Organic Wastes Amended with Sorbents Reduce N2O Emissions from Sugarcane Cropping
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Experiment
2.2. Poultry Litter and Amendments
2.3. Soil Analyses
2.4. Experimental Design
2.5. Sampling and Analysis of Greenhouse Gases
2.6. Soil Sampling and Analysis
2.7. Sugarcane Biomass
2.8. Laboratory Experiment
2.9. Data Analyses
3. Results
3.1. Field Experiment
3.1.1. Sugarcane Yield
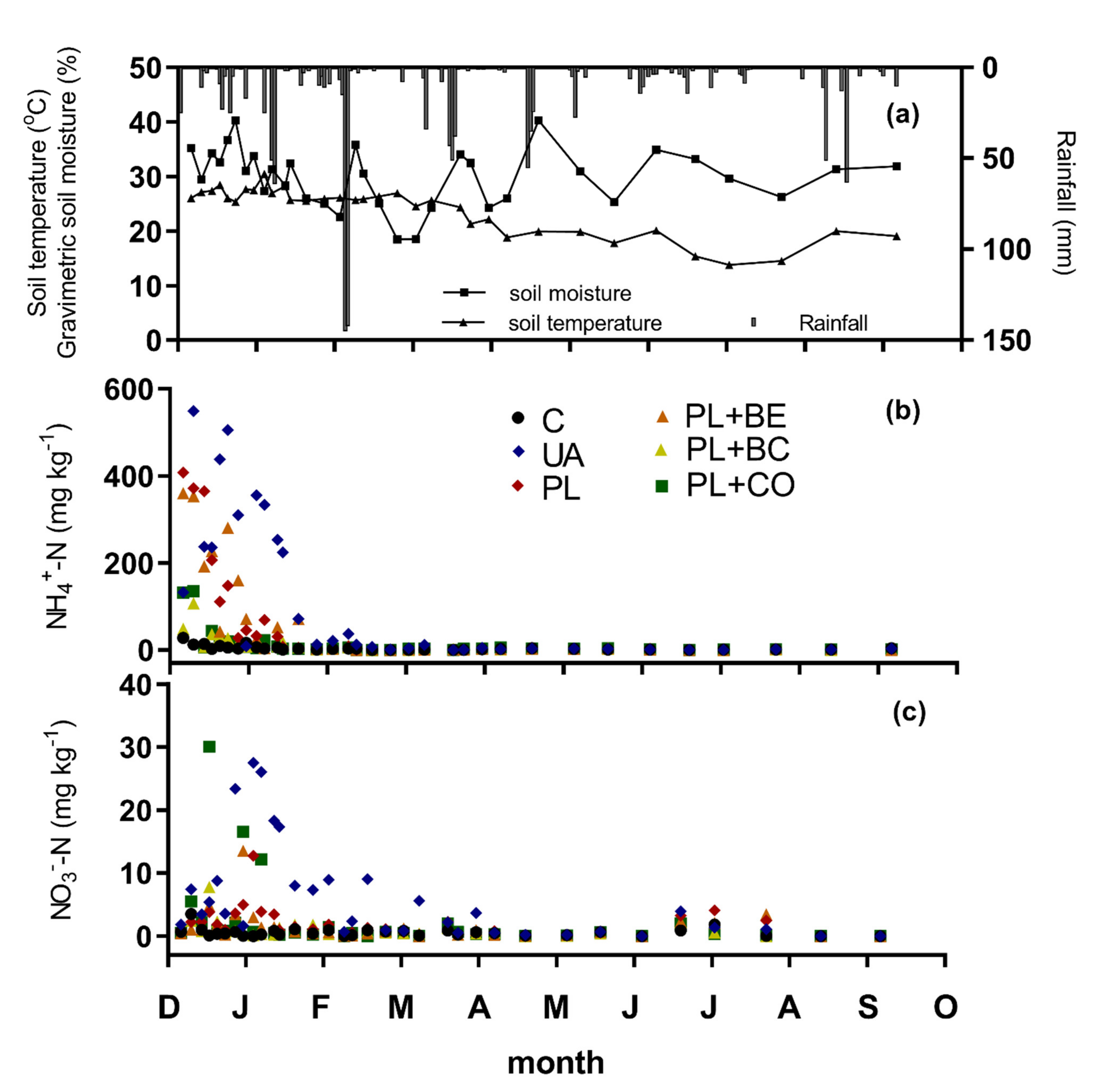
3.1.2. Environmental Variables and Mineral Soil Nitrogen
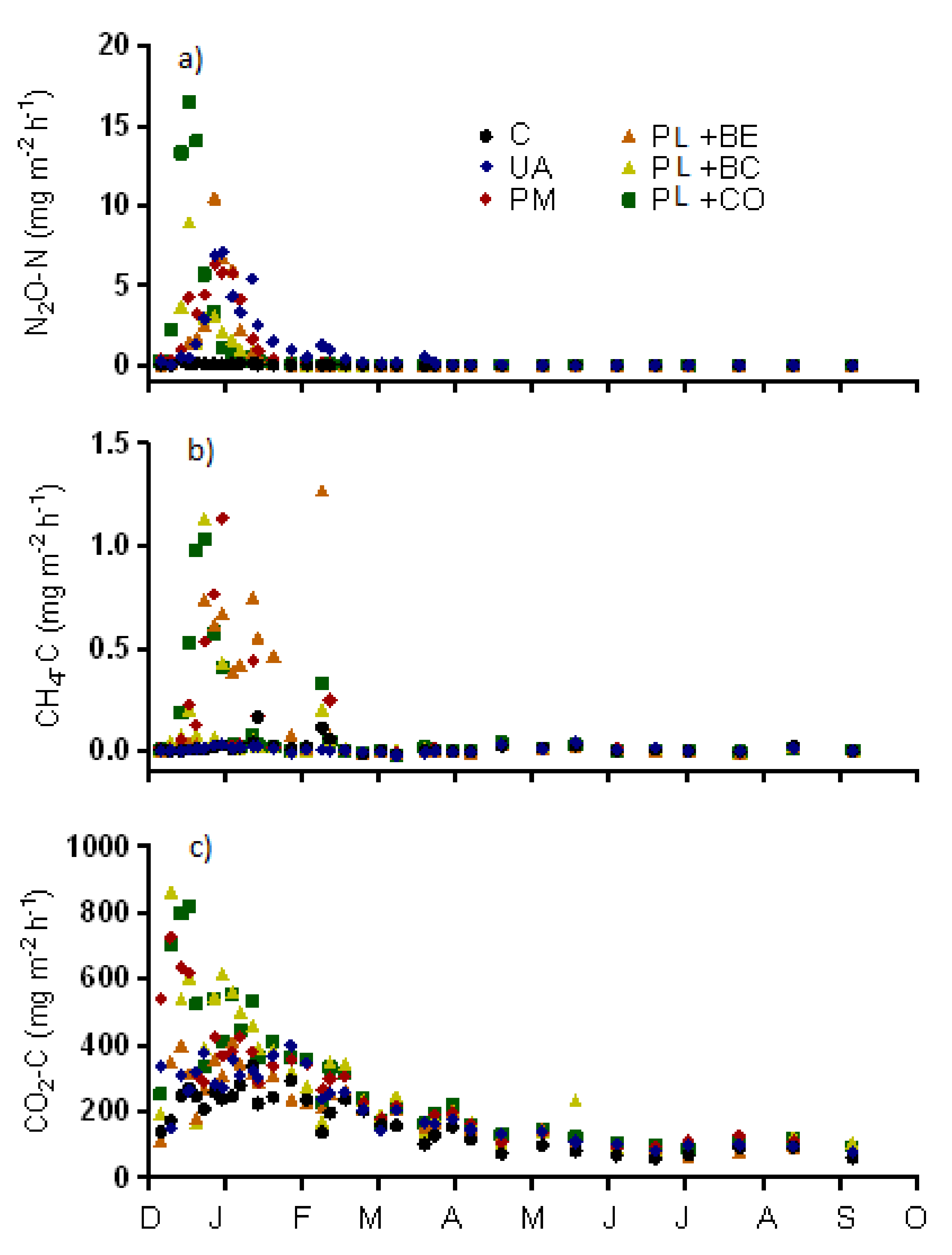
3.1.3. Greenhouse Gas Emissions
3.1.4. Cumulative N2O Emissions and Emission Factors
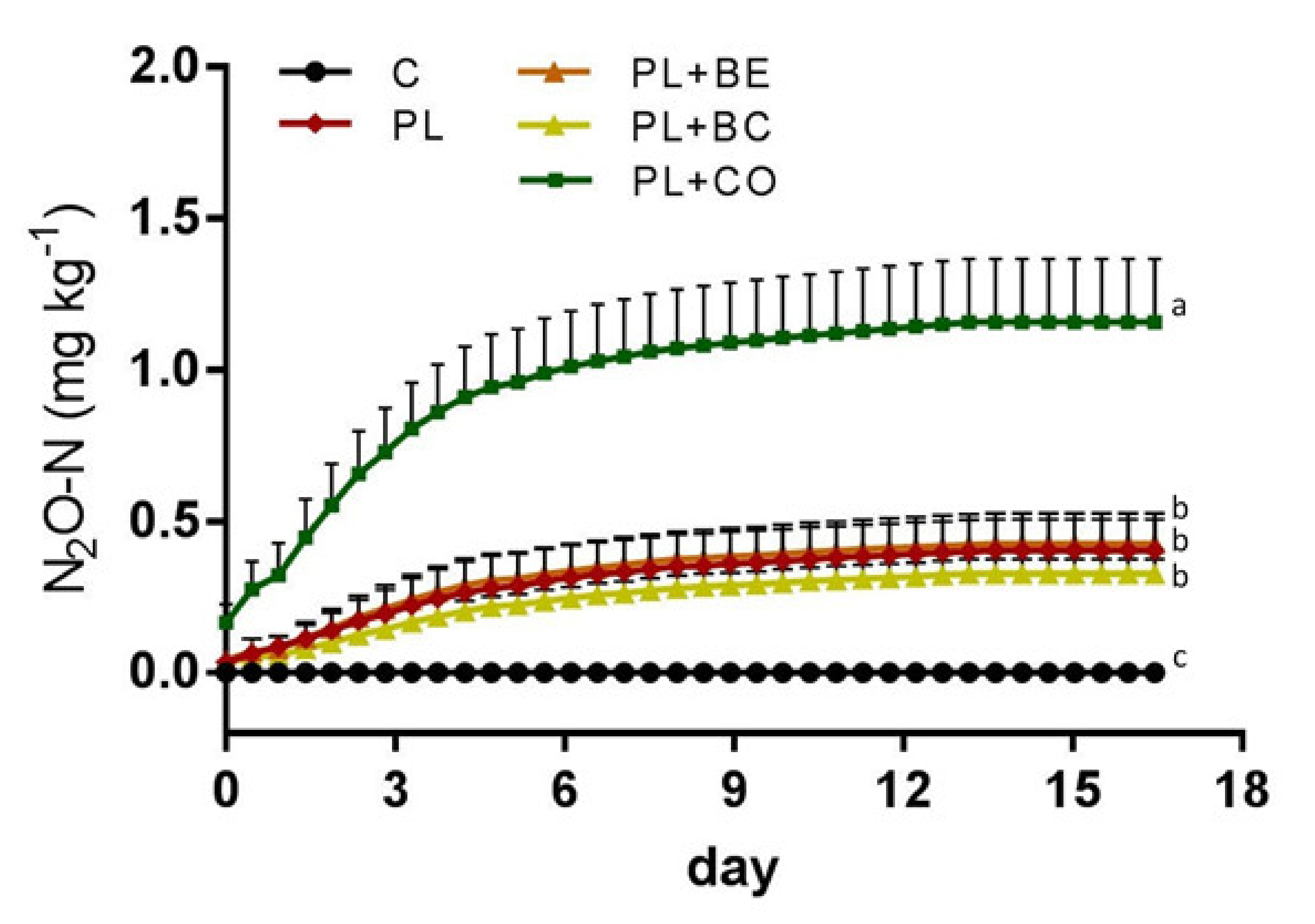
3.2. Laboratory Experiment
4. Discussion
4.1. Emissions of N2O and Other Greenhouse Gases from the Field Experiment
4.2. Can Field Level Emissions Be Predicted with Laboratory Incubations?
4.3. Implications and Recommendations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. Food and Agriculture Organization of the United Nations, Rome, Italy. 2017. Available online: http://www.fao.org/faostat/ (accessed on 1 January 2020).
- Robinson, N.; Brackin, R.; Vinall, K.; Soper, F.; Holst, J.; Gamage, H.; Paungfoo-Lonhienne, C.; Rennenberg, H.; Lakshmanan, P.; Schmidt, S. Nitrate Paradigm Does Not Hold Up for Sugarcane. PLoS ONE 2011, 6, e19045. [Google Scholar] [CrossRef] [Green Version]
- Prasertsak, P.; Freney, J.R.; Denmead, O.T.; Saffigna, P.G.; Prove, B.G.; Reghenzani, J.R. Effect of fertilizer placement on nitrogen loss from sugarcane in tropical Queensland. Nutr. Cycl. Agroecosyst. 2002, 62, 229–239. [Google Scholar] [CrossRef]
- Rasiah, V.; Armour, J.D.; Nelson, P.N. Nitrate in shallow fluctuating groundwater under sugarcane: Quantifying the lateral export quantities to surface waters. Agric. Ecosyst. Environ. 2013, 180, 103–110. [Google Scholar] [CrossRef]
- Freney, J.R.; Denmead, T. Factors Controlling Ammonia and Nitrous Oxide Emissions from Flooded Rice Fields. Ecol. Bull. 1992, 42, 188–194. [Google Scholar]
- Lisboa, C.C.; Butterbach-Bahl, K.; Mauder, M.; Kiese, R. Bioethanol production from sugarcane and emissions of greenhouse gases—Known and unknowns. GCB Bioenergy 2011, 3, 277–292. [Google Scholar] [CrossRef]
