez-Pedrerol, M.; Alastuey, A.; Sunyer, J.; Querol, X. Outdoor and indoor UFP in primary schools across Barcelona. Sci. Total Environ. 2014, 493, 943–953. [Google Scholar] [CrossRef]
- Lv, Y.; Wang, H.; Wei, S.; Zhang, L.; Zhao, Q. The Correlation between indoor and outdoor particulate matter of different building types in daqing, china. Procedia Eng. 2017, 205, 360–367. [Google Scholar] [CrossRef]
- Portela, N.B.; Teixeira, E.C.; Agudelo-Castañeda, D.M.; Civeira, M.D.S.; Silva, L.F.O.; Vigo, A.; Kumar, P. Indoor-outdoor relationships of airborne nanoparticles, BC and VOCs at rural and urban preschools. Environ. Pollut. 2021, 268, 115751. [Google Scholar] [CrossRef] [PubMed]
- Fuller, C.H.; Brugge, D.; Williams, P.L.; Mittleman, M.A.; Lane, K.; Durant, J.L.; Spengler, J.D. Indoor and outdoor measurements of particle number concentration in near-highway homes. J. Expo. Sci. Environ. Epidemiol. 2013, 23, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Statista. Total Number of People Employed in Offices in Germany from 2016 to 2020. Available online: https://0-www-statista-com.brum.beds.ac.uk/statistics/1022874/number-of-individuals-employed-in-offices-in-germany/ (accessed on 6 August 2021).
- Eurostat. Sit at Work? You Are One of 39%. Available online: https://ec.europa.eu/eurostat/web/products-eurostat-news/-/DDN-20190305-1 (accessed on 6 August 2021).
- Forsberg, H. Videoanalys NUS Området, Passerande Flöde för Fordonstrafik Samt för Övergångställe; Trafikia AB: Uppsala, Sweden, 2014. [Google Scholar]
- Chen, B.T.; Schwegler-Berry, D.; Cumpston, A.; Cumpston, J.; Friend, S.; Stone, S.; Keane, M. Performance of a scanning mobility particle sizer in measuring diverse types of airborne nanoparticles: Multi-walled carbon nanotubes, welding fumes, and titanium dioxide spray. J. Occup. Environ. Hyg. 2016, 13, 501–518. [Google Scholar] [CrossRef] [Green Version]
- Fierz, M.; Houle, C.; Steigmeier, P.; Burtscher, H. Design, Calibration, and Field Performance of a Miniature Diffusion Size Classifier. Aerosol Sci. Technol. 2011, 45, 1–10. [Google Scholar] [CrossRef]
- Ahlvik, P.; DISCmini and Grimm Inc., Stockholm, Sweden. Personal communication, 2021.
- Vert, C.; Meliefste, K.; Hoek, G. Outdoor ultrafine particle concentrations in front of fast food restaurants. J. Expo. Sci. Environ. Epidemiol. 2015, 26, 35–41. [Google Scholar] [CrossRef]
- Hong, K.Y.; Pinheiro, P.O.; Weichenthal, S. Predicting outdoor ultrafine particle number concentrations, particle size, and noise using street-level images and audio data. Environ. Int. 2020, 144, 106044. [Google Scholar] [CrossRef]
- Meier, R.; Clark, K.; Riediker, M. Comparative testing of a miniature diffusion size classifier to assess airborne ultrafine particles under field conditions. Aerosol Sci. Technol. 2013, 47, 22–28. [Google Scholar] [CrossRef] [Green Version]
