Antioxidant Capacity and Antigenotoxic Effect of Hibiscus sabdariffa L. Extracts Obtained with Ultrasound-Assisted Extraction Process
Abstract
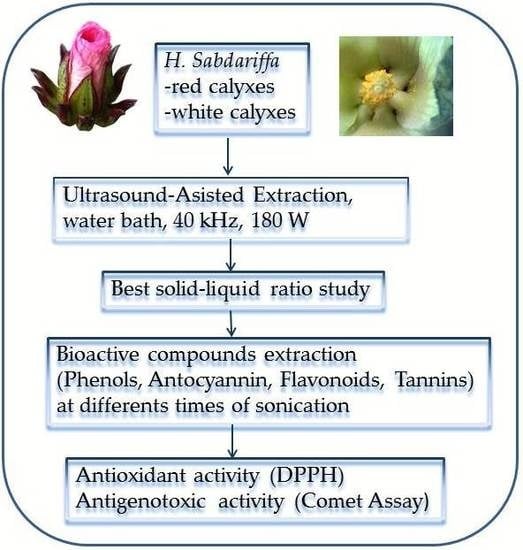
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Ultrasound-Assisted Extraction (UAE)
2.3. Solid-Liquid Extraction
2.4. Determination of Total Polyphenols
2.5. Total Flavonoid Contents
2.6. Determination of Anthocyanin
2.7. Total Condensed Tannin Contents (Proanthocyanidin)
2.8. DPPH Radical Scavenging Assay
2.9. Antimutagenic Test: Comet Assay
2.10. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| UAE | Ultrasound-assisted extraction |
| dw | Dry weight |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| CE | Catechin Equivalents |
| Cya3GE | Cyanidin-3-Glucoside equivalents |
| QUE | Quercetin Equivalents |
| μg | microgram |
| EMS | Ethyl methane sulfonate |
| DMSO | dimethylsulfoxide |
| S-L | Solid-liquid extraction |
| CA | Concentration of Anthocyanin |
| EDTA | Ethylenediaminetetraacetic acid |
| TRIS | trisaminomethane |
References
- Chinedu, S.N.; Olasumbo, A.C.; Eboji, O.K.; Emiloju, O.C.; Arinola, O.K.; Dania, D.I. Proximate and Phytochemical Analyses of Solanum aethiopicum L. and Solanum macrocarpon L. Fruits. Res. J. Chem. Sci. 2011, 3, 24–35. [Google Scholar]
- Crane, J.C. Roselle—A potentially important plant fiber. Econ. Bot. 1949, 3, 89–103. [Google Scholar] [CrossRef]
- Andzi Barhé, T.; Feuya Tchouya, G.R. Comparative study of the antioxidant activity of the total polyphenols extracted from Hibiscus Sabdariffa, L. Glycine max L. Merr., yellow tea and red wine through reaction with DPPH free radicals. Arab. J. Chem. 2016, 9, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Kao, E.-S.; Yang, M.-Y.; Hung, C.-H.; Huang, C.-N.; Wang, C.-J. Polyphenolic extract from Hibiscus sabdariffa reduces body fat by inhibiting hepatic lipogenesis and preadipocyte adipogenesis. Food Funct. 2016, 7, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Khaghani, S. Selective cytotoxicity and apoptogenic activity of Hibiscus sabdariffa aqueous extract against MCF-7 human breast cancer cell line. J. Cancer Ther. 2011, 394–400. [Google Scholar] [CrossRef] [Green Version]
- Borrás-Linares, I.; Fernández-Arroyo, S.; Arráez-Roman, D.; Palmeros-Suárez, P.A.; Del Val-Díaz, R.; Andrade-Gonzáles, I.; Fernández-Gutiérrez, A.; Gómez-Leyva, J.F.; Segura-Carretero, A. Characterization of phenolic compounds anthocyanidin, antioxidant and antimicrobial activity of 25 varieties of Mexican Roselle (Hibiscus sabdariffa). Ind. Crop. Prod. 2015, 69, 385–394. [Google Scholar] [CrossRef]
- Ching-Chuan, S.; Chau-Jong, W.; Kai-Hsun, H.; Yi-Ju, L.; Wei-Ming, C.; Yun-Ching, C. Anthocyanins from Hibiscus sabdariffa calyx attenuate in vitro and in vivo melanoma cancer metastasis. J. Funct. Foods 2018, 48, 614–663. [Google Scholar] [CrossRef]
- Plotto, A.; Mazuad, F.; Röttger, A.; Steffel, K. Hibiscus: Post-Production Management for Improved Market Access Organization; Food and Agriculture Organization of the United Nations (FAO), AGST: Rome, Italy, 2004.
