Long-Term Eutrophication and Dynamics of Bloom-Forming Microbial Communities during Summer HAB in Large Arctic Lake
Abstract
:1. Introduction
2. Materials and Methods
2.1. Hydrochemical Analysis
2.2. Prediction of Chlorophyll-a Concentrations and Visualization of TC/TN/TP/Chl-a Ratios
2.3. DNA Preparation and Processing
2.4. Analysis of Bacterial Communities
2.5. Analysis of the Publically Available Dolichospermum Genomes
3. Results
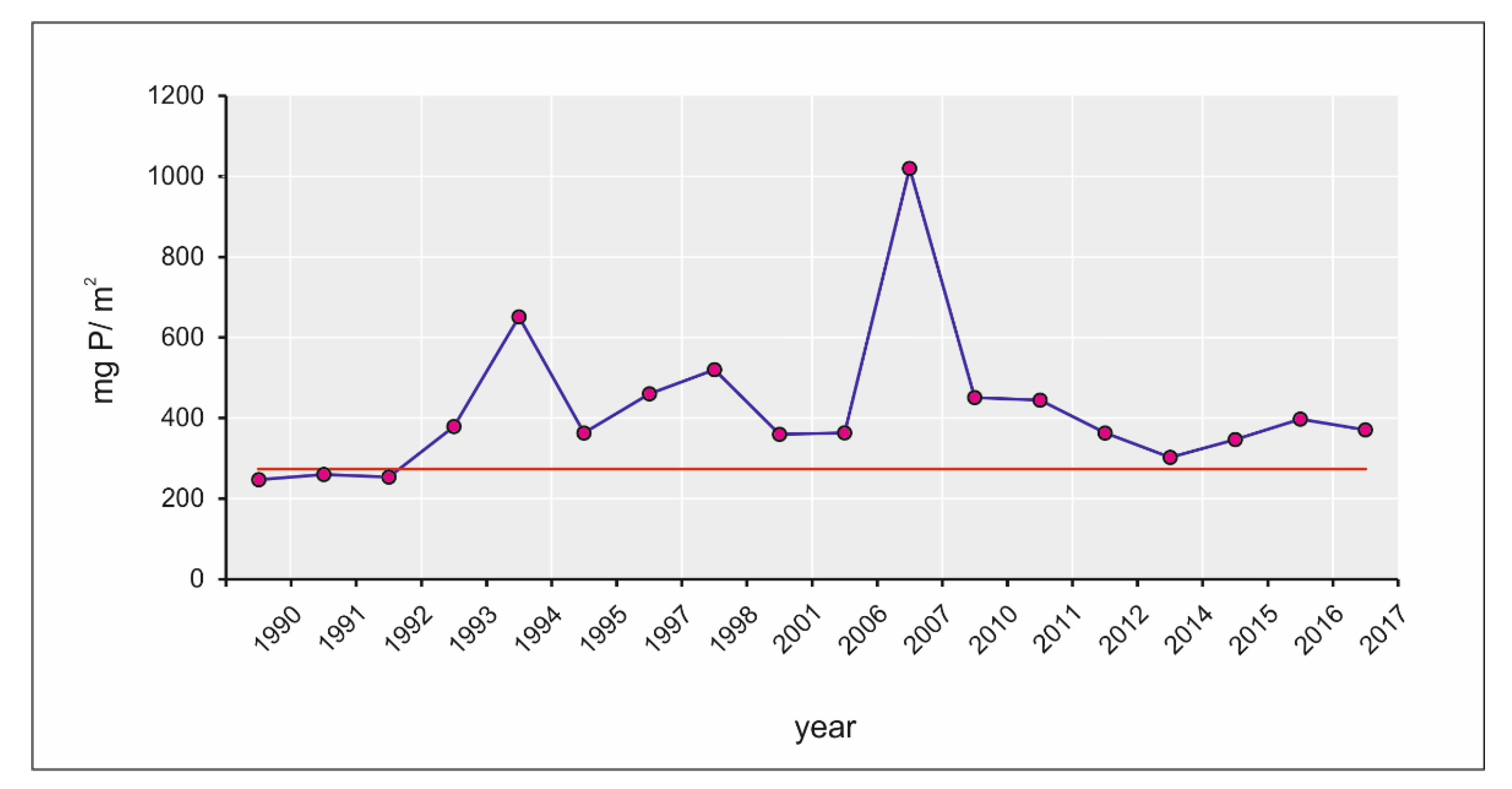
3.1. Eutrophication Dynamics
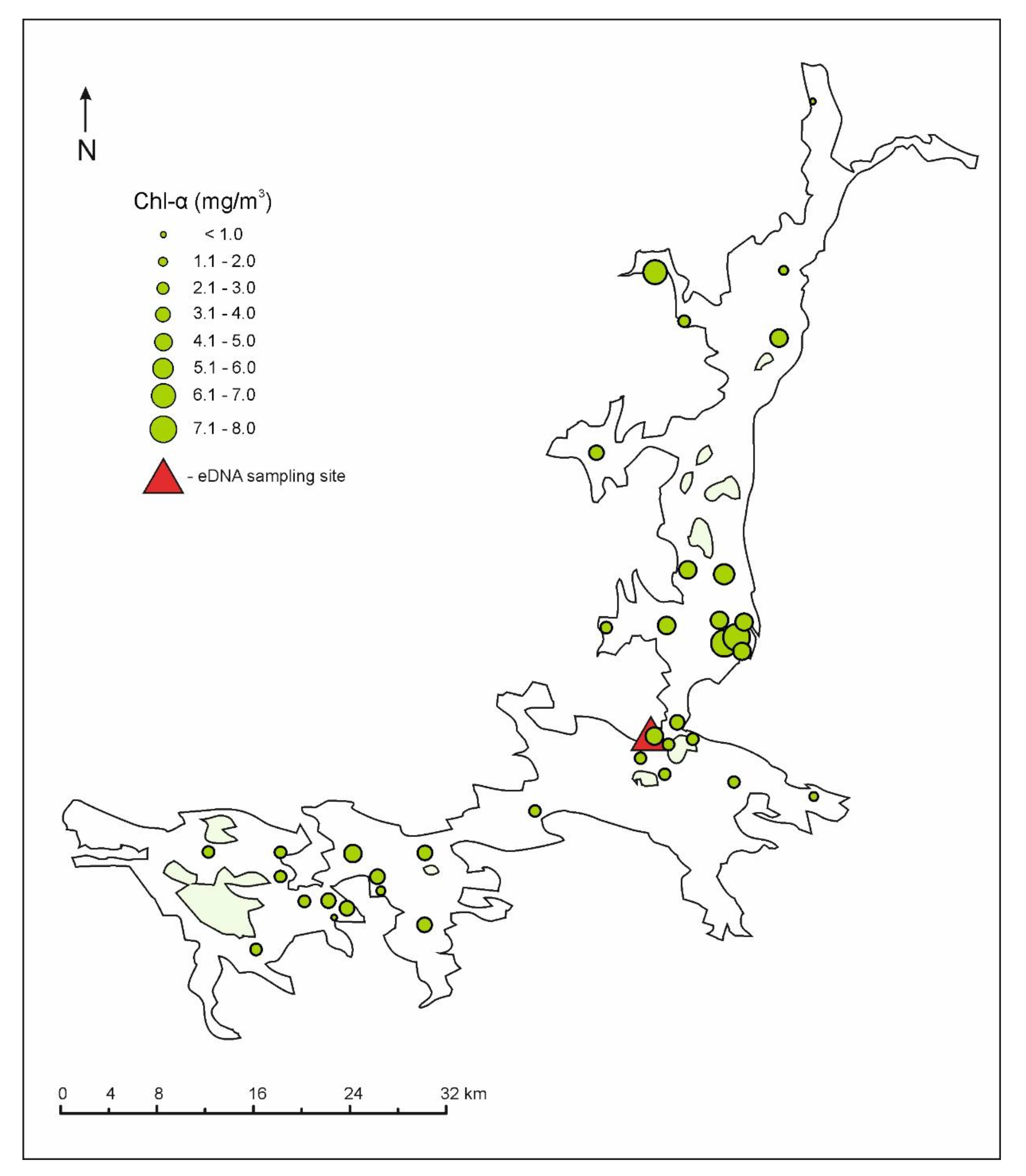
3.2. Nutrient Stoichiometry and Chlorophyll-a
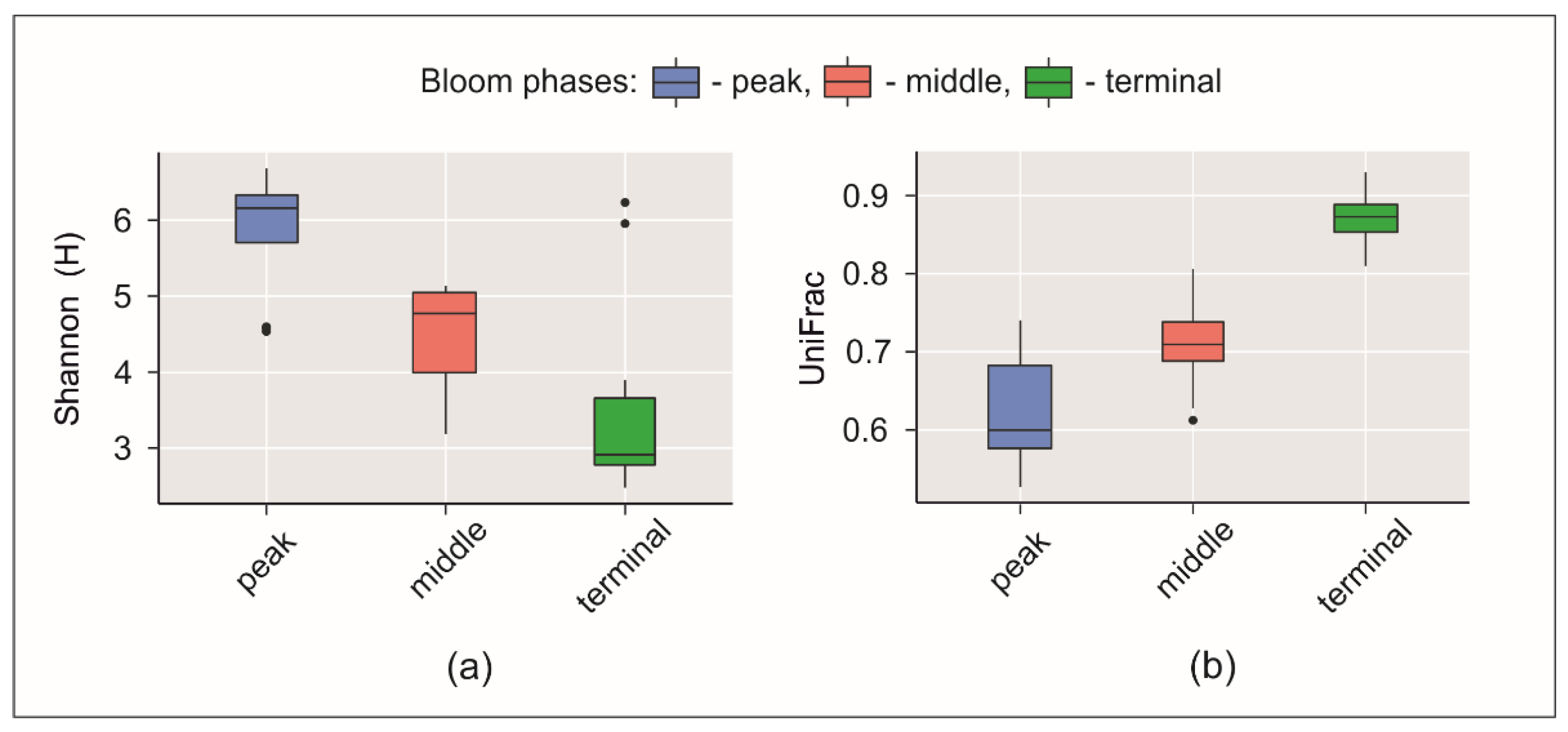
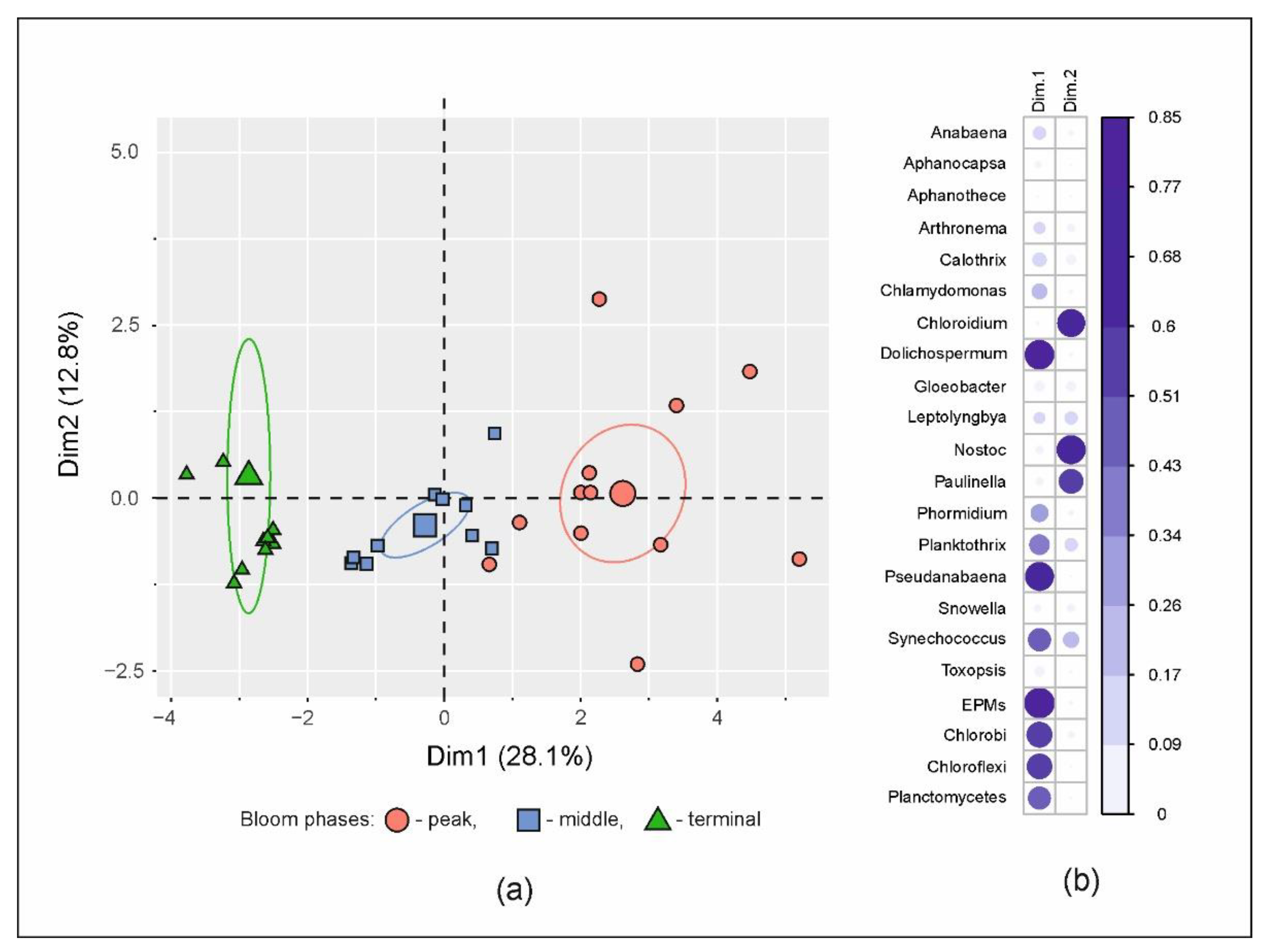
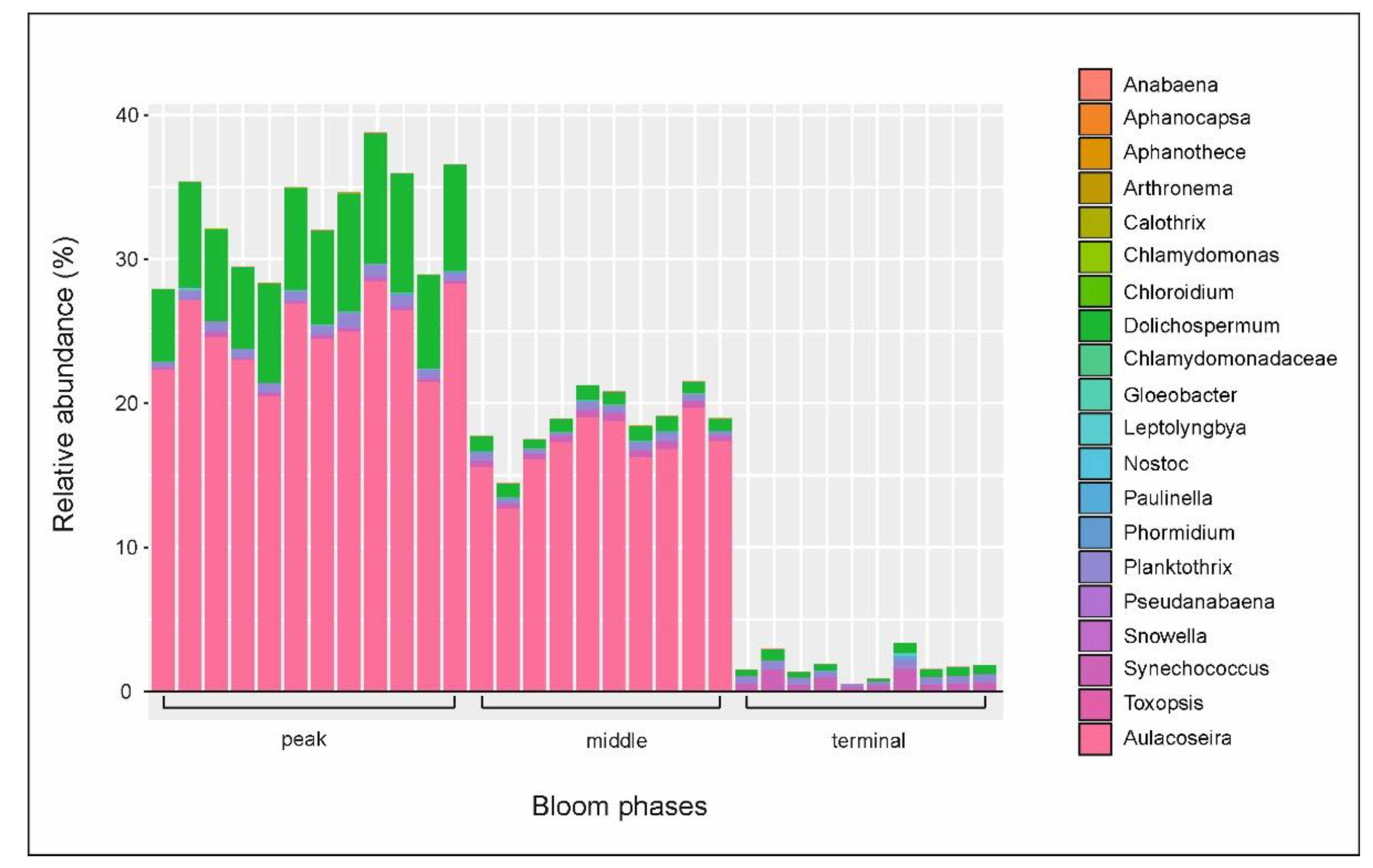
3.3. Microbial Communities
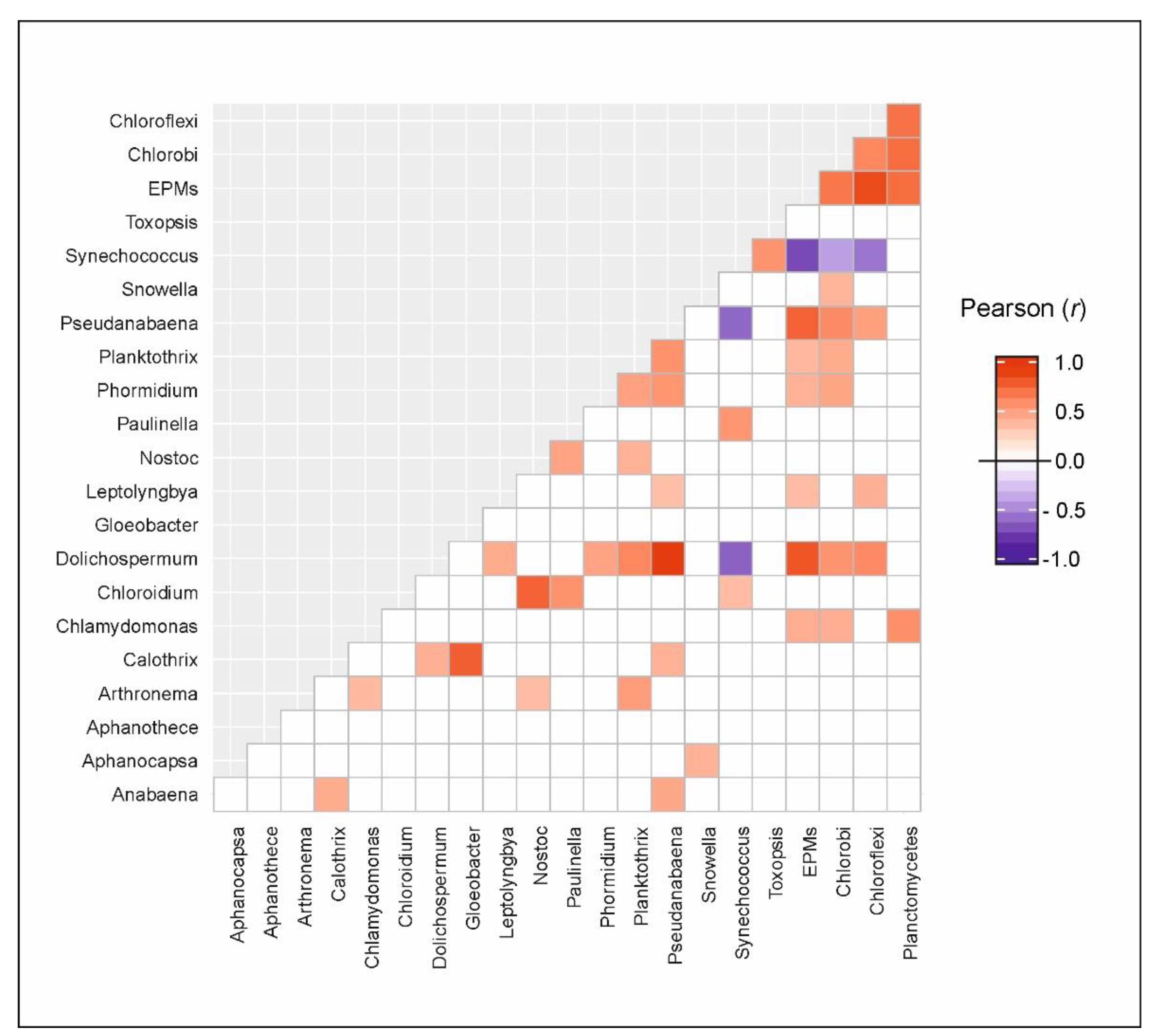
3.4. Putative Genetic Triats Beneath Simultaneous Blooming
4. Discussion
4.1. Selection of the Primers
4.2. Concentrations of Macronutrients and Chlorophyll-a
4.3. HABs in Arctic Waters
4.4. Putative Genetic Triats and Adaptations Beneath Simultaneous Blooming
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Denisov, D.; Kashulin, N. Cyanoprokaryota of the Imandra Lake (Kola Peninsula). Annu. Reporst Kola Sci. Cent. Russ. Acad. 2016, 7, 40–57. [Google Scholar]
- Kashulin, N.; Bekkelund, A.; Dauvalter, V.; Petrova, O. Apatite mining and processing production and eutrophication of the Arctic Lake Imandra. Arkt. Ekol. Ekon. 2019, 3, 16–34. [Google Scholar] [CrossRef]
- Kashulin, N.; Bekkelund, A.; Dauvalter, V. Characteristics of summertime spatial distribution of phosphorus, nitrogen and chlorophyll-a in a major eutrophic Arctic lake Imandra (Murmansk Region, Russia) as associated with harmful algal blooms. Biosfera 2020, 12, 63–92. [Google Scholar]
- Kravtsova, V. Lake Imandra water contamination dynamics from space images. IAHS Publ. Ser. Proc. Rep. Intern Assoc Hydrol. Sci. 1995, 230, 67–72. [Google Scholar]
- Dauvalter, V.; Kashulin, N. Geoecology of the lakes of Murmansk region. In MGTU; Murmansk State Technical University: Murmansk, Russia, 2014. [Google Scholar]
