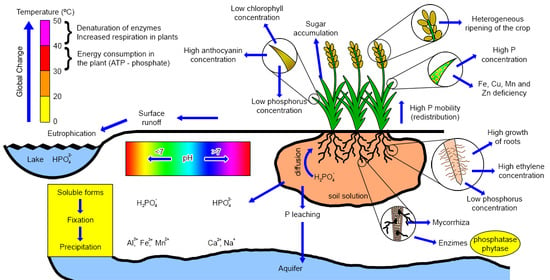
Phosphorus Dynamics in the Soil–Plant–Environment Relationship in Cropping Systems: A Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Selection of Search Criteria
2.1.1. Recent Results: Starting When? When Will We Apply Results from Other Years?
2.1.2. Priority Geographical Location
2.1.3. Agronomic Criteria
2.1.4. Databases
3. Characteristics of Soil P Pool Processes in the Soil
4. Phosphorus Dynamics in the Plant
5. Role of Soil Microbiota on P Processes
6. Phosphorus Dynamics and Effects of Climate Change
7. Efficiency of P Fertilizers in Agronomic Management and Environmental Impact
8. Alternative Sources of Phosphate Fertilizers of Potential Use in Agriculture
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Akiyama, K.; Matsuzaki, K.-I.; Hayashi, H. Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 2005, 435, 824–827. [Google Scholar] [CrossRef] [PubMed]
- Abel, S.; Ticconi, C.A.; Delatorre, C.A. Phosphate sensing in higher plants. Physiol. Plant. 2002, 115, 1–8. [Google Scholar] [CrossRef]
- Puga, M.I.; Rojas-Triana, M.; de Lorenzo, L.; Leyva, A.; Rubio, V.; Paz-Ares, J. Novel signals in the regulation of Pi starvation responses in plants: Facts and promises. Curr. Opin. Plant Biol. 2017, 39, 40–49. [Google Scholar] [CrossRef]
- Wang, F.; Deng, M.; Xu, J.; Zhu, X.; Mao, C. Molecular mechanism of phosphate signaling in plants. Semin. Cell Dev. Biol. 2018, 74, 114–122. [Google Scholar] [CrossRef]
- Fernandes, A.M.; Soratto, R.P.; Gonsales, J.R. Root morphology and phosphorus uptake by potato cultivars grown under deficient and sufficient phosphorus supply. Sci. Hortic. 2014, 180, 190–198. [Google Scholar] [CrossRef]
- Chen, F.-S.; Niklas, K.J.; Liu, Y.; Fang, X.-M.; Wan, S.-Z.; Wang, H. Nitrogen and phosphorus additions alter nutrient dynamics but not resorption efficiencies of Chinese fir leaves and twigs differing in age. Tree Physiol. 2015, 35, 1106–1117. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Xie, D.; Ni, J.; Zeng, X. Optimizing phosphate fertilizer application to reduce nutrient loss in a mustard (Brassica juncea var. tumida)-maize (Zea mays L.) rotation system in Three Gorges Reservoir area. Soil Tillage Res. 2019, 190, 78–85. [Google Scholar] [CrossRef]
- Adimassu, Z.; Mekonnen, K.; Yirga, C.; Kessler, A. Effect of soil bunds on runoff, soil and nutrient losses, and crop yield in the central highlands of ethiopia. Land Degrad. Dev. 2014, 25, 554–564. [Google Scholar] [CrossRef]
- Bai, J.; Ye, X.; Jia, J.; Zhang, G.; Zhao, Q.; Cui, B.; Liu, X. Phosphorus sorption-desorption and effects of temperature, pH and salinity on phosphorus sorption in marsh soils from coastal wetlands with different flooding conditions. Chemosphere 2017, 188, 677–688. [Google Scholar] [CrossRef] [PubMed]
- Dimkpa, C.O.; Singh, U.; Bindraban, P.S.; Adisa, I.O.; Elmer, W.H.; Gardea-Torresdey, J.L.; White, J.C. Addition-omission of zinc, copper, and boron nano and bulk oxide particles demonstrate element and size -specific response of soybean to micronutrients exposure. Sci. Total Environ. 2019, 665, 606–616. [Google Scholar] [CrossRef]
- Qin, H.-L.; Quan, Z.; Liu, X.-L.; Li, M.-D.; Zong, Y.; Wu, J.-S.; Wei, W.-X. Phosphorus Status and Risk of Phosphate Leaching Loss from Vegetable Soils of Different Planting Years in Suburbs of Changsha, China. Agric. Sci. China 2010, 9, 1641–1649. [Google Scholar] [CrossRef]