- Ariza-Flores, R.; Serrano-Altamirano, V.; Navarro-Galindo, S.; Ovando-Cruz, M.E.; Vázquez-García, E.; Barrios-Ayala, A.; Michel-Aceves, A.C.; Guzmán-Maldonado, S.H.; Otero-Sánchez, M.A. Mexican varieties of jamaica (Hibiscus sabdariffa L.) ‘alma blanca’ and ‘rosalíz’ light colored, and ‘cotzaltzin’ y ‘tecoanapa’ red colored. Rev. Fitotec. Mex. 2014, 37, 181–185. [Google Scholar]
- Muhlack, R.A.; Potumarthi, R.; Jeffery, D.W. Sustainable wineries through waste valorisation: A review of grape marc utilisation for value-added products. Waste Manag. 2018, 72, 99–118. [Google Scholar] [CrossRef]
- Bubalo, M.C.; Vidovi’c, S.; Radojˇci’c Redovnikovi’c, I.; Joki’c, S. New perspective in extraction of plant biologically active compounds by green solvents. Food Bioprod. Process. 2018, 109, 52–73. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Meullemiestre, A.; Turk, M.; Perino, S.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Review of Green Food Processing techniques. Preservation, transformation, and extraction. Innov. Food Sci. Emerg. Technol. 2017, 41, 357–377. [Google Scholar] [CrossRef]
- Romero-Díez, R.; Matosb, M.; Rodrigues, L.; Bronze, M.R.; Rodríguez-Rojo, S.; Cocero, M.J.; Matias, A.A. Microwave and ultrasound pre-treatments to enhance anthocyanins extraction from different wine lees. Food Chem. 2019, 272, 258–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Shen, B.J.; Xie, D.H.; Cai, B.C.; Qin, K.M.; Cai, H. Optimization of ultrasound-assisted extraction of phenolic compounds from Cimicifugae rhizoma with response surface methodology. Pharmacogn. Mag. 2015, 11, 682–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shirsath, S.R.; Sonawane, S.H.; Gogate, P.R. Intensification of extraction of natural products using ultrasonic irradiations—A review of current status. Chem. Eng. Process. 2012, 53, 10–23. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, Q.J.; Li, X.; Luo, Y.; Liu, H. Extraction and HPLC Characterization of Chlorogenic Acid from Tobacco Residuals. Sep. Sci. Technol. 2007, 42, 3481–3492. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.F.; Yang, X.H.; Zhao, L.D.; Wang, Y. Ultrasonic-assisted extraction of epimedin C from fresh leaves of Epimedium and extraction mechanism. Innov. Food Sci. Emerg. Technol. 2009, 10, 54–60. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoidcontents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- AOAC. Official Method 2005.02 Total Monomeric Anthocyanin Pigment Content of Fruit Juices, Beverages, Natural Colorants, and Wines. J. AOAC Int. 2005, 88, 1269. [Google Scholar]
- Broadhurst, R.B.; Jones, W.T. Analysis of condensed tannins using acidified vanillin. J. Sci. Food Agric. 1978, 48, 788–794. [Google Scholar] [CrossRef]
- Brand-Wiliams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Odumosu, P.; Ojerinde, S.; Egbuchiem, M. Polyphenolic contents of some instant tea brands and their anti-oxidant activities. J. Appl. Pharm. Sci. 2015, 5, 100–105. [Google Scholar] [CrossRef] [Green Version]
- Olive, P.L.; Bánath, J.P. The comet assay: A method to measure DNA damage in individual cells. Nat. Protoc. 2006, 1, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.-P.; Zhou, Y.; Zheng, J.; Li, S.; Li, A.-N.; Li, H.-B. Optimization of ultrasound-assisted extraction of natural antioxidants from the flower of Jatropha integerrima by response surface methodology. Molecules 2016, 21, 18–29. [Google Scholar] [CrossRef] [Green Version]