- Flynn, K.J.; Stoecker, D.K.; Mitra, A.; Raven, J.A.; Glibert, P.M.; Hansen, P.J.; Granéli, E.; Burkholder, J.M. Misuse of the phytoplankton–zooplankton dichotomy: The need to assign organisms as mixotrophs within plankton functional types. J. Plankton Res. 2013, 35, 3–11. [Google Scholar] [CrossRef] [Green Version]
- Voloshko, L. Spiniferomonas species (Chrysophyceae, Paraphysomonadaceae) in waterbodies of the Northern Russia. Bot. Zhurnal 2013, 98, 848–858. [Google Scholar]
- Voloshko, L. Chrysophycean algae in waterbodies of the Northern Russia. Genus Chrysosphaerella Bot. Zhurnal 2016, 101, 753–776. [Google Scholar]
- Denisov, D.; Kashulin, N. The phytoplankton communities in the vicinities of еру Kola nuclear power Plant (the Imandra Lake). Annu. Reporst Kola Sci. Cent. Russ. Acad. Sci. 2013, 3, 71–95. [Google Scholar]
- Morton, J.T.; Sanders, J.; Quinn, R.; McDonald, D.; Gonzalez, A.; Vázquez-Baeza, Y.; Navas-Molina, J.A.; Song, S.J.; Metcalf, J.L.; Hyde, E.R.; et al. Balance trees reveal microbial niche differentiation. MSystems 2017, 2, e00162-16. [Google Scholar] [CrossRef] [Green Version]
- Bedoshvili, Y.D.; Popkova, T.; Likhoshway, Y.V. Chloroplast structure of diatoms of different classes. Cell Tissue Biol. 2009, 3, 297–310. [Google Scholar] [CrossRef]
- Smutná, M.; Babica, P.; Jarque, S.; Hilscherová, K.; Maršálek, B.; Haeba, M.; Bláha, L. Acute, chronic and reproductive toxicity of complex cyanobacterial blooms in Daphnia magna and the role of microcystins. Toxicon 2014, 79, 11–18. [Google Scholar] [CrossRef]
- Vollenweider, R.A. Advances in defining critical loading levels for phosphorus in lake eutrophication. Memorie dell’Istituto Italiano di Idrobiologia Dott Marco de Marchi Verbania Pallanza 1976, 33, 53–83. [Google Scholar]
- Kirchner, W.; Dillon, P. An empirical method of estimating the retention of phosphorus in lakes. Water Resour. Res. 1975, 11, 182–183. [Google Scholar] [CrossRef]
- Cleveland, W.S.; Devlin, S.J. Locally weighted regression: An approach to regression analysis by local fitting. J. Am. Stat. Assoc. 1988, 83, 596–610. [Google Scholar] [CrossRef]