- Fink, J.R.; Inda, A.V.; Bavaresco, J.; Barrón, V.; Torrent, J.; Bayer, C. Adsorption and desorption of phosphorus in subtropical soils as affected by management system and mineralogy. Soil Tillage Res. 2016, 155, 62–68. [Google Scholar] [CrossRef]
- Li, Z.; Sheng, Y.; Yang, J.; Burton, E. Phosphorus release from coastal sediments: Impacts of the oxidation-reduction potential and sulfide. Mar. Pollut. Bull. 2016, 113, 176–181. [Google Scholar] [CrossRef]
- Barbieri, D.M.; Júnior, J.M.; Pereira, G.T.; La Scala, N.; Siqueira, D.S.; Panosso, A.R. Comportamento dos óxidos de ferro da fração argila e do fósforo adsorvido, em diferentes sistemas de colheita de cana-de-açúcar. Rev. Bras. Ciência Solo 2013, 37, 1557–1568. [Google Scholar] [CrossRef] [Green Version]
- De Oliveira, C.M.B.; Gatiboni, L.C.; Miquelluti, D.J.; Smyth, T.J.; Almeida, J.A. Capacidade máxima de adsorção de fósforo e constante de energia em Latossolo Bruno em razão de diferentes ajustes do modelo Langmuir. Rev. Bras. Ciência Solo 2014, 38, 1805–1815. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.; Kour, D.; Yadav, A.N.; Saxena, R.; Rai, P.; Jyoti, A.; Tomar, R.S. Biodiversity of methylotrophic microbial communities and their potential role in mitigation of abiotic stresses in plants. Biology 2019, 74, 287–308. [Google Scholar] [CrossRef]
- Rezakhani, L.; Motesharezadeh, B.; Tehrani, M.M.; Etesami, H.; Hosseini, H.M. Phosphate–solubilizing bacteria and silicon synergistically augment phosphorus (P) uptake by wheat (Triticum aestivum L.) plant fertilized with soluble or insoluble P source. Ecotoxicol. Environ. Saf. 2019, 173, 504–513. [Google Scholar] [CrossRef]
- Jansa, J.; Finlay, R.D.; Wallander, H.; Smith, F.A.; Smith, S.E. Role of Mycorrhizal Symbioses in Phosphorus Cycling. In Soil Biology; Springer: Berlin/Heidelberg, Germany, 2011; pp. 137–168. [Google Scholar]
- Reetz, H.F. Fertilizers and Their Efficient Use; International Fertilizer industry Association, IFA: Paris, France, 2016. [Google Scholar]
- Everaert, M.; da Silva, R.C.; Degryse, F.; McLaughlin, M.J.; Smolders, E. Limited Dissolved Phosphorus Runoff Losses from Layered Double Hydroxide and Struvite Fertilizers in a Rainfall Simulation Study. J. Environ. Qual. 2018, 47, 371–377. [Google Scholar] [CrossRef]
- Roose, E. Introduction a la Gestion Conservatoire de l’Eau, de la Biomasse et de la Fertilite des Sols (GCES); FAO: Rome, Italy, 1994. [Google Scholar]
- Bergström, L.; Kirchmann, H.; Djodjic, F.; Kyllmar, K.; Ulén, B.; Liu, J.; Andersson, H.; Aronsson, H.; Börjesson, G.; Kynkäänniemi, P.; et al. Turnover and Losses of Phosphorus in Swedish Agricultural Soils: Long-Term Changes, Leaching Trends, and Mitigation Measures. J. Environ. Qual. 2015, 44, 512–523. [Google Scholar] [CrossRef] [Green Version]
- Schindler, D.W.; Carpenter, S.; Chapra, S.C.; Hecky, R.E.; Orihel, D. Reducing Phosphorus to Curb Lake Eutrophication is a Success. Environ. Sci. Technol. 2016, 50, 8923–8929. [Google Scholar] [CrossRef]
- Roy, E.; Willig, E.; Richards, P.D.; Martinelli, L.; Vazquez, F.F.; Pegorini, L.; Spera, S.; Porder, S. Soil phosphorus sorption capacity after three decades of intensive fertilization in Mato Grosso, Brazil. Agric. Ecosyst. Environ. 2017, 249, 206–214. [Google Scholar] [CrossRef]