- Ferrero, L.; Cappelletti, D.; Busetto, M.; Mazzola, M.; Lupi, A.; Lanconelli, C.; Becagli, S.; Traversi, R.; Caiazzo, L.; Giardi, F.; et al. Vertical profiles of aerosol and black carbon in the Arctic: A seasonal phenomenology along 2 years (2011–2012) of field campaigns. Atmos. Chem. Phys. Discuss. 2016, 16, 12601–12629. [Google Scholar] [CrossRef] [Green Version]
- IBM. IBM SPSS Statistics for Windows; Version 26.0; IBM Corp: Armonk, NY, USA, 2019. [Google Scholar]
- Isaxon, C.; Gudmundsson, A.; Nordin, E.; Lönnblad, L.; Dahl, A.; Wieslander, G.; Bohgard, M.; Wierzbicka, A. Contribution of indoor-generated particles to residential exposure. Atmos. Environ. 2015, 106, 458–466. [Google Scholar] [CrossRef]
- Franco, J.F.; Rojas, N.Y.; Sarmiento, O.L.; Behrentz, E. Urban air pollution in school-related microenvironments in Bogota, Colombia. Ing. Investig. 2013, 33, 42–48. [Google Scholar] [CrossRef]
- Kukkonen, J.; López-Aparicio, S.; Segersson, D.; Geels, C.; Kangas, L.; Kauhaniemi, M.; Maragkidou, A.; Jensen, A.; Assmuth, T.; Karppinen, A.; et al. The influence of residential wood combustion on the concentrations of PM2.5 in four Nordic cities. Atmos. Chem. Phys. Discuss. 2020, 20, 4333–4365. [Google Scholar] [CrossRef] [Green Version]
- Morawska, L.; Ayoko, G.; Bae, G.; Buonanno, G.; Chao, Y.H.C.; Clifford, S.; Fu, S.C.; Hänninen, O.; He, C.; Isaxon, C.; et al. Airborne particles in indoor environment of homes, schools, offices and aged care facilities: The main routes of exposure. Environ. Int. 2017, 108, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Madureira, J.; Slezakova, K.; Costa, C.; Pereira, M.C.; Teixeira, J.P. Assessment of indoor air exposure among newborns and their mothers: Levels and sources of PM10, PM2.5 and ultrafine particles at 65 home environments. Environ. Pollut. 2020, 264, 114746. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Yao, M.; Luo, X.; Zhu, Y.; Liu, Z.; Zhuo, H.; Zhao, B. Outdoor-to-indoor transport of ultrafine particles: Measurement and model development of infiltration factor. Environ. Pollut. 2020, 267, 115402. [Google Scholar] [CrossRef]
- Kumar, P.; Ketzel, M.; Vardoulakis, S.; Pirjola, L.; Britter, R. Dynamics and dispersion modelling of nanoparticles from road traffic in the urban atmospheric environment—A review. J. Aerosol Sci. 2011, 42, 580–603. [Google Scholar] [CrossRef] [Green Version]
- Holøs, S.B.; Yang, A.; Lind, M.; Thunshelle, K.; Schild, P.; Mysen, M. VOC emission rates in newly built and renovated buildings, and the influence of ventilation—A review and meta-analysis. Int. J. Vent. 2018, 18, 153–166. [Google Scholar] [CrossRef]