- Team, R.C. R: A Language and Environment For Statistical Computing. 2013. Available online: http://www.R-project.org/ (accessed on 17 August 2021).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Smith, D.; Jarvie, H.; Bowes, M. Carbon, nitrogen, and phosphorus stoichiometry and eutrophication in River Thames tributaries, UK. Agric. Environ. Lett. 2017, 2, 20. [Google Scholar] [CrossRef]
- Hamilton, N.E.; Ferry, M. ggtern: Ternary diagrams using ggplot2. J. Stat. Softw. 2018, 87, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335. [Google Scholar] [CrossRef] [Green Version]
- DeSantis, T.Z.; Hugenholtz, P.; Larsen, N.; Rojas, M.; Brodie, E.L.; Keller, K.; Huber, T.; Dalevi, D.; Hu, P.; Andersen, G.L. Greengenes, a Chimera-Checked 16S rRNA Gene Database and Workbench Compatible with ARB. Appl. Environ. Microbiol. 2006, 72, 5069–5072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Caporaso, J.G. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2’s q2-feature-classifier plugin. Microbiome 2018, 6, 90. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischer, M.A.; Güllert, S.; Neulinger, S.C.; Streit, W.R.; Schmitz, R.A. Evaluation of 16S rRNA gene primer pairs for monitoring microbial community structures showed high reproducibility within and low comparability between datasets generated with multiple archaeal and bacterial primer pairs. Front. Microbiol. 2016, 7, 1297. [Google Scholar] [CrossRef] [PubMed]
- Bukin, Y.S.; Galachyants, Y.P.; Morozov, I.; Bukin, S.; Zakharenko, A.; Zemskaya, T. The effect of 16S rRNA region choice on bacterial community metabarcoding results. Sci. Data 2019, 6, 190007. [Google Scholar] [CrossRef] [Green Version]
- Wear, E.K.; Wilbanks, E.G.; Nelson, C.E.; Carlson, C.A. Primer selection impacts specific population abundances but not community dynamics in a monthly time-series 16S rRNA gene amplicon analysis of coastal marine bacterioplankton. Environ. Microbiol. 2018, 20, 2709–2726. [Google Scholar] [CrossRef] [Green Version]