- Toma, M.; Vinatoru, M.; Paniwnyk, L.; Mason, T.J. Investigation of the effects of ultrasound on vegetal tissues during solvent extraction. Ultrason. Sonochem. 2001, 8, 137–142. [Google Scholar] [CrossRef]
- Maran, J.P.; Manikandan, S.; Vigna Nivetha, C.; Dinesh, R. Ultrasound assisted extraction of bioactive compounds from Nephelium lappaceum L. fruit peel using central composite face centered response surface design. Arab. J. Chem. 2013, 10, S1145–S1157. [Google Scholar] [CrossRef] [Green Version]
- Xu, D.-P.; Zheng, J.; Zhou, Y.; Li, Y.; Li, S.; Li, H.-B. Ultrasound-assisted extraction of natural antioxidants from the flower of Limonium sinuatum: Optimization and comparison with conventional methods. Food Chem. 2017, 217, 552–559. [Google Scholar] [CrossRef]
- Yang, L.; Jiang, J.G.; Li, W.F.; Chen, J.; Wang, D.Y.; Zhu, L. Optimum extraction process of polyphenols from the bark of Phyllanthus emblica L. based on the response surface methodology. J. Sep. Sci. 2009, 32, 1437–1444. [Google Scholar] [CrossRef]
- Wijngaard, H.; Hossain, M.B.; Rai, D.K.; Brunton, N. Techniques to extract bioactive compounds from food by-products of plant origin. Food Res. Int. 2012, 46, 505–513. [Google Scholar] [CrossRef]
- Rodríguez-Rojo, S.; Visentin, A.; Maestri, D.; Cocero, M.J. Assisted extraction of rosemary antioxidants with green solvents. J. Food Eng. 2012, 109, 98–103. [Google Scholar] [CrossRef] [Green Version]
- Koffi, E.; Sea, T.; Dodehe, Y.; Soro, S. Effect of solvent type on extraction of polyphenols from twenty three Ivorian plants. J. Anim. Plant Sci. 2010, 5, 550–558. [Google Scholar]
- Anokwuru, C.P.; Esiaba, I.; Ajibaye, O.; Adesuyi, A.O. Polyphenolic Content and Antioxidant Activity of Hibiscus sabdariffa Calix. Res. J. Med. Plant 2011, 5, 557–566. [Google Scholar] [CrossRef]
- Reyes-Luengas, A.; Salinas-Moreno, Y.; Ovando-Cruz, M.E.; Arteaga-Garibay, R.I.; Martínez-Peña, M.D. Analysis of phenolic acids and antioxidant activity of aqueous extracts of jamaica (Hibiscus sabdariffa L.) varieties with calyxes of different colors. Agrociencia 2015, 49, 277–290. [Google Scholar]
- Almahy, H.A.; Abdel-Razik, H.H.; El-Badry, Y.A.; Ibrahim, A.M. Ultrasound-Assisted Extraction of Anthocyanin Pigments from Hibiscus sabdariffa (Rosella) and its Phytochemical Activity at Kingdom of Saudi Arabia. Int. J. Chem. Sci. 2017, 15, 196. [Google Scholar]
- Pinela, J.; Prieto, M.A.; Pereira, E.; Jabeur, I.; Filomena Barreiro, M.; Barros, L.; Ferreira Isabel, C.F.R. Optimization of heat-and ultrasound-assisted extraction of anthocyanins from Hibiscus sabdariffa calyces for natural food colorants. Food Chem. 2019, 275, 309–321. [Google Scholar] [CrossRef] [Green Version]
- Zong-cai, T.U.; Yue-bin, Y.; Ying, J.; Li, L.C.; Yuan-wen, M. Optimization of Technology for Ultrasonic-assisted Extraction of Anthocyanin from Roselle (Hibiscus Sabdariffa L.). Food Res. Dev. 2011, 10. [Google Scholar]
- Salazar-González, C.; Vergara-Balderas, F.T.; Ortega-Regules, A.E.; Guerrero-Beltrán, J.Á. Antioxidant properties and color of Hibiscus sabdariffa extracts. Cienc. Investig. Agrar. 2012, 39, 79–90. [Google Scholar] [CrossRef] [Green Version]
