Development of a Green Methodology for Simultaneous Extraction of Polyphenols and Pigments from Red Winemaking Solid Wastes (Pomace) Using a Novel Glycerol-Sodium Benzoate Deep Eutectic Solvent and Ultrasonication Pretreatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Deep Eutectic Solvent (DES) Synthesis
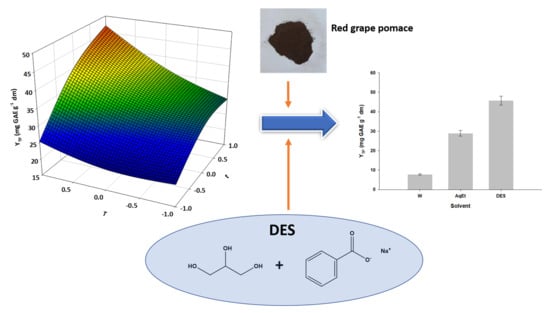
2.3. Red Grape Pomace (RGP) Collection and Handling
2.4. Effect of Water Proportion
2.5. Ultrasonication Pretreatment
2.6. Effect of Liquid-to-Solid Ratio (RL/S)
2.7. Combined Effect of Time and Temperature
2.8. Determinations
2.9. Liquid Chromatography—Diode Array–Mass Spectrometry (LC–DAD–MS)
2.10. High-Performance Liquid Chromatography (HPLC)
2.11. Statistical Processing
3. Results and Discussion
3.1. The Effect of DES/Water Proportion
3.2. The Effect of Ultrasonication Power (P)
3.3. The Effect of Liquid-to-Solid Ratio (RL/S)
3.4. Optimization of Extraction Time and Temperature
3.5. Polyphenolic Composition
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Osorio, L.L.D.R.; Flórez-López, E.; Grande-Tovar, C.D. The potential of selected agri-food loss and waste to contribute to a circular economy: Applications in the food, cosmetic and pharmaceutical industries. Molecules 2021, 26, 515. [Google Scholar] [CrossRef] [PubMed]
- Troilo, M.; Difonzo, G.; Paradiso, V.M.; Summo, C.; Caponio, F. Bioactive compounds from vine shoots, grape stalks, and wine lees: Their potential use in agro-food chains. Foods 2021, 10, 342. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and human health: The role of bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef] [PubMed]
- Makris, D.P. Green extraction processes for the efficient recovery of bioactive polyphenols from wine industry solid wastes–Recent progress. Cur. Opin. Green Sustain. Chem. 2018, 13, 50–55. [Google Scholar] [CrossRef]
- Chávez-González, M.L.; Sepúlveda, L.; Verma, D.K.; Luna-García, H.A.; Rodríguez-Durán, L.V.; Ilina, A.; Aguilar, C.N. Conventional and emerging extraction processes of flavonoids. Processes 2020, 8, 434. [Google Scholar] [CrossRef] [Green Version]
- Chemat, F.; Abert Vian, M.; Ravi, H.K.; Khadhraoui, B.; Hilali, S.; Perino, S.; Fabiano Tixier, A.-S. Review of alternative solvents for green extraction of food and natural products: Panorama, principles, applications and prospects. Molecules 2019, 24, 3007. [Google Scholar] [CrossRef] [Green Version]
- Ivanović, M.; Islamčević Razboršek, M.; Kolar, M. Innovative extraction techniques using deep eutectic solvents and analytical methods for the isolation and characterization of natural bioactive compounds from plant material. Plants 2020, 9, 1428. [Google Scholar] [CrossRef]
- Medina-Torres, N.; Ayora-Talavera, T.; Espinosa-Andrews, H.; Sánchez-Contreras, A.; Pacheco, N. Ultrasound assisted extraction for the recovery of phenolic compounds from vegetable sources. Agronomy 2017, 7, 47. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.-G.; Meullemiestre, A.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef]