- Parulekar, N.N.; Kolekar, P.; Jenkins, A.; Kleiven, S.; Utkilen, H.; Johansen, A.; Sawant, S.; Kulkarni-Kale, U.; Kale, M.; Sæbø, M. Characterization of bacterial community associated with phytoplankton bloom in a eutrophic lake in South Norway using 16S rRNA gene amplicon sequence analysis. PLoS ONE 2017, 12, e0173408. [Google Scholar] [CrossRef] [PubMed]
- Bennke, C.; Pollehne, F.; Müller, A.; Hansen, R.; Kreikemeyer, B.; Labrenz, M. The distribution of phytoplankton in the Baltic Sea assessed by a prokaryotic 16S rRNA gene primer system. J. Plankton Res. 2018, 40, 244–254. [Google Scholar] [CrossRef]
- Lomas, M.W.; Glibert, P.M. Comparisons of nitrate uptake, storage, and reduction in marine diatoms and flagellates. J. Phycol. 2000, 36, 903–913. [Google Scholar] [CrossRef]
- Busseni, G.; Vieira, F.R.J.; Amato, A.; Pelletier, E.; Karlusich, J.J.P.; Ferrante, M.I.; Wincker, P.; Rogato, A.; Bowler, C.; Sanges, R.; et al. Meta-Omics Reveals Genetic Flexibility of Diatom Nitrogen Transporters in Response to Environmental Changes. Mol. Biol. Evol. 2019, 36, 2522–2535. [Google Scholar] [CrossRef] [Green Version]
- Kamp, A.; Stief, P.; Bristow, L.; Thamdrup, B.; Glud, R.N. Intracellular Nitrate of Marine Diatoms as a Driver of Anaerobic Nitrogen Cycling in Sinking Aggregates. Front. Microbiol. 2016, 7, 1669. [Google Scholar] [CrossRef] [PubMed]
- Vincent, W.F.; Quesada, A. Cyanobacteria in High Latitude Lakes, Rivers and Seas. In Ecology of Cyanobacteria II; Springer: Berlin/Heidelberg, Germany, 2012; pp. 371–385. [Google Scholar]
- Lu, J.; Zhu, B.; Struewing, I.; Xu, N.; Duan, S. Nitrogen–phosphorus-associated metabolic activities during the development of a cyanobacterial bloom revealed by metatranscriptomics. Sci. Rep. 2019, 9, 2480. [Google Scholar] [CrossRef] [Green Version]
- Davis, T.W.; Bullerjahn, G.; Tuttle, T.; McKay, R.; Watson, S.B. Effects of Increasing Nitrogen and Phosphorus Concentrations on Phytoplankton Community Growth and Toxicity DuringPlanktothrixBlooms in Sandusky Bay, Lake Erie. Environ. Sci. Technol. 2015, 49, 7197–7207. [Google Scholar] [CrossRef]
- Jankowiak, J.; Hattenrath-Lehmann, T.; Kramer, B.J.; Ladds, M.; Gobler, C.J. Deciphering the effects of nitrogen, phosphorus, and temperature on cyanobacterial bloom intensification, diversity, and toxicity in western Lake Erie. Limnol. Oceanogr. 2019, 64, 1347–1370. [Google Scholar] [CrossRef] [Green Version]