- Bergmeier, D.; Dalberto Berres, P.H.; Filippi, D.; Bilibio, D.; Bettiol, V.R.; Wagner Luiz, P. Extraction of total polyphenols from hibiscus (Hibiscus sabdariffa L.) and waxweed/‘sete-sangrias’ (Cuphea carthagenensis) and evaluation of their antioxidant potential. Acta Sci. Technol. 2014, 36, 545–551. [Google Scholar] [CrossRef] [Green Version]
- Sirag, N.; Elhadi, M.M.; Algaili, M.A.; Hozeifa, M.H.; Mohamed, O. Determination of total phenolic content and antioxidant activity of Roselle (Hibiscus sabdariffa L.) Calyx ethanolic extract. Stand. Res. J. Pharm. Pharmacol. 2014, 2, 34–39. [Google Scholar]
- Cai, Y.; Luo, Q.; Sun, M.; Corke, H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004, 74, 2157–2184. [Google Scholar] [CrossRef]
- Rebaya, A.; Belghith Souad, I.; Baghdikian, B.; Mahiou Leddet, V.; Mabrouki, F.; Olivier, E.; Kalthoum Cherif, J.; Trabelsi Ayadi, M. Total Phenolic, Total Flavonoid, Tannin Content, and Antioxidant Capacity of Halimium halimifolium (Cistaceae). J. Appl. Pharm. Sci. 2016, 5, 52–57. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.N.; Chan, K.C.; Lin, W.T.; Su, S.L.; Wang, C.J.; Peng, C.H. Hibiscus sabdariffa inhibits vascular smooth muscle cell proliferation and migration induced by high glucose—A mechanism involves connective tissue growth factor signals. J. Agric. Food Chem. 2009, 57, 3073–3079. [Google Scholar] [CrossRef] [PubMed]
- Mahadevan, N.; Shivali; Kamboj, P. Hibiscus sabdariffa Linn. A ovierview. Nat. Prod. Radiance 2009, 8, 77–83. [Google Scholar]
- Tsai, P.J.; Mcintosh, J.; Pearce, P.; Camden, B.; Jordan, B.R. Anthocyanin and antioxidant capacity in roselle (Hibiscus sabdariffa L.) extract. Food Res. Int. 2002, 35, 351–356. [Google Scholar] [CrossRef]
- Christian, K.R.; Jachson, J.C. Changes in total phenolic and monomeric anthocyanin composition and antioxidant activity of three varieties of sorrel (Hibiscus sabdariffa) during maturity. J. Food Compos. Anal. 2009, 22, 663–667. [Google Scholar] [CrossRef]
- Galicia-Flores, L.A.; Salinas-Moreno, Y.; Espinoza-García, B.M.; Sánchez-Feria, C. Caracterización fisicoquímica y actividad antioxidante de extractos de jamaica (Hibiscus sabdariffa L.) nacional e importada. Rev. Chapingo Ser. Hortic. 2008, 14, 121–129. [Google Scholar]
- Fernández-Arroyo, S.; Rodríguez-Medina, I.C.; Beltrán-Debón, R.; Pasini, F.; Joven, J.; Micol, V.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Quantification of the polyphenolic fraction and in vitro antioxidant and in vivo anti-hyperlipemic activities of Hibiscus sabdariffa aqueous extract. Food Res. Int. 2011, 44, 1490–1495. [Google Scholar] [CrossRef]
- Ramírez-Rodríguez, M.M.; Plaza, M.L.; Azeredo, A.; Balaban, M.O.; Marshall, M.R. Physicochemical and phytochemical properties of cold and hot water extraction from Hibiscus sabdariffa. J. Food Sci. 2011, 76, 428–435. [Google Scholar] [CrossRef]
- Prenesti, E.; Berto, S.; Daniele, P.G.; Toso, S. Antioxidant power quantification of decoction and cold infusions of Hibiscus sabdariffa flowers. Food Chem. 2007, 100, 433–438. [Google Scholar] [CrossRef] [Green Version]