- Allen, D.E.; Kingston, G.; Rennenberg, H.; Dalal, R.C.; Schmidt, S. Effect of nitrogen fertilizer management and waterlogging on nitrous oxide emission from subtropical sugarcane soils. Agric. Ecosyst. Environ. 2010, 136, 209–217. [Google Scholar] [CrossRef]
- Denmead, O.T.; Macdonald, B.C.T.; Bryant, G.; Naylor, T.; Wilson, S.; Griffith, D.W.T.; Wang, W.J.; Salter, B.; White, I.; Moody, P.W. Emissions of methane and nitrous oxide from Australian sugarcane soils. Agric. For. Meteorol. 2010, 150, 748–756. [Google Scholar] [CrossRef]
- Takeda, N.; Friedl, J.; Rowlings, D.; De Rosa, D.; Scheer, C.; Grace, P. Exponential response of nitrous oxide (N2O) emissions to increasing nitrogen fertiliser rates in a tropical sugarcane cropping system. Agric. Ecosyst. Environ. 2021, 313, 107376. [Google Scholar] [CrossRef]
- Wang, W.J.; Salter, B.; Reeves, S.H.; Brieffies, T.C.; Perna, J. Nitrous oxide emissions from a sugarcane soil under different fallow and nitrogen fertiliser management regimes. Proc. Aust. Soc. Sugar Cane Technol. 2012, 34, 1–8. [Google Scholar]
- Wang, W.; Park, G.; Reeves, S.; Zahmel, M.; Heenan, M.; Salter, B. Nitrous oxide emission and fertiliser nitrogen efficiency in a tropical sugarcane cropping system applied with different formulations of urea. Soil Res. 2016, 54, 572–584. [Google Scholar] [CrossRef] [Green Version]
- Tian, H.; Xu, R.; Canadell, J.G.; Thompson, R.L.; Winiwarter, W.; Suntharalingam, P.; Davidson, E.A.; Ciais, P.; Jackson, R.B.; Janssens-Maenhout, G.; et al. A comprehensive quantification of global nitrous oxide sources and sinks. Nature 2020, 586, 248–271. [Google Scholar] [CrossRef]
- Maraseni, T.N.; Qu, J. An international comparison of agricultural nitrous oxide emissions. J. Clean. Prod. 2016, 135, 1256–1266. [Google Scholar] [CrossRef]
- Thompson, R.L.; Lassaletta, L.; Patra, P.K.; Wilson, C.; Wells, K.C.; Gressent, A.; Koffi, E.N.; Chipperfield, M.P.; Winiwarter, W.; Davidson, E.A.; et al. Acceleration of global N2O emissions seen from two decades of atmospheric inversion. Nat. Clim. Chang. 2019, 9, 993–998. [Google Scholar] [CrossRef]