- Nørgaard, A.; Kudal, J.; Kofoed-Sørensen, V.; Koponen, I.; Wolkoff, P. Ozone-initiated VOC and particle emissions from a cleaning agent and an air freshener: Risk assessment of acute airway effects. Environ. Int. 2014, 68, 209–218. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.-H.; Kabir, E.; Jahan, S.A. Airborne bioaerosols and their impact on human health. J. Environ. Sci. 2018, 67, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Chen, R.; Huo, L.; Zhao, L.; Bai, R.; Long, D.; Pui, D.Y.H.; Rang, W.; Chen, C.; Xiaofei, S.; et al. Evaluation of nanoparticles emitted from printers in a lean chamber, a copy center and office rooms: Health risks of indoor air quality. J. Nanosci. Nanotechnol. 2015, 15, 9554–9564. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.-S.; Ryu, M.H.; Carlsten, C. Ultrafine particles: Unique physicochemical properties relevant to health and disease. Exp. Mol. Med. 2020, 52, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Birmili, W.; Wehner, B.; Daniels, A.; Weinhold, K.; Wang, L.; Merkel, M.; Kecorius, S.; Tuch, T.; Franck, U.; et al. Particle mass concentrations and number size distributions in 40 homes in germany: Indoor-to-outdoor relationships, diurnal and seasonal variation. Aerosol Air Qual. Res. 2020, 20, 576–589. [Google Scholar] [CrossRef]
- Kumar, P.; Morawska, L.; Birmili, W.; Paasonen, P.; Hu, M.; Kulmala, M.; Harrison, R.M.; Norford, L.; Britter, R. Ultrafine particles in cities. Environ. Int. 2014, 66, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Olivares, G.; Johansson, C.; Ström, J.; Hansson, H.-C. The role of ambient temperature for particle number concentrations in a street canyon. Atmos. Environ. 2007, 41, 2145–2155. [Google Scholar] [CrossRef]
- Mandin, C.; Trantallidi, M.; Cattaneo, A.; Canha, N.; Mihucz, V.G.; Szigeti, T.; Mabilia, R.; Perreca, E.; Spinazzè, A.; Fossati, S.; et al. Assessment of indoor air quality in office buildings across Europe—The OFFICAIR study. Sci. Total Environ. 2017, 579, 169–178. [Google Scholar] [CrossRef] [Green Version]
- Giemsa, E.; Soentgen, J.; Kusch, T.; Beck, C.; Münkel, C.; Cyrys, J.; Pitz, M. Influence of local sources and meteorological parameters on the spatial and temporal distribution of ultrafine particles in Augsburg, Germany. Front. Environ. Sci. 2021, 8, 280. [Google Scholar] [CrossRef]
- Saha, P.K.; Robinson, E.S.; Shah, R.U.; Zimmerman, N.; Apte, J.S.; Robinson, A.L.; Presto, A.A. Reduced ultrafine particle concentration in urban air: Changes in nucleation and anthropogenic emissions. Environ. Sci. Technol. 2018, 52, 6798–6806. [Google Scholar] [CrossRef] [PubMed]