- Wan, L.; Chen, X.; Deng, Q.; Yang, L.; Li, X.; Zhang, J.; Song, C.; Zhou, Y.; Cao, X. Phosphorus strategy in bloom-forming cyanobacteria (Dolichospermum and Microcystis) and its role in their succession. Harmful Algae 2019, 84, 46–55. [Google Scholar] [CrossRef]
- Cordeiro, R.S.; Vaz, I.C.; Magalhães, S.M.; Barbosa, F.A. Effects of nutritional conditions on lipid production by cyanobacteria. Anais da Academia Brasileira de Ciências 2017, 89, 2021–2031. [Google Scholar] [CrossRef]
- Arias, D.M.; Uggetti, E.; García-Galán, M.J.; García, J. Production of polyhydroxybutyrates and carbohydrates in a mixed cyanobacterial culture: Effect of nutrients limitation and photoperiods. New Biotechnol. 2018, 42, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Kashtan, N.; Roggensack, S.E.; Rodrigue, S.; Thompson, J.W.; Biller, S.J.; Coe, A.; Ding, H.; Marttinen, P.; Malmstrom, R.R.; Stocker, R.; et al. Single-Cell Genomics Reveals Hundreds of Coexisting Subpopulations in Wild Prochlorococcus. Science 2014, 344, 416–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Segawa, T.; Takeuchi, N.; Fujita, K.; Aizen, V.B.; Willerslev, E.; Yonezawa, T. Demographic analysis of cyanobacteria based on the mutation rates estimated from an ancient ice core. Heredity 2018, 120, 562. [Google Scholar] [CrossRef]
- Pinevich, A.V.; Averina, S.G.; Gavrilova, O.V.; Migunova, A.V. Baeocytes in the cyanobacterium Pleurocapsa sp.: Characterization of the differentiated cells produced by multiple fission. Microbiology 2008, 77, 62–68. [Google Scholar] [CrossRef]
- Tandon, R.; Kesarwani, S.; Tiwari, G. Baeocytes and pseudofilamentous stages of Pleurocapsa concharum Hansgirg (Cyanobacteria, Pleurocapsales) in culture. Nova Hedwig. 2016, 102, 429–437. [Google Scholar] [CrossRef]
- Kaplan-Levy, R.N.; Hadas, O.; Summers, M.L.; Rücker, J.; Sukenik, A. Akinetes: Dormant cells of cyanobacteria. In Dormancy and Resistance in Harsh Environments; Springer: Berlin/Heidelberg, Germany, 2010; pp. 5–27. [Google Scholar]