- Nayak, A.; Bhushan, B.; Rosales, A.; Turienzo, L.R.; Cortina, J. Valorisation potential of Cabernet grape pomace for the recovery of polyphenols: Process intensification, optimisation and study of kinetics. Food Bioprod. Proc. 2018, 109, 74–85. [Google Scholar] [CrossRef]
- Kaltsa, O.; Lakka, A.; Grigorakis, S.; Karageorgou, I.; Batra, G.; Bozinou, E.; Lalas, S.; Makris, D.P. A green extraction process for polyphenols from elderberry (Sambucus nigra) flowers using deep eutectic solvent and ultrasound-assisted pretreatment. Molecules 2020, 25, 921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cherif, M.M.; Grigorakis, S.; Halahlah, A.; Loupassaki, S.; Makris, D.P. High-efficiency extraction of phenolics from wheat waste biomass (bran) by combining deep eutectic solvent, ultrasound-assisted pretreatment and thermal treatment. Environ. Proc. 2020, 7, 845–859. [Google Scholar] [CrossRef]
- Slim, Z.; Jancheva, M.; Grigorakis, S.; Makris, D.P. Polyphenol extraction from Origanum dictamnus using low-transition temperature mixtures composed of glycerol and organic salts: Effect of organic anion carbon chain length. Chem. Eng. Com. 2018, 205, 1494–1506. [Google Scholar] [CrossRef]
- Kimura, T.; Sakamoto, T.; Leveque, J.-M.; Sohmiya, H.; Fujita, M.; Ikeda, S.; Ando, T. Standardization of ultrasonic power for sonochemical reaction. Ultrason. Sonochem. 1996, 3, S157–S161. [Google Scholar] [CrossRef]
- Lakka, A.; Grigorakis, S.; Karageorgou, I.; Batra, G.; Kaltsa, O.; Bozinou, E.; Lalas, S.; Makris, D.P. Saffron processing wastes as a bioresource of high-value added compounds: Development of a green extraction process for polyphenol recovery using a natural deep eutectic solvent. Antioxidants 2019, 8, 586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trasanidou, D.; Apostolakis, A.; Makris, D.P. Development of a green process for the preparation of antioxidant and pigment-enriched extracts from winery solid wastes using response surface methodology and kinetics. Chem. Eng. Com. 2016, 203, 1317–1325. [Google Scholar] [CrossRef]
- Dourtoglou, V.G.; Mamalos, A.; Makris, D.P. Storage of olives (Olea europaea) under CO2 atmosphere: Effect on anthocyanins, phenolics, sensory attributes and in vitro antioxidant properties. Food Chem. 2006, 99, 342–349. [Google Scholar] [CrossRef]
- Makris, D.; Kefalas, P. Characterization of polyphenolic phytochemicals in red grape pomace. Int. J. Waste Resour. 2013, 126. [Google Scholar] [CrossRef] [Green Version]
- Lu, W.; Liu, S. Choline chloride–based deep eutectic solvents (Ch-DESs) as promising green solvents for phenolic compounds extraction from bioresources: State-of-the-art, prospects, and challenges. Biomass Conver. Bioref. 2020. [CrossRef]
- Qiao, L.; Ye, X.; Sun, Y.; Ying, J.; Shen, Y.; Chen, J. Sonochemical effects on free phenolic acids under ultrasound treatment in a model system. Ultrason. Sonochem. 2013, 20, 1017–1025. [Google Scholar] [CrossRef]