- Olstrup, H.; Johansson, C.; Forsberg, B.; Åström, C. Association between mortality and short-term exposure to particles, ozone and nitrogen dioxide in Stockholm, Sweden. Int. J. Environ. Res. Public Health 2019, 16, 1028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gidhagen, L.; Johansson, C.; Langner, J.; Foltescu, V. Urban scale modeling of particle number concentration in Stockholm. Atmos. Environ. 2005, 39, 1711–1725. [Google Scholar] [CrossRef]
- Karl, M.; Kukkonen, J.; Keuken, M.P.; Lützenkirchen, S.; Pirjola, L.; Hussein, T. Modeling and measurements of urban aerosol processes on the neighborhood scale in Rotterdam, Oslo and Helsinki. Atmos. Chem. Phys. Discuss. 2016, 16, 4817–4835. [Google Scholar] [CrossRef] [Green Version]
- Von Barany, D.; Kuhlbusch, T.A.; Monz, C.; Dziurowitz, N.; Pelzer, J.; Vossen, K.; Dietrich, S.; Götz, U.; Kiesling, H.-J.; Asbach, C.; et al. Comparability of portable nanoparticle exposure monitors. Ann. Occup. Hyg. 2012, 56, 606–621. [Google Scholar] [CrossRef] [Green Version]
- Asbach, C.; Kaminski, H.; Fissan, H.; Monz, C.; Dahmann, D.; Mülhopt, S.; Paur, H.-R.; Kiesling, H.J.; Herrmann, F.; Voetz, M.; et al. Comparison of four mobility particle sizers with different time resolution for stationary exposure measurements. J. Nanoparticle Res. 2009, 11, 1593–1609. [Google Scholar] [CrossRef]
- Ozgen, S.; Becagli, S.; Bernardoni, V.; Caserini, S.; Caruso, D.; Corbella, L.; Dell’Acqua, M.; Fermo, P.; Gonzalez, R.; Lonati, G.; et al. Analysis of the chemical composition of ultrafine particles from two domestic solid biomass fired room heaters under simulated real-world use. Atmos. Environ. 2017, 150, 87–97. [Google Scholar] [CrossRef] [Green Version]
- Corsini, E.; Vecchi, R.; Marabini, L.; Fermo, P.; Becagli, S.; Bernardoni, V.; Caruso, D.; Corbella, L.; Dell’Acqua, M.; Galli, C.L.; et al. The chemical composition of ultrafine particles and associated biological effects at an alpine town impacted by wood burning. Sci. Total Environ. 2017, 587–588, 223–231. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Lawler, M.J.; Hao, J.; Smith, J.N.; Jiang, J. Composition of ultrafine particles in urban beijing: Measurement using a thermal desorption chemical ionization mass spectrometer. Environ. Sci. Technol. 2021, 55, 2859–2868. [Google Scholar] [CrossRef] [PubMed]
- Pedata, P.; Stoeger, T.; Zimmermann, R.; Peters, A.; Oberdörster, G.; D’Anna, A. Are we forgetting the smallest, sub 10 nm combustion generated particles? Part Fibre Toxicol. 2015, 12, 1–4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sirignano, M.; Conturso, M.; Magno, A.; Di Iorio, S.; Mancaruso, E.; Vaglieco, B.M.; D’Anna, A. Evidence of sub-10 nm particles emitted from a small-size diesel engine. Exp. Therm. Fluid Sci. 2018, 95, 60–64. [Google Scholar] [CrossRef]