- Yang, L.; Deng, Y.; Wang, X.; Zhang, W.; Shi, X.; Chen, X.; Lakshmanan, P.; Zhang, F. Global direct nitrous oxide emissions from the bioenergy crop sugarcane (Saccharum spp. inter-specific hybrids). Sci. Total Environ. 2021, 752, 141795. [Google Scholar] [CrossRef] [PubMed]
- Garside, A.L.; Bell, M.J. Growth and yield responses to amendments to the sugarcane monoculture: Effects of crop, pasture and bare fallow breaks and soil fumigation on plant and ratoon crops. Crop Pasture Sci. 2011, 62, 396–412. [Google Scholar] [CrossRef]
- Singh, K.; Suman, A.; Singh, P.; Srivastav, T. Improving quality of sugarcane-growing soils by organic amendments under subtropical climatic conditions of India. Biol. Fertil. Soils 2007, 44, 367–376. [Google Scholar] [CrossRef]
- Brock, C.; Hoyer, U.; Leithold, G.; Hülsbergen, K.-J. The humus balance model (HU-MOD): A simple tool for the assessment of management change impact on soil organic matter levels in arable soils. Nutr. Cycl. Agroecosyst. 2012, 92, 239–254. [Google Scholar] [CrossRef]
- Wiedemann, S.; McGahan, E.; Murphy, C. Environmental impacts and resource use from Australian pork production assessed using life-cycle assessment. 1. Greenhouse gas emissions. Anim. Prod. Sci. 2016, 56, 1418–1431. [Google Scholar] [CrossRef] [Green Version]
- Wiedemann, S.; McGahan, E.; Murphy, C. Resource use and environmental impacts from Australian chicken meat production. J. Clean. Prod. 2017, 140, 675–684. [Google Scholar] [CrossRef] [Green Version]
- Maillard, E.; Angers, D. Animal manure application and soil organic carbon stocks: A meta-analysis. Glob. Chang. Biol. 2014, 20, 666–679. [Google Scholar] [CrossRef] [PubMed]
- Jensen, L.S. Animal Manure Fertiliser Value, Crop Utilisation and Soil Quality Impacts. In Animal Manure Recycling; Sommer, S.G., Christensen, M.L., Schmidt, T., Jensen, L.S., Eds.; Wiley: Hoboken, NJ, USA, 2013; pp. 295–328. [Google Scholar]
- Akiyama, H.; Tsuruta, H. Nitrous Oxide, Nitric Oxide, and Nitrogen Dioxide Fluxes from Soils after Manure and Urea Application. J. Environ. Qual. 2003, 32, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Rochette, P.; Angers, D.A.; Chantigny, M.H.; Gagnon, B.; Bertrand, N. N2O fluxes in soils of contrasting textures fertilized with liquid and solid dairy cattle manures. Can. J. Soil Sci. 2008, 88, 175–187. [Google Scholar] [CrossRef] [Green Version]