- Cavalcante, H.; Araújo, F.; Noyma, N.; Becker, V. Phosphorus fractionation in sediments of tropical semiarid reservoirs. Sci. Total Environ. 2018, 619-620, 1022–1029. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Ou, Y.; Wang, L.; Wu, H.; Yan, B.; Li, Y. Distribution and release of phosphorus fractions associated with soil aggregate structure in restored wetlands. Chemosphere 2019, 223, 319–329. [Google Scholar] [CrossRef]
- Parent, S.; Dossou-Yovo, W.; Ziadi, N.; Leblanc, M.; Tremblay, G.; Pellerin, A.; Parent, L. Corn response to banded phosphorus fertilizers with or without manure application in Eastern Canada. Agron. J. 2020, 112, 2176–2187. [Google Scholar] [CrossRef]
- Manghabati, H.; Weis, W.; Göttlein, A. Changes in phosphorus concentration in needles of adult Norway spruce–nutrient re-translocation or dilution effect? Eur. J. For. Res. 2019, 138, 539–546. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Zhou, X.; Dong, L.; Guo, J.; Chen, Y.; Zhang, Y.; Wu, L.; Xu, M. iTRAQ-based analysis of the Arabidopsis proteome reveals insights into the potential mechanisms of anthocyanin accumulation regulation in response to phosphate deficiency. J. Proteom. 2018, 184, 39–53. [Google Scholar] [CrossRef]
- Mo, X.; Zhang, M.; Liang, C.; Cai, L.; Tian, J. Integration of metabolome and transcriptome analyses highlights soybean roots responding to phosphorus deficiency by modulating phosphorylated metabolite processes. Plant Physiol. Biochem. 2019, 139, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Cuq, S.; Lemetter, V.; Kleiber, D.; Levasseur-Garcia, C. Assessing macro- (P, K, Ca, Mg) and micronutrient (Mn, Fe, Cu, Zn, B) concentration in vine leaves and grape berries of vitis vinifera by using near-infrared spectroscopy and chemometrics. Comput. Electron. Agric. 2020, 179, 105841. [Google Scholar] [CrossRef]
- Spohn, M. Increasing the organic carbon stocks in mineral soils sequesters large amounts of phosphorus. Glob. Chang. Biol. 2020, 26, 4169–4177. [Google Scholar] [CrossRef]
- Divito, G.A.; Sadras, V.O. How do phosphorus, potassium and sulphur affect plant growth and biological nitrogen fixation in crop and pasture legumes? A meta-analysis. Field Crop. Res. 2014, 156, 161–171. [Google Scholar] [CrossRef]
- Marra, L.M.; Soares, C.R.F.S.; De Oliveira, S.M.; Ferreira, P.A.A.; Soares, B.L.; Carvalho, R.D.F.; Lima, J.; Moreira, F.M.D.S. Biological nitrogen fixation and phosphate solubilization by bacteria isolated from tropical soils. Plant Soil 2012, 357, 289–307. [Google Scholar] [CrossRef]
- Wurzburger, N.; Bellenger, J.P.; Kraepiel, A.M.L.; Hedin, L.O. Molybdenum and Phosphorus Interact to Constrain Asymbiotic Nitrogen Fixation in Tropical Forests. PLoS ONE 2012, 7, e33710. [Google Scholar] [CrossRef]
- Mus, F.; Crook, M.B.; Garcia, K.; Costas, A.G.; Geddes, B.A.; Kouri, E.D.; Paramasivan, P.; Ryu, M.-H.; Oldroyd, G.E.; Poole, P.S.; et al. Symbiotic Nitrogen Fixation and the Challenges to Its Extension to Nonlegumes. Appl. Environ. Microbiol. 2016, 82, 3698–3710. [Google Scholar] [CrossRef] [Green Version]
- Hammond, L.L.; Chien, S.H.; Roy, A.H.; Mokwunye, A.U. Solubility and agronomic effectiveness of partially acidulated phosphate rocks as influenced by their iron and aluminium oxide content. Nutr. Cycl. Agroecosystems 1989, 19, 93–98. [Google Scholar] [CrossRef]