- Nosko, O.; Vanhanen, J.; Olofsson, U. Emission of 1.3–10 nm airborne particles from brake materials. Aerosol Sci. Technol. 2016, 51, 91–96. [Google Scholar] [CrossRef] [Green Version]
- Johnson, A.R. Laser and 3-Dimensional Printers: Characterizing Emissions and Occupational Exposures; West Virginia University: Morgantown, WV, USA, 2018. [Google Scholar]
- Weldon, B.A.; Faustman, E.M.; Oberdörster, G.; Workman, T.; Griffith, W.C.; Kneuer, C.; Yu, I.J. Occupational exposure limit for silver nanoparticles: Considerations on the derivation of a general health-based value. Nanotoxicology 2016, 10, 945–956. [Google Scholar] [CrossRef] [PubMed]
- OSHA. Occupational Safety and Health Administration. Working Safely with Nanomaterials; Occupational Safety and Health Administration: Washington, DC, USA, 2013.
- Schulte, P.A.; Murashov, V.; Zumwalde, R.; Kuempel, E.D.; Geraci, C.L. Occupational exposure limits for nanomaterials: State of the art. J. Nanoparticle Res. 2010, 12, 1971–1987. [Google Scholar] [CrossRef]
- Van Veelen, W.; Streekstra, W.-H.; Van Broekhuizen, P.; Schulte, P.; Reijnders, L. Exposure limits for nanoparticles: Report of an international workshop on nano reference values. Ann. Occup. Hyg. 2012, 56, 515–524. [Google Scholar] [CrossRef] [Green Version]
- Broekhuizen, P.; Hendrikx, B. Nano reference values in the Netherlands. Gefahrst. Reinhalt. Luft 2013, 73, 407–414. [Google Scholar]
- Wientzek, S. Emission Test of a XEROX Everyday Imitation Toner Cartridge on HP Printer; Fraunhofer Wil-helm-Klauditz-Institute: Braunschweig, Germany, 2020. [Google Scholar]
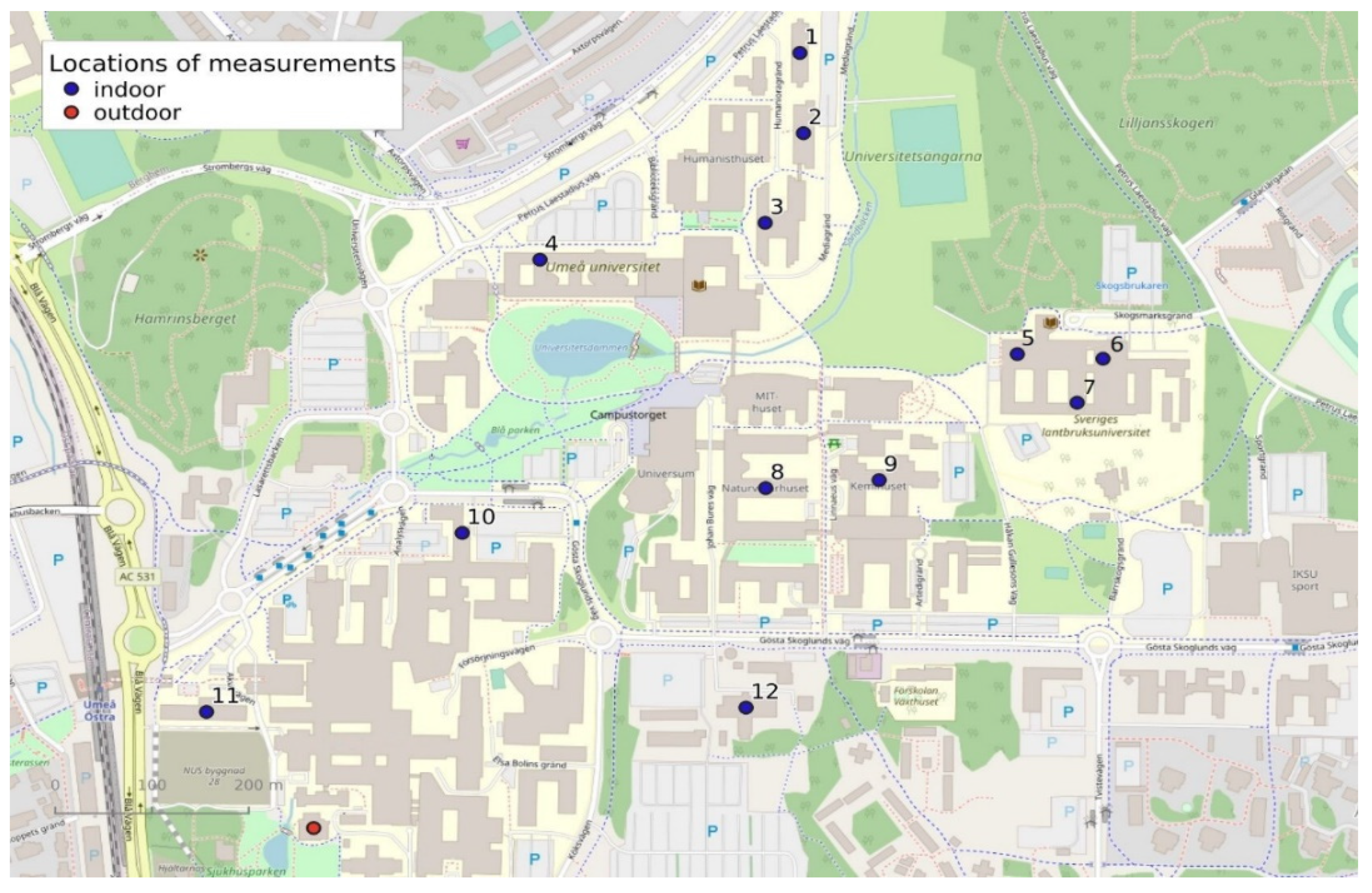


| Building | Year of Construction | Energy Consumption (kWh/m2/year) | Type of Ventilation | Filter Class | Distance from Busy Street (m) |
|---|---|---|---|---|---|
| 1 | 2017 | NA 1 | MVHR | NA 1 | 850 |
| 2 | 2002 | 118 | MVHR | F7 | 790 |
| 3 | 1992 | 139 | MVHR | F7 | 700 |
| 4 | 1968 | 106 | MVHR | F7 | 500 |
| 5, 6, 7 2 | 1978 | 154 | MVHR | F7 | 860, 920, 970 |
| 8 | 1966 | 121 | MVHR | F7 | 570 |
| 9 | 1963 | 286 | MVHR | F7 | 680 |
| 10 | 1960 | 270 | MVHR | M6 | 250 |
| 11 3 | 1935 | 160 | MVHR | F7 and F9 | 130 |
| 12 | 1969 | 206 | MVHR | F7 | 550 |
| Buildings 1–12 | Average Number Concentration (Particles per cm3) | Average Particle Size (nm) | I/O Ratio | Correlation (Pearson) | ||
|---|---|---|---|---|---|---|
| Indoor | Outdoor | Indoor | Outdoor | between I/O Concentration | ||
| Heating season | ||||||
| 1 | 3288 | 5548 | 54 | 43 | 0.59 | −0.09 |
| 2 | 316 | 4020 | 69 | 38 | 0.08 | 0.39 |
| 3 | 911 | 3396 | 60 | 35 | 0.27 | 0.30 |
| 4 | 182 | 1208 | 64 | 30 | 0.15 | 0.47 |
| 5 | 253 | 3895 | 96 | 30 | 0.06 | 0.89 |
| 6 | 459 | 5546 | 92 | 48 | 0.09 | −0.15 |
| 7 | 398 | 4331 | 66 | 33 | 0.09 | 0.41 |
| 8 | 396 | 4388 | 74 | 39 | 0.09 | 0.47 |
| 9 | 575 | 2944 | 72 | 51 | 0.20 | 0.16 |