- Qiao, L.; Sun, Y.; Chen, R.; Fu, Y.; Zhang, W.; Li, X.; Chen, J.; Shen, Y.; Ye, X. Sonochemical effects on 14 flavonoids common in citrus: Relation to stability. PLoS ONE 2014, 9, e87766. [Google Scholar]
- Wang, P.; Cheng, C.; Ma, Y.; Jia, M. Degradation behavior of polyphenols in model aqueous extraction system based on mechanical and sonochemical effects induced by ultrasound. Separ. Purif. Technol. 2020, 247, 116967. [Google Scholar] [CrossRef]
- Tomšik, A.; Pavlić, B.; Vladić, J.; Ramić, M.; Brindza, J.; Vidović, S. Optimization of ultrasound-assisted extraction of bioactive compounds from wild garlic (Allium ursinum L.). Ultrason. Sonochem. 2016, 29, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zheng, J.; Gan, R.-Y.; Zhou, T.; Xu, D.-P.; Li, H.-B. Optimization of ultrasound-assisted extraction of antioxidants from the mung bean coat. Molecules 2017, 22, 638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Athanasiadis, V.; Grigorakis, S.; Lalas, S.; Makris, D.P. Stability effects of methyl β-cyclodextrin on Olea europaea leaf extracts in a natural deep eutectic solvent. Eur. Food Res. Technol. 2018, 244, 1783–1792. [Google Scholar] [CrossRef]
- Karageorgou, I.; Grigorakis, S.; Lalas, S.; Makris, D.P. The effect of 2-hydroxypropyl β-cyclodextrin on the stability of polyphenolic compounds from Moringa oleifera Lam leaf extracts in a natural low-transition temperature mixture. Nova Biotech. Chim. 2018, 17, 29–37. [Google Scholar] [CrossRef]
- Grigorakis, S.; Halahlah, A.; Makris, D.P. Stability of Salvia fruticosa Mill. Polyphenols and antioxidant activity in a citrate-based natural deep eutectic solvent. Nova Biotech. Chim. 2020, 19, 200–207. [Google Scholar]
- Shewale, S.; Rathod, V.K. Extraction of total phenolic content from Azadirachta indica or (neem) leaves: Kinetics study. Prep. Biochem. Biotech. 2018, 48, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Jancheva, M.; Grigorakis, S.; Loupassaki, S.; Makris, D.P. Optimised extraction of antioxidant polyphenols from Satureja thymbra using newly designed glycerol-based natural low-transition temperature mixtures (LTTMs). J. Applied Res. Med. Arom. Plants 2017, 6, 31–40. [Google Scholar] [CrossRef]
- Dedousi, M.; Mamoudaki, V.; Grigorakis, S.; Makris, D.P. Ultrasound-assisted extraction of polyphenolic antioxidants from olive (Olea europaea) leaves using a novel glycerol/sodium-potassium tartrate low-transition temperature mixture (LTTM). Environments 2017, 4, 31. [Google Scholar] [CrossRef] [Green Version]
- Rajha, H.N.; El Darra, N.; Hobaika, Z.; Boussetta, N.; Vorobiev, E.; Maroun, R.G.; Louka, N. Extraction of total phenolic compounds, flavonoids, anthocyanins and tannins from grape byproducts by response surface methodology. Influence of solid-liquid ratio, particle size, time, temperature and solvent mixtures on the optimization process. Food Nutr. Sci. 2014, 2014. [Google Scholar] [CrossRef] [Green Version]