- Prochnow, L.I.; Chien, S.H.; Taylor, R.W.; Carmona, G.; Henao, J.; Dillard, E.F. Characterization and Agronomic Evaluation of Single Superphosphates Varying in Iron Phosphate Impurities. Agron. J. 2003, 95, 293–302. [Google Scholar] [CrossRef]
- Barreto, M.S.C.; Mattiello, E.M.; Santos, W.O.; Melo, L.C.A.; Vergütz, L.; Novais, R.F. Agronomic efficiency of phosphate fertilizers produced by the re-use of a metallurgical acid residue. J. Environ. Manag. 2018, 208, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Shaviv, A.; Mikkelsen, R.L. Controlled-release fertilizers to increase efficiency of nutrient use and minimize environmental degradation–A review. Nutr. Cycl. Agroecosystems 1993, 35, 1–12. [Google Scholar] [CrossRef]
- Shaviv, A. Advances in controlled-release fertilizers. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2001; Volume 71, pp. 1–49. [Google Scholar]
- Eduah, J.O.; Henriksen, S.W.; Nartey, E.K.; Abekoe, M.K.; Andersen, M.N. Nonlinear sorption of phosphorus onto plant biomass-derived biochars at different pyrolysis temperatures. Environ. Technol. Innov. 2020, 19, 100808. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, B.; Chen, J.; Li, Y.; Creamer, A.E.; Chen, H. Slow-release fertilizer encapsulated by graphene oxide films. Chem. Eng. J. 2014, 255, 107–113. [Google Scholar] [CrossRef]
- Andelkovic, I.B.; Kabiri, S.; Tavakkoli, E.; Kirby, J.K.; McLaughlin, M.J.; Losic, D. Graphene oxide-Fe(III) composite containing phosphate–A novel slow release fertilizer for improved agriculture management. J. Clean. Prod. 2018, 185, 97–104. [Google Scholar] [CrossRef]
- Meyer, G.; Frossard, E.; Mäder, P.; Nanzer, S.; Randall, D.; Udert, K.M.; Oberson, A. Water soluble phosphate fertilizers for crops grown in calcareous soils–an outdated paradigm for recycled phosphorus fertilizers? Plant Soil 2018, 424, 367–388. [Google Scholar] [CrossRef]
- Fardeau, J.C.; Martinez, J. Pig Slurry Applications: Effects on Bioavailable Phosphorus and Cation Concentration in Soil Solution [P Dyer, isotopic exchange, P migration, K migration]. Agron. Fr. 1996, 16, 153–166. [Google Scholar] [CrossRef]
- Barrow, N.J. A mechanistic model for describing the sorption and desorption of phosphate by soil. J. Soil Sci. 1983, 34, 733–750. [Google Scholar] [CrossRef]
- Gustafsson, J.P.; Mwamila, L.B.; Kergoat, K. The pH dependence of phosphate sorption and desorption in Swedish agricultural soils. Geoderma 2012, 189-190, 304–311. [Google Scholar] [CrossRef] [Green Version]
- Hesterberg, D. Macroscale Chemical Properties and X-Ray Absorption Spectroscopy of Soil Phosphorus. In Global Change and Forest Soils; Elsevier: Amsterdam, The Netherlands, 2010; Volume 34, pp. 313–356. [Google Scholar]
- Balemi, T.; Negisho, K. Management of soil phosphorus and plant adaptation mechanisms to phosphorus stress for sustainable crop production: A review. J. Soil Sci. Plant Nutr. 2012, 12, 547–562. [Google Scholar] [CrossRef] [Green Version]
- Han, W.; Fang, J.; Guo, D.; Zhang, Y. Leaf nitrogen and phosphorus stoichiometry across 753 terrestrial plant species in China. New Phytol. 2005, 168, 377–385. [Google Scholar] [CrossRef]
- Vengavasi, K.; Pandey, R. Root exudation potential in contrasting soybean genotypes in response to low soil phosphorus availability is determined by photo-biochemical processes. Plant Physiol. Biochem. 2018, 124, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chi, L.; Mendoza-Vega, J.; Huerta, E.; Álvarez-Solís, J.D. Effect of Long-Term Sugarcane (Saccharum Spp.) Cultivation on Chemical and Physical Properties of Soils in Belize. Commun. Soil Sci. Plant Anal. 2017, 7, 123. [Google Scholar] [CrossRef]