- Velthof, G.L.; Kuikman, P.J.; Oenema, O. Nitrous oxide emission from animal manures applied to soil under controlled conditions. Biol. Fertil. Soils 2003, 37, 221–230. [Google Scholar] [CrossRef]
- Xia, F.; Mei, K.; Xu, Y.; Zhang, C.; Dahlgren, R.A.; Zhang, M. Response of N2O emission to manure application in field trials of agricultural soils across the globe. Sci. Total Environ. 2020, 733, 139390. [Google Scholar] [CrossRef]
- Zhou, M.; Zhu, B.; Wang, S.; Zhu, X.; Vereecken, H.; Brüggemann, N. Stimulation of N2O emission by manure application to agricultural soils may largely offset carbon benefits: A global meta-analysis. Glob. Chang. Biol. 2017, 23, 4068–4083. [Google Scholar] [CrossRef]
- Clough, T.J.; Condron, L.M.; Kammann, C.; Müller, C. A Review of Biochar and Soil Nitrogen Dynamics. Agronomy 2013, 3, 275–293. [Google Scholar] [CrossRef] [Green Version]
- Zaman, M.; Zaman, S.; Nguyen, M.L.; Smith, T.J.; Nawaz, S. The effect of urease and nitrification inhibitors on ammonia and nitrous oxide emissions from simulated urine patches in pastoral system: A two-year study. Sci. Total Environ. 2013, 465, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Pratt, C.; Redding, M.; Hill, J.; Brown, G.; Westermann, M. Clays Can Decrease Gaseous Nutrient Losses from Soil-Applied Livestock Manures. J. Environ. Qual. 2016, 45, 638–645. [Google Scholar] [CrossRef]
- Chin, A.; Schmidt, S.; Buckley, S.; Pirie, R.; Redding, M.; Laycock, B.; Luckman, P.; Batstone, D.J.; Robinson, N.; Brackin, R. Sorbents can tailor nitrogen release from organic wastes to match the uptake capacity of crops. Sci. Total Environ. 2018, 645, 1474–1483. [Google Scholar] [CrossRef]
- Cayuela, M.L.; van Zwieten, L.; Singh, B.P.; Jeffery, S.; Roig, A.; Sánchez-Monedero, M.A. Biochar’s role in mitigating soil nitrous oxide emissions: A review and meta-analysis. Agric. Ecosyst. Environ. 2014, 191, 5–16. [Google Scholar] [CrossRef]
- Dalal, R.C.; Gibson, I.; Allen, D.E.; Menzies, N.W. Green waste compost reduces nitrous oxide emissions from feedlot manure applied to soil. Agric. Ecosyst. Environ. 2010, 136, 273–281. [Google Scholar] [CrossRef]
