Facile Solution Synthesis, Processing and Characterization of n- and p-Type Binary and Ternary Bi–Sb Tellurides
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis and Processing of Thermoelectric Nanopowders
2.2. Consolidation of Thermoelectric Nanopowders Using Spark Plasma Sintering (SPS)
2.3. Structural and Morphological Characterization
2.4. Transport Property Evaluation
3. Results and Discussion
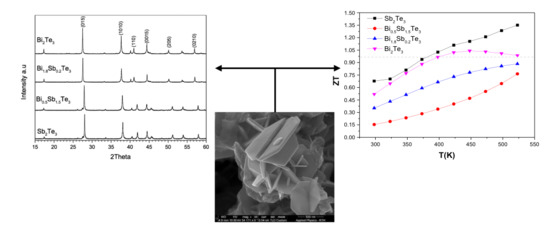
3.1. Structural Analysis
3.2. Morphology and Surface Analysis
3.3. Transport Property Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Boyer, A.; Cissé, E. Properties of thin film thermoelectric materials: Application to sensors using the Seebeck effect. Mater. Sci. Eng. B 1992, 13, 103–111. [Google Scholar]
- Dresselhaus, M.S.; Dresselhaus, G.; Sun, X.; Zhang, Z.; Cronin, S.B.; Koga, T. Low dimensional thermoelectric materials. Phys. Solid State 1999, 41, 679–682. [Google Scholar] [CrossRef]
- Hicks, L.D.; Dresselhaus, M.S. Thermoelectric figure of merit of a one-dimensional conductor. Phys. Rev. B-Condens. Matter Mater. Phys. 1993, 47, 16631–16634. [Google Scholar]
- Hicks, L.D.; Dresselhaus, S.M. Effect of quantum-well structures on the thermoelectric figure of merit. Phys. Rev. 1993, 47, 12727–12731. [Google Scholar]
- Venkatasubramanian, R.; Siivola, E.; Colpitts, T.; Quinn, B.O. Thin-film thermoelectric devices with high room-temperature figures of merit. Nature 2001, 413, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.M.; Sun, X.; Dresselhaus, M. Theoretical investigation of thermoelectric transport properties of cylindrical Bi nanowires. Phys. Rev. B-Condens. Matter Mater. Phys. 2000, 62, 4610–4623. [Google Scholar]
- Mamur, H.; Bhuiyan, M.R.A.; Korkmaz, F.; Nil, M. A review on bismuth telluride (Bi2Te3) nanostructure for thermoelectric applications. Renew. Sustain. Energy Rev. 2018, 82, 4159–4169. [Google Scholar] [CrossRef]
- Madavali, B.; Kim, H.S.; Lee, K.H.; Isoda, Y.; Gascoin, F.; Hong, S.J. Large scale production of high efficient and robust p-type Bi–Sb–Te based thermoelectric materials by powder metallurgy. Mater. Des. 2016, 112, 485–494. [Google Scholar] [CrossRef]
- Serrano-Sánchez, F.; Gharsallah, M.; Nemes, N.M.; Biskup, N.; Varela, M.; Martínez, J.L.; Fernández-Díaz, M.T.; Alonso, J.A. Enhanced figure of merit in nanostructured (Bi,Sb)2Te3 with optimized composition, prepared by a straightforward arc-melting procedure. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Gharsallah, M.; Serrano-Sánchez, F.; Bermúdez, J.; Nemes, N.M.; Martínez, J.L.; Elhalouani, F.; Alonso, J.A. Nanostructured Bi2Te3 Prepared by a Straightforward Arc-Melting Method. Nanoscale Res. Lett. 2016, 11, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Yang, J.; Jiang, Q.; Fu, L.; Xiao, Y.; Li, W.; Zhang, D.; Zhou, Z.; Cheng, Y. Melting and solidification of bismuth antimony telluride under a high magnetic field: A new route to high thermoelectric performance. Nano Energy 2015, 15, 709–718. [Google Scholar] [CrossRef]