- Kapkov, V.; Vasilieva, S.; Lobakova, E. Growth of Toxic Cyanobacteria Dolichospermum flos-aquae (Anabaena flos-aquae) in the Waters of the Boreal Zone. Mosc. Univ. Biol. Sci. Bull. 2019, 74, 15–20. [Google Scholar] [CrossRef]
- Salmaso, N.; Boscaini, A.; Capelli, C.; Cerasino, L.; Milan, M.; Putelli, S.; Tolotti, M. Historical colonization patterns of Dolichospermum lemmermannii (Cyanobacteria) in a deep lake south of the Alps. Adv. Oceanogr. Limnol. 2015, 6. [Google Scholar] [CrossRef] [Green Version]
- Kravchuk, E.S.; Ivanova, E.A.; Gladyshev, M.I. Seasonal dynamics of akinetes of Anabaena flos-aquae in bottom sediments and water column of small Siberian reservoir. Aquat. Ecol. 2006, 40, 325–336. [Google Scholar] [CrossRef]
- Perez, R.; Forchhammer, K.; Salerno, G.; Maldener, I. Clear differences in metabolic and morphological adaptations of akinetes of two Nostocales living in different habitats. Microbiology 2016, 162, 214–223. [Google Scholar] [CrossRef]
- Watzer, B.; Forchhammer, K. Cyanophycin synthesis optimizes nitrogen utilization in the unicellular cyanobacterium Synechocystis sp. strain PCC 6803. Appl. Environ. Microbiol. 2018, 84, e01298-18. [Google Scholar] [CrossRef] [Green Version]
- Perez, R.; Wörmer, L.; Sass, P.; Maldener, I. A highly asynchronous developmental program triggered during germination of dormant akinetes of filamentous diazotrophic cyanobacteria. FEMS Microbiol. Ecol. 2018, 94, fix131. [Google Scholar] [CrossRef]
- Park, C.-H.; Park, M.-H.; Kim, K.H.; Park, J.-H.; Kwon, D.-R.; Kim, N.Y.; Lim, B.-J.; Hwang, S.-J. Akinete germination chamber: An experimental device for cyanobacterial akinete germination and plankton emergence. Harmful Algae 2018, 72, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Lee, W.; Kim, Y.-O.; Lee, H.-O.; Han, M.-S. Relationship between akinete germination and vegetative population of Anabaena flos-aquae (Nostocales, Cyanobacteria) in Seokchon reservoir (Seoul, Korea). Arch. Hydrobiol. 2005, 163, 49–64. [Google Scholar] [CrossRef]