- Bezerra, A.A.D.C.; Filho, R.S.D.C.; De Oliveira, S.R.M.; Filho, F.R.F. Morphophysiological biometry and grain production in cowpea under different phosphorus levels. Comun. Sci. 2018, 9, 275–281. [Google Scholar] [CrossRef]
- Le Noë, J.; Billen, G.; Esculier, F.; Garnier, J. Long-term socioecological trajectories of agro-food systems revealed by N and P flows in French regions from 1852 to 2014. Agric. Ecosyst. Environ. 2018, 265, 132–143. [Google Scholar] [CrossRef]
- Adam, M.; Dzotsi, K.; Hoogenboom, G.; Traore, P.C.S.; Porter, C.; Rattunde, H.; Nebie, B.; Leiser, W.; Weltzien, E.; Jones, J. Modelling varietal differences in response to phosphorus in West African sorghum. Eur. J. Agron. 2018, 100, 35–43. [Google Scholar] [CrossRef]
- Vandamme, E.; Rose, T.; Saito, K.; Jeong, K.; Wissuwa, M. Integration of P acquisition efficiency, P utilization efficiency and low grain P concentrations into P-efficient rice genotypes for specific target environments. Nutr. Cycl. Agroecosyst. 2016, 104, 413–427. [Google Scholar] [CrossRef]
- Kleinman, P.J.A. The Persistent Environmental Relevance of Soil Phosphorus Sorption Saturation. Curr. Pollut. Rep. 2017, 3, 141–150. [Google Scholar] [CrossRef] [Green Version]
- Edwards, A.C.; Withers, P.J.A. Soil phosphorus management and water quality: A UK perspective. Soil Use Manag. 1998, 14, 124–130. [Google Scholar] [CrossRef]
- Heathwaite, A.; Quinn, P.; Hewett, C. Modelling and managing critical source areas of diffuse pollution from agricultural land using flow connectivity simulation. J. Hydrol. 2005, 304, 446–461. [Google Scholar] [CrossRef]
- Withers, P.J.A.; Van Dijk, K.C.; Neset, T.-S.S.; Nesme, T.; Oenema, O.; Rubæk, G.H.; Schoumans, O.F.; Smit, B.; Pellerin, S. Stewardship to tackle global phosphorus inefficiency: The case of Europe. Ambio 2015, 44, 193–206. [Google Scholar] [CrossRef] [Green Version]
- Uusitalo, R.; Lemola, R.; Turtola, E. Surface and Subsurface Phosphorus Discharge from a Clay Soil in a Nine-Year Study Comparing No-Till and Plowing. J. Environ. Qual. 2018, 47, 1478–1486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bieroza, M.; Heathwaite, A.L. Seasonal variation in phosphorus concentration–discharge hysteresis inferred from high-frequency in situ monitoring. J. Hydrol. 2015, 524, 333–347. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Xu, C.-C.; Ridoutt, B.; Wang, X.-C.; Ren, P.-A. Nitrogen and phosphorus losses and eutrophication potential associated with fertilizer application to cropland in China. J. Clean. Prod. 2017, 159, 171–179. [Google Scholar] [CrossRef]
- Hart, M.R.; Quin, B.F.; Nguyen, M.L. Phosphorus Runoff from Agricultural Land and Direct Fertilizer Effects: A Review. J. Environ. Qual. 2004, 33, 1954–1972. [Google Scholar] [CrossRef]
- Shigaki, F.; Sharpley, A.; Prochnow, L.I. Source-Related Transport of Phosphorus in Surface Runoff. J. Environ. Qual. 2006, 35, 2229–2235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumaragamage, D.; Flaten, D.; Akinremi, O.O.; Sawka, C.; Zvomuya, F. Soil test phosphorus changes and phosphorus runoff losses in incubated soils treated with livestock manures and synthetic fertilizer. Can. J. Soil Sci. 2011, 91, 375–384. [Google Scholar] [CrossRef]