- Cao, Y.Q.; Zhao, X.B.; Zhu, T.J.; Zhang, X.B.; Tu, J.P. Syntheses and thermoelectric properties of Bi2Te3 Sb2Te3 bulk nanocomposites with laminated nanostructure. Appl. Phys. Lett. 2008, 92, 90–93. [Google Scholar]
- Cao, Y.Q.; Zhu, T.J.; Zhao, X.B.; Zhang, X.B.; Tu, J.P. Nanostructuring and improved performance of ternary Bi–Sb–Te thermoelectric materials. Appl. Phys. A Mater. Sci. Process. 2008, 92, 321–324. [Google Scholar]
- Jiang, Y.; Zhu, Y.J. Bi2Te3 nanostructures prepared by microwave heating. J. Cryst. Growth 2007, 306, 351–355. [Google Scholar]
- Shi, W.; Zhou, L.; Song, S.; Yang, J.; Zhang, H. Hydrothermal synthesis and thermoelectric transport properties of impurity-free antimony telluride hexagonal nanoplates. Adv. Mater. 2008, 20, 1892–1897. [Google Scholar]
- Li, S.; Zhang, S.; He, Z.; Toprak, M.; Stiewe, C.; Muhammed, M.; Müller, E. Novel solution route synthesis of low thermal conductivity nanocrystalline bismuth telluride. J. Nanosci. Nanotechnol. 2010, 10, 7658–7662. [Google Scholar] [CrossRef]
- Li, S.; Toprak, M.S.; Soliman, H.M.; Zhou, J.; Muhammed, M.; Platzek, D.; Müller, E. Fabrication of nanostructured thermoelectric bismuth telluride thick films by electrochemical deposition. Chem. Mater. 2006, 18, 3627–3633. [Google Scholar]
- Yu, H.; Gibbons, P.C.; Buhro, W.E. Bismuth, tellurium, and bismuth telluride nanowires. J. Mater. Chem. 2004, 14, 595–602. [Google Scholar]
- Li, S.; Liang, Y.; Qin, J.; Toprak, M.; Muhammed, M. Template electrodeposition of ordered bismuth telluride nanowire arrays. J. Nanosci. Nanotechnol. 2009, 9, 1543–1547. [Google Scholar]
- Prieto, A.L.; Sander, M.S.; Martín-González, M.S.; Gronsky, R.; Sands, T.; Stacy, A.M. Electrodeposition of ordered Bi2Te3 nanowire arrays. J. Am. Chem. Soc. 2001, 123, 7160–7161. [Google Scholar]
- Xiao, F.; Yoo, B.; Kyu, H.L.; Myung, N.V. Synthesis of Bi2Te3 nanotubes by galvanic displacement. J. Am. Chem. Soc. 2007, 129, 10068–10069. [Google Scholar]
- Zhao, X.B.; Ji, X.H.; Zhang, Y.H.; Zhu, T.J.; Tu, J.P.; Zhang, X.B. Bismuth telluride nanotubes and the effects on the thermoelectric properties of nanotube-containing nanocomposites. Appl. Phys. Lett. 2005, 86, 062111. [Google Scholar]
- Zhang, C.; Peng, Z.; Li, Z.; Yu, L.; Khor, K.A.; Xiong, Q. Controlled growth of bismuth antimony telluride BixSb2−xTe3 nanoplatelets and their bulk thermoelectric nanocomposites. Nano Energy 2015, 15, 688–696. [Google Scholar]
- Harpeness, R.; Gedanken, A. Microwave-assisted synthesis of nanosized Bi2Se3. New J. Chem. 2003, 27, 1191–1193. [Google Scholar] [CrossRef]