- Ramm, J.; Rücker, J.; Knie, M.; Nixdorf, B. Lost in the dark: Estimation of the akinete pool for the recruitment of Nostocales populations (cyanobacteria) in a temperate deep lake. J. Plankton Res. 2017, 39, 392–403. [Google Scholar] [CrossRef] [Green Version]
- Hildebrand, M.; Lerch, S.J.L.; Shrestha, R.P. Understanding Diatom Cell Wall Silicification—Moving Forward. Front. Mar. Sci. 2018, 5, 125. [Google Scholar] [CrossRef] [Green Version]
- Flori, S.; Jouneau, P.-H.; Bailleul, B.; Gallet, B.; Estrozi, L.F.; Moriscot, C.; Bastien, O.; Eicke, S.; Schober, A.; Bártulos, C.R.; et al. Plastid thylakoid architecture optimizes photosynthesis in diatoms. Nat. Commun. 2017, 8, 15885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olli, K.; Klais-Peets, R.; Tamminen, T. Rehabilitating the Cyanobacteria—Niche partitioning, resource use efficiency and phytoplankton community structure during diazotrophic cyanobacterial blooms. J. Ecol. 2015, 103, 1153–1164. [Google Scholar] [CrossRef]
- Caputo, A.A.A.; Nylander, J.A.; Foster, R. The genetic diversity and evolution of diatom-diazotroph associations highlights traits favoring symbiont integration. FEMS Microbiol. Lett. 2019, 366, 297. [Google Scholar] [CrossRef] [Green Version]
- Croft, M.T.; Lawrence, A.D.; Raux-Deery, E.; Warren, M.; Smith, A. Algae acquire vitamin B12 through a symbiotic relationship with bacteria. Nat. Cell Biol. 2005, 438, 90–93. [Google Scholar] [CrossRef]
- Wagner-Döbler, I.; Ballhausen, B.; Berger, M.; Brinkhoff, T.; Buchholz, I.; Bunk, B.; Cypionka, H.; Daniel, R.; Drepper, T.; Gerdts, G.; et al. The complete genome sequence of the algal symbiont Dinoroseobacter shibae: A hitchhiker’s guide to life in the sea. ISME J. 2010, 4, 61–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kagami, M.; Yoshida, T.; Gurung, T.; Urabe, J. Direct and indirect effects of zooplankton on algal composition in in situ grazing experiments. Oecologia 2002, 133, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, S.; Hansson, L.-A. Development of tolerance against toxic cyanobacteria in Daphnia. Aquat. Ecol. 2004, 38, 37–44. [Google Scholar] [CrossRef]