- Isbell, R.F. The Australian Soil Classification, Australian Soil and Land Survey Handbook; CSIRO Publishing: Collingwood, VIC, Australia, 2002. [Google Scholar]
- WRB. World reference base for soil resources, 2014. In International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; Update, 2015; FAO: Rome, Italy, 2015. [Google Scholar]
- Pratt, C.; Redding, M.; Hill, J.; Mudge, S.R.; Westermann, M.; Paungfoo-Lonhienne, C.; Schmidt, S. Assessing refrigerating and freezing effects on the biological/chemical composition of two livestock manures. Agric. Ecosyst. Environ. 2014, 197, 288–292. [Google Scholar] [CrossRef]
- Gee, G.W.; Or, D. Particle-Size Analysis. In Methods of Soil Analysis Part 4—Physical Methods; Dane, J.H., Topp, G.C., Eds.; Soil Science Society of America, Inc.: Madison, WI, USA, 2002; pp. 255–293. [Google Scholar]
- Wilke, B.M. Determination of Chemical and Physical Soil Properties. In Manual of Soil Analysis. Monitoring and Assessing Soil Bioremediation; Margesin, R., Schinner, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 47–95. [Google Scholar]
- Rayment, G.E.; Lyons, D.J. Soil Chemical Methods: Australasia; CSIRO Publishing: Collingwood, VIC, Australia, 2011. [Google Scholar]
- Sumner, M.E.; Miller, W.P. Cation exchange capacity and exchange coefficients. In Methods of Soil Analysis. Part 3. Chemical Methods; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Eds.; ASA and SSSA: Madison, WI, USA, 1996; pp. 1221–1229. [Google Scholar]
- Kandeler, E.; Gerber, H. Short-term assay of soil urease activity using colorimetric determination of ammonium. Biol. Fertil. Soils 1988, 6, 68–72. [Google Scholar] [CrossRef]
- Miranda, K.M.; Espey, M.G.; Wink, D.A. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide 2001, 5, 62–71. [Google Scholar] [CrossRef]
- Joergensen, R.G.; Brookes, P.C. Quantification of Soil Microbial Biomass by Fumigation-Extraction. In Monitoring and Assessing Soil Bioremediation; Springer: Berlin/Heidelberg, Germany, 2005; pp. 281–295. [Google Scholar]
- Sugar Research Australia. Nutrient Management Guidelines for Sugarcane in the Bundaberg, Isis and Maryborough Districts. 2013. Available online: http://www.sugarresearch.com.au/icms_docs/194343_SIX_EASY_STEPS_Nutrient_Guidelines_for_SOUTHERN_DISTRICTS.pdf (accessed on 1 January 2020).