- Zhou, B.; Zhao, Y.; Pu, L.; Zhu, J. Microwave-assisted synthesis of nanocrystalline Bi2Te3. Mater. Chem. Phys. 2006, 96, 192–196. [Google Scholar]
- Yao, Q.; Zhu, Y.; Chen, L.; Sun, Z.; Chen, X. Microwave-assisted synthesis and characterization of Bi2Te3 nanosheets and nanotubes. J. Alloys Compd. 2009, 481, 91–95. [Google Scholar]
- Pradhan, S.; Das, R.; Bhar, R.; Bandyopadhyay, R.; Pramanik, P. A simple fast microwave-assisted synthesis of thermoelectric bismuth telluride nanoparticles from homogeneous reaction-mixture. J. Nanoparticle Res. 2017, 69, 19. [Google Scholar]
- Semenkovich, S.A.; Melekh, B.T. Thermodynamic Properties of Bi2Te3, Bi2Se3, Sb2Te3 and Sb2Se3. In Chemical Bonds in Solids; Springer: New York, NY, USA, 1972; pp. 159–162. [Google Scholar]
- Kosmulski, M. Isoelectric points and points of zero charge of metal (hydr)oxides: 50 years after Parks’ review. Adv. Colloid Interface Sci. 2016, 238, 1–61. [Google Scholar] [CrossRef]
- Crist, B.V. Handbook of Monochromatic XPS Spectra—The Elements and Native Oxides; Volume 1 in PDF; XPS International LLC: Mountain View, CA, USA, 1999. [Google Scholar]
- Scheele, M.; Oeschler, N.; Veremchuk, I.; Reinsberg, K.G.; Kreuziger, A.M.; Kornowski, A.; Broekaert, J.; Klinke, C.; Weller, H. ZT enhancement in solution-grown Sb(2−x)BixTe3 nanoplatelets. ACS Nano 2010, 4, 4283–4291. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Dyck, J.S.; Hernandez, B.M.; Burda, C. Enhancing thermoelectric performance of ternary nanocrystals through adjusting carrier concentration. J. Am. Chem. Soc. 2010, 132, 4982–4983. [Google Scholar] [CrossRef]
- Poudel, B.; Hao, Q.; Ma, Y.; Lan, Y.; Minnich, A.; Yu, B.; Yan, X.; Wang, D.; Muto, A.; Vashaee, D.; et al. High-thermoelectric performance of nanostructured bismuth antimony telluride bulk alloys. Science 2008, 320, 634–638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Z.; Wu, H.; Zhu, T.; Fu, C.; Liu, X.; Hu, L.; He, J.; He, J.; Zhao, X. Attaining high mid-Temperature performance in (Bi,Sb)2Te3 thermoelectric materials via synergistic optimization. NPG Asia Mater. 2016, 8, e302. [Google Scholar] [CrossRef]
- Sehr, R.; Testardi, L.R. The optical properties of p-type Bi2Te3-Sb2Te3 alloys between 2–15 microns. J. Phys. Chem. Solids 1962, 23, 1219–1224. [Google Scholar] [CrossRef]
- Hu, L.P.; Zhu, T.J.; Wang, Y.G.; Xie, H.H.; Xu, Z.J.; Zhao, X.B. Shifting up the optimum figure of merit of p-type bismuth telluride-based thermoelectric materials for power generation by suppressing intrinsic conduction. NPG Asia Mater. 2014, 6, e88. [Google Scholar] [CrossRef] [Green Version]
- Charoenphakdee, A.; Yamanaka, S.; Snyder, G.J. Enhancement of Thermoelectric of the Electronic Density of States. Science 2008, 321, 1457–1461. [Google Scholar]