- Liao, W.; MaNutt, M.A.; Zhu, W.G. The comet assay: A sensitive method for detecting DNA damage in individual cells. Methods 2009, 48, 46–53. [Google Scholar] [CrossRef]
- Wang, C.J.; Wang, J.M.; Lin, W.L.; Chu, C.Y.; Chau, F.P.; Tseng, T.H. Protective effect of Hibiscus anthoyanins against tert-butl hydroperoxide-induced hepatic toxicity in rats. Food Chem. Toxicol. 2000, 38, 411–416. [Google Scholar] [CrossRef]
- Lazze, M.; Pizzala, R.; Savio, M.; Stivala, L.; Prosperi, E.; Bianchi, L. Anthocyanins Project against DNA damage induced by tert-butyl-hidroperoxide in rat smooth muscle and hematoma cells. Mutat. Res. Genet. Toxicol. Environ. Mutagenesis 2003, 535, 103–115. [Google Scholar] [CrossRef]
- Tseng, T.H.; Wang, C.J.; Kao, E.S.; Chu, H.Y. Hibiscus protocatechuic acid protects against oxidative damage induced by t-butylhydroperoxide in rat primary hepatocytes. Chem. Biol. Interact. 1996, 101, 137–148. [Google Scholar] [CrossRef]
- Saleh, I.A.; Vinatoru, M.; Mason, T.J.; Abdel-Azim, N.S.; Aboutabl, F.M.; Hammoda, F.M. A possible general mechanism for ultrasound-assisted extraction (UAE) suggested from the results of UAE of chlorogenic acid from Cynara ecolymus L. (artichoke) leaves. Ultrason. Sonochemistry 2016, 31, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Suslick, K.S.; Hammerton, D.A.; Cline, R.E. Sonochemical hot spot. J. Am. Chem. Soc. 1986, 108, 5641–5642. [Google Scholar] [CrossRef]
- Flint, E.B.; Suslick, K.S. The Temperature of Cavitation. Science 1991, 253, 1397–1399. [Google Scholar] [CrossRef] [PubMed]
- Himmel, M.E.; Ding, S.Y.; Johnson, D.K.; Adney, W.S.; Nimlos, M.R.; Brady, J.W.; Foust, T.D. Biomass recalcitrance: Engineering plants and enzymes for biofuels production. Science 2007, 315, 804. [Google Scholar] [CrossRef] [Green Version]
- Jambrak, A.R.; Mason, T.J.; Vesna, L.; Paniwnyk, L.; Hercega, Z. Effect of ultrasound treatment on particle size and molecular weight of whey proteins. J. Food Eng. 2014, 121, 15–23. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.-G.; Meullemiestre, A.; Fabiano-Tixier, A.-S.; Albert-Vian, M. Ultrasound assiste extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochemistry 2017, 34, 540–560. [Google Scholar] [CrossRef]
- Esclapez, M.D.; García-Pérez, J.V.; Mulet, A.; Cárcel, J.A. Ultrasound-Assisted Extraction of Natural Products. Food Eng. Rev. 2011, 3, 108–120. [Google Scholar] [CrossRef]
- Medina-Torres, N.; Ayora-Talavera, T.; Espinosa-Andrews, H.; Sánchez-Contreras, A.; Pacheco, N. Ultrasound Assisted Extraction for the Recovery of Phenolic Compounds from Vegetable Sources. Agronomy 2017, 7, 1–19. [Google Scholar] [CrossRef]



| Time of Sonication | Solids Yield, g/100 g dw | Polyphenols mg GAE/g dw | Flavonoids mg CE/g dw | Anthocyanins mg Cya3G)/g dw | Tannins mg CE/g dw | DPPH, % |
|---|---|---|---|---|---|---|
| Red Calyx | ||||||
| 20 | 9.98 ± 0.39 a | 11.464 ± 0.90 a | 2.201 ± 0.33 a | 1.271 ± 0.54 a | 0.344 ± 0.16 a | 18.40 ± 10.54 a |
| 40 | 14.28 ± 2.77 a | 12.711 ± 0.11 b | 4.186 ± 1.03 b | 1.855 ± 1.07 b | 0.368 ± 10.15 a | 34.36 ± 2.19 b |
| 60 | 20.84 ± 2.26 b | 13.019 ± 0.12 b | 4.416 ± 1.04 b | 1.804 ± 1.05 b | 0.592 ± 0.02 b | 74.58 ± 6.62 c |
| 120 | 28.44 ± 2.26 c | 12.947 ± 0.18 b | 4.981 ± 0.88 b | 1.763 ± 1.02 b | 0.745 ± 0.038 b | 72.07 ± 1.27 c |