- González-Centeno, M.; Comas-Serra, F.; Femenia, A.; Rosselló, C.; Simal, S. Effect of power ultrasound application on aqueous extraction of phenolic compounds and antioxidant capacity from grape pomace (Vitis vinifera L.): Experimental kinetics and modeling. Ultrason. Sonochem. 2015, 22, 506–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pintać, D.; Majkić, T.; Torović, L.; Orčić, D.; Beara, I.; Simin, N.; Mimica-Dukić, N.; Lesjak, M. Solvent selection for efficient extraction of bioactive compounds from grape pomace. Ind. Crops Prod. 2018, 111, 379–390. [Google Scholar] [CrossRef]
- Liazid, A.; Guerrero, R.; Cantos, E.; Palma, M.; Barroso, C. Microwave assisted extraction of anthocyanins from grape skins. Food Chem. 2011, 124, 1238–1243. [Google Scholar] [CrossRef]
- Makris, D.; Boskou, G.; Chiou, A.; Andrikopoulos, N. An investigation on factors affecting recovery of antioxidant phenolics and anthocyanins from red grape (Vitis vinifera L.) pomace employing water/ethanol-based solutions. Am. J. Food Technol 2008, 3, 164–173. [Google Scholar] [CrossRef] [Green Version]
- Monrad, J.K.; Srinivas, K.; Howard, L.R.; King, J.W. Design and optimization of a semicontinuous hot–cold extraction of polyphenols from grape pomace. J. Agric. Food Chem. 2012, 60, 5571–5582. [Google Scholar] [CrossRef] [PubMed]
- Monrad, J.K.; Howard, L.R.; King, J.W.; Srinivas, K.; Mauromoustakos, A. Subcritical solvent extraction of anthocyanins from dried red grape pomace. J. Agric. Food Chem. 2010, 58, 2862–2868. [Google Scholar] [CrossRef]
- Georgantzi, C.; Lioliou, A.-E.; Paterakis, N.; Makris, D.P. Combination of lactic acid-based deep eutectic solvents (DES) with β-cyclodextrin: Performance screening using ultrasound-assisted extraction of polyphenols from selected native Greek medicinal plants. Agronomy 2017, 7, 54. [Google Scholar] [CrossRef] [Green Version]
- Makris, D.P.; Psarra, E.; Kallithraka, S.; Kefalas, P. The effect of polyphenolic composition as related to antioxidant capacity in white wines. Food Res. Inter. 2003, 36, 805–814. [Google Scholar] [CrossRef]
- Kefalas, P.; Makris, D. Liquid chromatography-mass spectrometry techniques in flavonoid analysis: Recent advances. In Natural Antioxidant Phenols: Sources, Structure-Activity Relationship, Current Trends in Analysis and Characterisation; Research Signpost: Kerala, India, 2006; pp. 69–123. [Google Scholar]









| Independent Variables | Code Units | Coded Variable Level | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| t (min) | X1 | 10 | 115 | 240 |
| T (°C) | X2 | 50 | 65 | 80 |
| Term | Estimate | Standard Error | t Ratio | Probability > t |
|---|---|---|---|---|
| YTP | ||||
| Intercept | 32.591053 | 0.635778 | 51.26 | <0.0001 * |
| t | 7.53 | 0.505966 | 14.88 | <0.0001 * |
| T | 5.2416667 | 0.505966 | 10.36 | 0.0001 * |
| t × T | 3.33 | 0.619679 | 5.37 | 0.0030 * |
| T × t | −3.752632 | 0.778665 | −4.82 | 0.0048 * |
| T × T | 2.0723684 | 0.778665 | 2.66 | 0.0448 * |
| Lack-of-fit | 0.2644 | |||
| YTPm | ||||
| Intercept | 1.7926316 | 0.04981 | 35.99 | <0.0001 * |
| t | 0.4333333 | 0.03964 | 10.93 | 0.0001 * |
| T | 0.2016667 | 0.03964 | 5.09 | 0.0038 * |