- Redding, M.R.; Shorten, P.R.; Lewis, R.; Pratt, C.; Paungfoo-Lonhienne, C.; Hill, J. Soil N availability, rather than N deposition, controls indirect N2O emissions. Soil Biol. Biochem. 2016, 95, 288–298. [Google Scholar] [CrossRef] [Green Version]
- Hill, J.; Redding, M.; Pratt, C. A novel and effective technology for mitigating nitrous oxide emissions from land-applied manures. Anim. Prod. Sci. 2016, 56, 362–369. [Google Scholar] [CrossRef] [Green Version]
- Reeves, S.; Wang, W.; Salter, B.; Halpin, N. Quantifying nitrous oxide emissions from sugarcane cropping systems: Optimum sampling time and frequency. Atmos. Environ. 2016, 136, 123–133. [Google Scholar] [CrossRef] [Green Version]
- IPCC. IPCC Guidelines for National Greenhouse Gas Inventories. Volume 4: Agriculture, Forestry and Other Land Uses; Report, Intergovernmental Panel on Climate Change; IPCC National Greenhouse Gas Inventories Programme; IPCC: Kanagawa, Japan, 2006. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016; Available online: https://www.R-project.org/ (accessed on 1 January 2020).
- Carmo, J.B.; Filoso, S.; Zotelli, L.C.; de Sousa Neto, E.R.; Pitombo, L.M.; Duarte-Neto, P.J.; Vargas, V.P.; Andrade, C.A.; Gava, G.J.C.; Rossetto, R.; et al. Infield greenhouse gas emissions from sugarcane soils in Brazil: Effects from synthetic and organic fertilizer application and crop trash accumulation. GCB Bioenergy 2012, 5, 267–280. [Google Scholar] [CrossRef]
- Soares, J.R.; Cantarella, H.; Vargas, V.P.; Carmo, J.B.; Martins, A.A.; Sousa, R.M.; Andrade, C.A. Enhanced-efficiency fertilizers in nitrous oxide emissions from urea applied to sugarcane. J. Environ. Qual. 2014, 44, 423–430. [Google Scholar] [CrossRef] [Green Version]
- Soares, J.R.; Cassman, N.A.; Kielak, A.M.; Pijl, A.; Carmo, J.B.; Lourenco, K.S.; Laanbroek, H.J.; Cantarella, H.; Kuramae, E.E. Nitrous oxide emission related to ammonia-oxidizing bacteria and mitigation options from N fertilization in a tropical soil. Sci. Rep. 2016, 6, 30349. [Google Scholar] [CrossRef] [Green Version]
- Redding, M.R.; Lewis, R.; Kearton, T.; Smith, O. Manure and sorbent fertilisers increase on-going nutrient availability relative to conventional fertilisers. Sci. Total Environ. 2016, 569–570, 927–936. [Google Scholar] [CrossRef] [Green Version]
- Bourg, I.C.; Sposito, G.; Bourg, A.C.M. Tracer diffusion in compacted, water-saturated bentonite. Clays Clay Miner. 2006, 54, 363–374. [Google Scholar] [CrossRef]
- Venterea, R.T.; Clough, T.J.; Coulter, J.A.; Breuillin-Sessoms, F.; Wang, R.; Sadowsky, M.J. Corrigendum: Ammonium sorption and ammonia inhibition of nitrite-oxidizing bacteria explain contrasting soil N2O production. Sci. Rep. 2016, 6, 20720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ros, M.; Teresa, M.; Carlos Garcia, H. Soil microbial activity after restoration of a semiarid soil by organic amendments. Soil Biol. Biochem. 2003, 35, 463–469. [Google Scholar] [CrossRef]
- Akiyama, K.; McTaggart, I.P.; Ball, B.C.; Scott, A. N2O, NO, and NH3 emissions from soil after the application of organic fertilizers, urea and water. Water Air Soil Pollut. 2004, 156, 112–129. [Google Scholar] [CrossRef]
- Khalil, M.I.; Rosenani, A.B.; Van Cleemput, O.; Fauziah, C.I.; Shamshuddin, J. Nitrous Oxide Emissions from an Ultisol of the Humid Tropics under Maize—Groundnut Rotation. J. Environ. Qual. 2002, 31, 1071–1078. [Google Scholar] [CrossRef]