- Gibson, C.; Fitzsimons, A. Induction of the resting phase in the planktonic diatom Aulacoseira subarctica in very low light. Br. Phycol. J. 1990, 25, 329–334. [Google Scholar] [CrossRef]




| Hmax (m) | Z (m) | Slake (km2) | V (km3) | Scathc (km2) | R (m/s) | t (Year) | CLP (mg P/m2; Year) | Q(P)max (ton P; Yyear) | M(P) (mg P/m2; Year) | L(P) (ton P; Year) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Entire Lake | 67 | 13.3 | 813 | 10.81 | 12300 | 145 | 2.38 | 333 | 270 | 22 | 1507 |
| Bolshaya I. | 67 | 14.7 | 312 | 4.58 | 4993 | 145 | 1.00 | 368 | 115 | 23 | 999 |
| Jokostrovskaya I | 42 | 10.9 | 352 | 3.84 | 6070 | 145 | 0.84 | 273 | 96 | 16 | 332 |
| Babinskaya I. | 43.5 | 16.3 | 149 | 2.42 | 1238 | 145 | 0.53 | 408 | 61 | 49 | 176 |
| pH | NH4 (µgN/l) | NO3 (µgN/) | NO3: NH3 | TN (µgN/l) | Nopr (µgN/l) | PO4 (µgP/l) | TP (µgP/l) | TN:TP | |
|---|---|---|---|---|---|---|---|---|---|
| Bolshaya I. | 7.5 6.8–8.4 | 7.0 1.0–114 | 5.0 0–1156 | 3.4 0–39.0 | 196 119–1629 | 188 117–365 | 3.0 1.0–153 | 27.0 7.0–251 | 8.4 2.5–18.9 |
| Jokostrovskaya I. | 7.3 7.1–7.4 | 10.0 1.0–58.0 | 2.0 0–30.0 | 1.0 0–12.0 | 152 111–259 | 138 84–199 | 1.0 0–2.0 | 7.0 5.0–12.0 | 21.6 14.7–34.0 |
| Babinskaya I. | 7.3 7.0–7.5 | 9.0 2.0–22.0 | 7.0 0–78.0 | 2.8 0–21.7 | 156 66.0–318 | 126 44–250 | 1.0 0–3.0 | 5.0 3.0–22.0 | 30.6 5.7–63.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kashulin, N.; Kashulina, T.; Bekkelund, A. Long-Term Eutrophication and Dynamics of Bloom-Forming Microbial Communities during Summer HAB in Large Arctic Lake. Environments 2021, 8, 82. https://0-doi-org.brum.beds.ac.uk/10.3390/environments8080082
Kashulin N, Kashulina T, Bekkelund A. Long-Term Eutrophication and Dynamics of Bloom-Forming Microbial Communities during Summer HAB in Large Arctic Lake. Environments. 2021; 8(8):82. https://0-doi-org.brum.beds.ac.uk/10.3390/environments8080082
Chicago/Turabian StyleKashulin, Nikolay, Tatiana Kashulina, and Alexander Bekkelund. 2021. "Long-Term Eutrophication and Dynamics of Bloom-Forming Microbial Communities during Summer HAB in Large Arctic Lake" Environments 8, no. 8: 82. https://0-doi-org.brum.beds.ac.uk/10.3390/environments8080082