| (S-L) * | 33.00 ± 1.697 | 65.287 ± 0.015 | 0.448 ± 0.084 | 0.902 ± 0.151 | 0.188 ± 0.002 | 52.89 ± 4.31 |
| White Calyx | ||||||
| 20 | 6.64 ± 0.89 a | 4.914 ± 0.015 ª | 0.582 ± 0.48 ª | 0.00 ± 0.05 a | 0.003 ± 0.02 a | 12.76 ± 3.35 a |
| 40 | 6.42 ± 8.51 a | 12.581 ± 0.11 b | 3.525 ± 1.34 ª | 0.003 ± 0.01 a | 0.00 ± 0.00 a | 8.11 ± 1.60 a |
| 60 | 13.74 ± 5.12 ª | 12.744 ± 0.08 b,c | 4.527 ± 0.85 b | 0.005 ± 0.00 a | 0.021 ± 0.0 b | 36.95 ± 0.41 b |
| 120 | 21.94 ± 5.80 a | 12.953 ± 0.08 c | 4.868 ± 2.01 b | 0.00 ± 0.07 a | 0.049 ± 0.01 c | 35.29 ± 5.55 b |
| (S-L) * | 20.00 ± 2.828 | 57.244 ± 3.341 | 0.217 ± 0.018 | 0.005 ± 0.00 | 0.000 ± 0.00 | 24.64 ± 12.297 |
| Extraction Conditions | Solid Yield % | Phe | Fla | Anth | Tan | DPPH % | Reference |
|---|---|---|---|---|---|---|---|
| mg/g dw(*) | |||||||
| Ultrasound assisted extraction | |||||||
| - 500 mg, 50 mL (ratio 1:10), Ethanol-water 80:20, 30 °C, 20 min | --- | 6.90 | 4.05 | 17.53 | --- | --- | [35] |
| - Red dried calyces 1.5 g, solid: liquid ratio 230 g/Lt, ethanol-water (26.1–41.7) % (v/v), 426.9 W, 20 kHz, 45 min | --- | --- | --- | 52.94 | --- | --- | [36] |
| - Roselle dried calyx, solid-liquid ratio 1:25 (g/mL), Temperature 50 °C, time 22.5–45 min, ultrasonic power 80 W | --- | --- | --- | 5.66 | --- | --- | [37] |
| Solid- Liquid Extraction | |||||||
| - 20 g, 250 mL ethanol, 72 h. | 12.8 | 27.6 | 33.8 | --- | --- | 69.00 | [33] |
| - 2.5 Red Purple, dry calyces, 25 mL Ethanol: water solutions (50:50 and 70:30 %, v/v), 2 h. | --- | 26.49 | --- | 2.21 | --- | --- | [38] |
| - 2.5 g light red dry calyxes, 100 mL water, boiled 15 min | --- | 15.5 | --- | 4.52 | 0.43 | 65.00 | [34] |
| - 2.5 g dark red dry calyces, 100 mL water, boiled 15 min | --- | 36.50 | --- | 10.99 | 1.04 | 85.00 | [34] |
| - 2.5 g white dry calyxes (“Alma blanca”), 100 mL water, boiled 15 min | --- | 13.5 | --- | 0.20 | 0.24 | 65.00 | [34] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peredo Pozos, G.I.; Ruiz-López, M.A.; Zamora Nátera, J.F.; Álvarez Moya, C.; Barrientos Ramírez, L.; Reynoso Silva, M.; Rodríguez Macías, R.; García-López, P.M.; González Cruz, R.; Salcedo Pérez, E.; et al. Antioxidant Capacity and Antigenotoxic Effect of Hibiscus sabdariffa L. Extracts Obtained with Ultrasound-Assisted Extraction Process. Appl. Sci. 2020, 10, 560. https://0-doi-org.brum.beds.ac.uk/10.3390/app10020560
Peredo Pozos GI, Ruiz-López MA, Zamora Nátera JF, Álvarez Moya C, Barrientos Ramírez L, Reynoso Silva M, Rodríguez Macías R, García-López PM, González Cruz R, Salcedo Pérez E, et al. Antioxidant Capacity and Antigenotoxic Effect of Hibiscus sabdariffa L. Extracts Obtained with Ultrasound-Assisted Extraction Process. Applied Sciences. 2020; 10(2):560. https://0-doi-org.brum.beds.ac.uk/10.3390/app10020560
Chicago/Turabian StylePeredo Pozos, Gregorio Iván, Mario Alberto Ruiz-López, Juan Francisco Zamora Nátera, Carlos Álvarez Moya, Lucia Barrientos Ramírez, Mónica Reynoso Silva, Ramón Rodríguez Macías, Pedro Macedonio García-López, Ricardo González Cruz, Eduardo Salcedo Pérez, and et al. 2020. "Antioxidant Capacity and Antigenotoxic Effect of Hibiscus sabdariffa L. Extracts Obtained with Ultrasound-Assisted Extraction Process" Applied Sciences 10, no. 2: 560. https://0-doi-org.brum.beds.ac.uk/10.3390/app10020560