- Khalil, M.; Rosenani, A.; Van Cleemput, O.; Boeckx, P.; Shamshuddin, J.; Fauziah, C. Nitrous oxide production from an Ultisol of the humid tropics treated with different nitrogen sources and moisture regimes. Biol. Fertil. Soils 2002, 36, 59–65. [Google Scholar]
- Wang, L.; Pan, Z.; Xu, H.; Wang, C.; Gao, L.; Zhao, P.; Dong, Z.; Zhang, J.; Cui, G.; Wang, S.; et al. The influence of nitrogen fertiliser rate and crop rotation on soil methane flux in rain-fed potato fields in Wuchuan County, China. Sci. Total Environ. 2015, 537, 93–99. [Google Scholar] [CrossRef]
- Sistani, K.R.; Jn-Baptiste, M.; Lovanh, N.; Cook, K.L. Atmospheric Emissions of Nitrous Oxide, Methane, and Carbon Dioxide from Different Nitrogen Fertilizers. J. Environ. Qual. 2011, 40, 1797–1805. [Google Scholar] [CrossRef] [Green Version]
- Pratt, C.; Redding, M.; Hill, J.; Shilton, A.; Chung, M.; Guieysse, B. Good science for improving policy: Greenhouse gas emissions from agricultural manures. Anim. Prod. Sci. 2015, 55, 691–701. [Google Scholar] [CrossRef] [Green Version]
- Jiao, S.; Chen, W.; Wang, J.; Du, N.; Li, Q.; Wei, G. Soil microbiomes with distinct assemblies through vertical soil profiles drive the cycling of multiple nutrients in reforested ecosystems. Microbiome 2018, 6, 146. [Google Scholar] [CrossRef] [Green Version]
- Firestone, M.K.; Davidson, E.A. Microbiological Basis of NO and N2O Production and Consumption in Soil. In Exchange of Trace Gases between Terrestrial Ecosystems and the Atmosphere; Andreae, M.O., Schimel, D.S., Eds.; John Wiley & Sons Ltd.: New York, NY, USA, 1989; pp. 7–21. [Google Scholar]
- Meyer, R.; Risgaard-Petersen, N.; Allen, D. Correlation between anammox activity and microscale distribution of nitrite in a subtropical mangrove sediment. Appl. Environ. Microbiol. 2005, 71, 6142–6149. [Google Scholar] [CrossRef] [Green Version]
- Felber, R.; Leifeld, J.; Horák, J.; Neftel, A. Nitrous oxide emission reduction with greenwaste biochar: Comparison of laboratory and field experiments. Eur. J. Soil Sci. 2013, 65, 128–138. [Google Scholar] [CrossRef]
- Fuchs, K.; Merbold, L.; Buchmann, N.; Bellocchi, G.; Bindi, M.; Brilli, L.; Conant, R.; Dorich, C.; Ehrhardt, F.; Fitton, N.; et al. Evaluating the potential of legumes to mitigate N2O emissions from permanent grassland using process-based models. Glob. Biogeochem. Cycles 2020, 34, e2020GB006561. [Google Scholar] [CrossRef]
- Zhang, A. Effect of biochar amendment on yield and methane and nitrous oxide emissions from a rice paddy from Tai Lake plain, China. Agric. Ecosyst. Environ. 2010, 139, 469–475. [Google Scholar] [CrossRef]
- Brackin, R.; Näsholm, T.; Robinson, N.; Guillou, S.; Vinall, K.; Lakshmanan, P.; Schmidt, S.; Inselsbacher, E. Nitrogen fluxes at the root-soil interface show a mismatch of nitrogen fertilizer supply and sugarcane root uptake capacity. Sci. Rep. 2015, 5, 15727. [Google Scholar] [CrossRef] [Green Version]
- Dalal, R.C.; Gibson, I.; Menzies, N.W. Nitrous oxide emission from feedlot manure and green waste compost applied to Vertisols. Biol. Fertil Soils 2009, 45, 809–819. [Google Scholar] [CrossRef]
- Janssen, B.H. Nitrogen mineralization in relation to C:N ratio and decomposability of organic materials. Plant Soil 1996, 181, 39–45. [Google Scholar] [CrossRef]