- Wang, H.; Gibbs, Z.M.; Takagiwa, Y.; Snyder, G.J. Tuning bands of PbSe for better thermoelectric efficiency. Energy Environ. Sci. 2014, 7, 804–811. [Google Scholar] [CrossRef] [Green Version]



| Samples | BE [eV] [30] | Fraction | Assigned to [31] | iep (pH) |
|---|---|---|---|---|
| Bi2Te3 | 157 | 13.11 | Bi | 6.3 |
| 159 | 9.83 | Bi–O (Bi2O3) | ||
| 573 | 8.77 | Te | ||
| 577 | 19.68 | Te–O (TeO2) | ||
| Bi1.8 Sb0.2Te3 | 157 | 6.06 | Bi | 6 |
| 528.3 | 2.08 | Sb | ||
| 573 | 6.5 | Te | ||
| 159 | 4.56 | Bi–O (Bi2O3) | ||
| 530 | 1.42 | Sb–O (Sb2O3) | ||
| 577 | 10.35 | Te–O (TeO2) | ||
| Bi0.5 Sb1.5Te3 | 157 | 2.38 | Bi | 4.5 |
| 528.3 | 12.64 | Sb | ||
| 573 | 11.46 | Te | ||
| 159 | 2.38 | Bi–O (Bi2O3) | ||
| 530 | 8.43 | Sb–O (Sb2O3) | ||
| 577 | 15.7 | Te–O (TeO2) | ||
| Sb2Te3 | 528.3 | 14.92 | Sb | 3.3 |
| 573 | 8.38 | Te | ||
| 530 | 9.94 | Sb–O (Sb2O3) | ||
| 577 | 22.13 | Te–O (TeO2) |
| Sample | Tsint (°C) | thold (min) | Psint (MPa) | ρ % | T (K) | κ (W m−1 K−1) | S (μVK−1) | σ (Ω−1 m−1) | PF (μWK2 cm−1) | ZT |
|---|---|---|---|---|---|---|---|---|---|---|
| Bi2Te3 | 400 | 1 | 50 | 78 | 300 | 0.94788 | −124.624 | 106356.58 | 20.35 | 0.52 |
| 440 | 0.87553 | −159.466 | 80018.39 | 16.52 | 1.04 | |||||
| Bi1.8Sb0.2Te3 | 400 | 1 | 50 | 83 | 300 | 1.33091 | −89.190 | 197582.37 | 17.41 | 0.35 |
| 523 | 1.05234 | −128.942 | 104707.34 | 15.72 | 0.87 | |||||
| Bi0.5Sb1.5Te3 | 400 | 1 | 50 | 78 | 300 | 0.85478 | 104.873 | 40077.90 | 6.81 | 0.15 |
| 523 | 0.46612 | 157.339 | 27504.00 | 4.41 | 0.76 | |||||
| Sb2Te3 | 400 | 1 | 50 | 81 | 300 | 1.11645 | 104.893 | 230549.91 | 21.94 | 0.68 |
| 523 | 0.84984 | 152.565 | 94246.95 | 25.37 | 1.37 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamawandi, B.; Ballikaya, S.; Batili, H.; Roosmark, V.; Orlovská, M.; Yusuf, A.; Johnsson, M.; Szukiewicz, R.; Kuchowicz, M.; Toprak, M.S. Facile Solution Synthesis, Processing and Characterization of n- and p-Type Binary and Ternary Bi–Sb Tellurides. Appl. Sci. 2020, 10, 1178. https://0-doi-org.brum.beds.ac.uk/10.3390/app10031178
Hamawandi B, Ballikaya S, Batili H, Roosmark V, Orlovská M, Yusuf A, Johnsson M, Szukiewicz R, Kuchowicz M, Toprak MS. Facile Solution Synthesis, Processing and Characterization of n- and p-Type Binary and Ternary Bi–Sb Tellurides. Applied Sciences. 2020; 10(3):1178. https://0-doi-org.brum.beds.ac.uk/10.3390/app10031178
Chicago/Turabian StyleHamawandi, Bejan, Sedat Ballikaya, Hazal Batili, Viking Roosmark, Martina Orlovská, Aminu Yusuf, Mats Johnsson, Rafal Szukiewicz, Maciej Kuchowicz, and Muhammet S. Toprak. 2020. "Facile Solution Synthesis, Processing and Characterization of n- and p-Type Binary and Ternary Bi–Sb Tellurides" Applied Sciences 10, no. 3: 1178. https://0-doi-org.brum.beds.ac.uk/10.3390/app10031178