| 10 | 1127 | 6004 | 69 | 51 | 0.19 | 0.73 |
| 11 | 2211 | 5353 | 44 | 55 | 0.42 | −0.08 |
| 12 | 465 | 4349 | 78 | 47 | 0.11 | 0.28 |
| Non-heating season | ||||||
| 1 | 869 | 3572 | 73 | 34 | 0.24 | 0.07 |
| 2 | 323 | 3929 | 86 | 45 | 0.08 | 0.19 |
| 3 | 1170 | 3002 | 70 | 44 | 0.39 | 0.39 |
| 4 | 649 | 3814 | 89 | 54 | 0.18 | −0.03 |
| 5 | 726 | 3572 | 64 | 34 | 0.20 | −0.06 |
| 6 | 1241 | 3244 | 59 | 35 | 0.38 | 0.16 |
| 7 | 463 | 2537 | 69 | 34 | 0.18 | 0.34 |
| 8 | 566 | 4332 | 88 | 43 | 0.13 | 0.04 |
| 9 | 1139 | 3347 | 95 | 65 | 0.35 | 0.74 |
| 10 | 879 | 2589 | 102 | 61 | 0.35 | 0.44 |
| 11 | 575 | 3582 | 73 | 44 | 0.16 | 0.13 |
| 12 | 598 | 2131 | 91 | 49 | 0.29 | 0.06 |
| Buildings 1–12 | Indoor Concentration (Particles per cm3) | Outdoor Concentration (Particles per cm3) | Distance from Busy Street (m) | ||
|---|---|---|---|---|---|
| Maximum | Minimum | Maximum | Minimum | ||
| Heating Season | |||||
| 1 | 32,357 | 462 | 283,275 | 560 | 850 |
| 2 | 3274 | 0 | 36,129 | 256 | 790 |
| 3 | 4454 | 45 | 176,261 | 72 | 700 |
| 4 | 1806 | 19 | 21,809 | 33 | 500 |
| 5 | 1093 | 0 | 432,664 | 57 | 860 |
| 6 | 2542 | 0 | 164,311 | 500 | 920 |
| 7 | 1596 | 0 | 71,732 | 361 | 970 |
| 8 | 1765 | 46 | 155,486 | 121 | 570 |
| 9 | 2621 | 12 | 208,114 | 147 | 680 |
| 10 | 5652 | 67 | 53,763 | 54 | 250 |
| 11 | 27,461 | 368 | 164,311 | 166 | 130 |
| 12 | 3878 | 55 | 202,679 | 317 | 550 |
| Non-Heating Season | |||||
| 1 | 5291 | 0 | 149,128 | 179 | 850 |
| 2 | 1325 | 45 | 353,910 | 768 | 790 |
| 3 | 4578 | 156 | 118,288 | 240 | 700 |
| 4 | 44,613 | 0 | 198,795 | 710 | 500 |
| 5 | 2461 | 114 | 149,128 | 179 | 860 |
| 6 | 5528 | 228 | 88,736 | 278 | 920 |
| 7 | 1456 | 88 | 64,052 | 303 | 970 |
| 8 | 1945 | 118 | 522,546 | 4332 | 570 |
| 9 | 3547 | 392 | 30,931 | 638 | 680 |
| 10 | 2802 | 63 | 32,344 | 717 | 250 |
| 11 | 4784 | 94 | 241,334 | 683 | 130 |
| 12 | 4093 | 249 | 201,487 | 831 | 550 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orru, H.; Hagenbjörk, A.; Olstrup, H. Indoor and Outdoor Nanoparticle Concentrations in an Urban Background Area in Northern Sweden: The NanoOffice Study. Environments 2021, 8, 75. https://0-doi-org.brum.beds.ac.uk/10.3390/environments8080075
Orru H, Hagenbjörk A, Olstrup H. Indoor and Outdoor Nanoparticle Concentrations in an Urban Background Area in Northern Sweden: The NanoOffice Study. Environments. 2021; 8(8):75. https://0-doi-org.brum.beds.ac.uk/10.3390/environments8080075
Chicago/Turabian StyleOrru, Hans, Annika Hagenbjörk, and Henrik Olstrup. 2021. "Indoor and Outdoor Nanoparticle Concentrations in an Urban Background Area in Northern Sweden: The NanoOffice Study" Environments 8, no. 8: 75. https://0-doi-org.brum.beds.ac.uk/10.3390/environments8080075