- Cooperband, L.; Bollero, G.; Coale, F. Effect of poultry litter and composts on soil nitrogen and phosphorus availability and corn production. Nutr. Cycl. Agroecosyst. 2002, 62, 185–194. [Google Scholar] [CrossRef]



| Treatment | NH4+-N (mg kg−1 Soil) | NO3−-N (mg kg−1 Soil) |
|---|---|---|
| no-N control | 4.98 ± 7.78 d | 0.62 ± 0.83 c |
| Mineral (urea) fertiliser | 151 ± 216 a | 7.80 ± 10.10 a |
| Poultry litter | 74.0 ± 153 b | 2.15 ± 3.71 b |
| Poultry litter + bentonite | 75.2 ± 152 b | 1.63 ± 4.92 c |
| Poultry litter + biochar | 14.1 ± 26.9 c | 1.07 ± 2.59 c |
| Poultry litter + compost | 17.4 ± 52.8 c | 3.18 ± 8.86 b |
| N Applied as | N Applied with | ||||
|---|---|---|---|---|---|
| Treatment | Fertiliser or Poultry Litter (kg ha−1) | Sorbent or Compost (kg ha−1) | Total N Applied (kg ha−1) | Total N2O-N Emissions (kg ha−1) | Emission Factor (% of Applied N) |
| no-N control u | n/a | n/a | 0 | 0.84 ± 0.30 b | n/a |
| Urea (UA) | 160 | n/a | 160 | 13.67 ± 6.82 a | 8.02 ± 4.26 |
| Poultry litter (PL) | 160 | n/a | 160 | 11.67 ± 6.75 a | 6.77 ± 4.22 |
| PL + bentonite (BE) | 160 | 1.83 | 162 | 9.74 ± 5.97 a | 5.50 ± 3.69 |
| PL + biochar (BC) | 160 | 37.6 | 197 | 7.48 ± 3.73 a | 3.36 ± 1.89 |
| PL + compost (CO) | 160 | 59.6 | 220 | 15.65 ± 10.21 a | 6.75 ± 4.65 |
| Treatment | Cumulative N2O-N Emissions (mg kg−1) |
|---|---|
| Control | <0.01 ± 0.00 a |
| Poultry litter | 0.40 ± 0.10 b |
| Poultry litter + bentonite | 0.43 ± 0.10 b |
| Poultry litter + biochar | 0.33 ± 0.05 b |
| Poultry litter + compost | 1.16 ± 0.21 c |
| Laboratory Incubation | Field Experiment | |||
|---|---|---|---|---|
| Treatment | Cumulative CO2-C Emissions (mg kg−1) | Cumulative CH4-C Emissions (mg kg−1) | Cumulative CO2-C Emissions (kg ha−1) | Cumulative CH4-C Emissions (kg ha−1) |
| Control | 64.4 ± 35.2 b | 353.1 ± 37.4 b | 5321 ± 154 b | 0.61 ± 0.11 ab |
| Poultry litter | 563.1 ± 87.6 a | 853.1 ± 87.9 a | 7554 ± 743 a | 2.06 ± 0.96 a |
| Poultry litter + bentonite | 572.9 ± 67.6 a | 862.7 ± 65.3 a | 6664 ± 554 ab | 2.75 ± 3.12 a |
| Poultry litter + biochar | 601.9 ± 63.4 a | 881.6 ± 60.6 a | 7040 ± 1360 a | 1.19 ± 1.12 ab |
| Poultry litter + compost | 646.2 ± 52.3 a | 921.2 ± 51.6 a | 7176 ± 1011 a | 1.56 ± 1.66 ab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Westermann, M.; Brackin, R.; Robinson, N.; Salazar Cajas, M.; Buckley, S.; Bailey, T.; Redding, M.; Kochanek, J.; Hill, J.; Guillou, S.; et al. Organic Wastes Amended with Sorbents Reduce N2O Emissions from Sugarcane Cropping. Environments 2021, 8, 78. https://0-doi-org.brum.beds.ac.uk/10.3390/environments8080078
Westermann M, Brackin R, Robinson N, Salazar Cajas M, Buckley S, Bailey T, Redding M, Kochanek J, Hill J, Guillou S, et al. Organic Wastes Amended with Sorbents Reduce N2O Emissions from Sugarcane Cropping. Environments. 2021; 8(8):78. https://0-doi-org.brum.beds.ac.uk/10.3390/environments8080078
Chicago/Turabian StyleWestermann, Maren, Richard Brackin, Nicole Robinson, Monica Salazar Cajas, Scott Buckley, Taleta Bailey, Matthew Redding, Jitka Kochanek, Jaye Hill, Stéphane Guillou, and et al. 2021. "Organic Wastes Amended with Sorbents Reduce N2O Emissions from Sugarcane Cropping" Environments 8, no. 8: 78. https://0-doi-org.brum.beds.ac.uk/10.3390/environments8080078