| t × T | 0.09 | 0.048549 | 1.85 | 0.1229 |
| T × t | −0.211579 | 0.061005 | −3.47 | 0.0179 * |
| T × T | 0.0834211 | 0.061005 | 1.37 | 0.2297 |
| Lack-of-fit | 0.0567 |
| Design Point | Independent Variables | Responses | ||||
|---|---|---|---|---|---|---|
| X1(t, min) | X2(T, °C) | YTP(mg GAE g−1dm) | YTPm(mg MvE g−1dm) | |||
| Measured | Predicted | Measured | Predicted | |||
| 1 | −1 (10) | −1 (50) | 21.23 | 21.47 | 1.16 | 1.12 |
| 2 | −1 (10) | 1 (80) | 24.64 | 25.29 | 1.29 | 1.34 |
| 3 | 1 (240) | −1 (50) | 29.57 | 29.87 | 1.81 | 1.81 |
| 4 | 1 (240) | 1 (80) | 46.30 | 47.01 | 2.30 | 2.39 |
| 5 | −1 (10) | 0 (65) | 22.20 | 21.31 | 1.16 | 1.15 |
| 6 | 1 (240) | 0 (65) | 37.38 | 36.37 | 2.10 | 2.01 |
| 7 | 0 (115) | −1 (50) | 29.96 | 29.42 | 1.63 | 1.67 |
| 8 | 0 (115) | 1 (80) | 41.27 | 39.91 | 2.22 | 2.08 |
| 9 | 0 (115) | 0 (65) | 31.45 | 32.59 | 1.73 | 1.79 |
| 10 | 0 (115) | 0 (65) | 32.93 | 32.59 | 1.76 | 1.79 |
| 11 | 0 (115) | 0 (65) | 31.49 | 32.59 | 1.79 | 1.79 |
| Compound | Yield (μg g−1 dm) | ||
|---|---|---|---|
| Water | AqEt | DES | |
| Non-pigment phenolics | |||
| Gallic acid | 128.31 ± 9.62 | 135.72 ± 9.22 | 4675.15 ± 254.33 |
| Caftaric acid | 68.88 ± 5.17 | 80.96 ± 5.50 | 356.17 ± 19.38 |
| Catechin | 104.32 ± 7.82 | 230.08 ± 15.62 | 1630.40 ± 88.69 |
| Rutin | n.d. | 40.72 ± 2.77 | 169.46 ± 9.22 |
| Quercetin | 8.43 ± 0.63 | 268.07 ± 18.20 | 1799.77 ± 97.91 |
| Sum | 309.94 | 755.56 | 8630.94 |
| Anthocyanin pigments | |||
| Malvidin 3-O-glucoside | 23.68 ± 1.78 | 215.93 ± 14.66 | 1488.66 ± 80.98 |
| Malvidin 3-O-glucoside acetate | n.d. | 21.51 ± 1.46 | n.d. |
| Malvidin 3-O-glucoside p-coumarate | n.d. | 572.61 ± 38.88 | 2398.83 ± 130.50 |
| Sum | 23.68 | 810.04 | 3887.50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alibade, A.; Lakka, A.; Bozinou, E.; Lalas, S.I.; Chatzilazarou, A.; Makris, D.P. Development of a Green Methodology for Simultaneous Extraction of Polyphenols and Pigments from Red Winemaking Solid Wastes (Pomace) Using a Novel Glycerol-Sodium Benzoate Deep Eutectic Solvent and Ultrasonication Pretreatment. Environments 2021, 8, 90. https://0-doi-org.brum.beds.ac.uk/10.3390/environments8090090
Alibade A, Lakka A, Bozinou E, Lalas SI, Chatzilazarou A, Makris DP. Development of a Green Methodology for Simultaneous Extraction of Polyphenols and Pigments from Red Winemaking Solid Wastes (Pomace) Using a Novel Glycerol-Sodium Benzoate Deep Eutectic Solvent and Ultrasonication Pretreatment. Environments. 2021; 8(9):90. https://0-doi-org.brum.beds.ac.uk/10.3390/environments8090090
Chicago/Turabian StyleAlibade, Aggeliki, Achillia Lakka, Eleni Bozinou, Stavros I. Lalas, Arhontoula Chatzilazarou, and Dimitris P. Makris. 2021. "Development of a Green Methodology for Simultaneous Extraction of Polyphenols and Pigments from Red Winemaking Solid Wastes (Pomace) Using a Novel Glycerol-Sodium Benzoate Deep Eutectic Solvent and Ultrasonication Pretreatment" Environments 8, no. 9: 90. https://0-doi-org.brum.beds.ac.uk/10.3390/environments8090090