- Liu, J.; Cui, L.; Losic, D. Graphene and graphene oxide as new nanocarriers for drug delivery applications. Acta Biomater. 2013, 9, 9243–9257. [Google Scholar] [CrossRef] [PubMed]
- Vadas, P.A.; Good, L.W.; Moore, P.A.; Widman, N. Estimating Phosphorus Loss in Runoff from Manure and Fertilizer for a Phosphorus Loss Quantification Tool. J. Environ. Qual. 2009, 38, 1645–1653. [Google Scholar] [CrossRef]
- Sattari, S.Z.; Van Ittersum, M.; Giller, E.K.; Zhang, F.; Bouwman, A.F. Key role of China and its agriculture in global sustainable phosphorus management. Environ. Res. Lett. 2014, 9, 054003. [Google Scholar] [CrossRef] [Green Version]
- Filippelli, G.M. The Global Phosphorus Cycle: Past, Present, and Future. Elements 2008, 4, 89–95. [Google Scholar] [CrossRef]
- Hammond, J.P.; White, P. Sucrose transport in the phloem: Integrating root responses to phosphorus starvation. J. Exp. Bot. 2007, 59, 93–109. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, I.; Marschner, H. Mechanism of phosphorus-induced zinc deficiency in cotton. III. Changes in physiological availability of zinc in plants Is mail. Physiol. Plant. 1987, 70, 13–20. [Google Scholar] [CrossRef]
- Netzer, F.; Pozzi, L.; Dubbert, D.; Herschbach, C. Improved photosynthesis and growth of poplar during nitrogen fertilization is accompanied by phosphorus depletion that indicates phosphorus remobilization from older stem tissues. Environ. Exp. Bot. 2019, 162, 421–432. [Google Scholar] [CrossRef]
- Carstensen, A.; Herdean, A.; Schmidt, S.B.; Sharma, A.; Spetea, C.; Pribil, M.; Husted, S. The Impacts of Phosphorus Deficiency on the Photosynthetic Electron Transport Chain. Plant Physiol. 2018, 177, 271–284. [Google Scholar] [CrossRef] [Green Version]
- Laliberté, E.; Zemunik, G.; Turner, B.L. Environmental filtering explains variation in plant diversity along resource gradients. Science 2014, 345, 1602–1605. [Google Scholar] [CrossRef] [PubMed]
- Ramaekers, L.; Remans, R.; Rao, I.M.; Blair, M.W.; Vanderleyden, J. Strategies for improving phosphorus acquisition efficiency of crop plants. Field Crop. Res. 2010, 117, 169–176. [Google Scholar] [CrossRef]
- Haling, R.E.; Brown, L.K.; Bengough, G.; Young, I.M.; Hallett, P.; White, P.; George, T.S. Root hairs improve root penetration, root–soil contact, and phosphorus acquisition in soils of different strength. J. Exp. Bot. 2013, 64, 3711–3721. [Google Scholar] [CrossRef] [Green Version]
- Neumann, G. The Role of Ethylene in Plant Adaptations for Phosphate Acquisition in Soils-A Review. Front. Plant Sci. 2016, 6, 1224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Huang, G.; Meng, Q.; Ma, L.; Yuan, L.; Wang, F.; Zhang, W.; Cui, Z.; Shen, J.; Chen, X.; et al. Integrated soil and plant phosphorus management for crop and environment in China. A review. Plant Soil 2011, 349, 157–167. [Google Scholar] [CrossRef]
- Lynch, J.P. Root Phenes for Enhanced Soil Exploration and Phosphorus Acquisition: Tools for Future Crops. Plant Physiol. 2011, 156, 1041–1049. [Google Scholar] [CrossRef] [Green Version]
- Bayuelo-Jiménez, J.S.; Ochoa-Cadavid, I. Phosphorus acquisition and internal utilization efficiency among maize landraces from the central Mexican highlands. Field Crop. Res. 2014, 156, 123–134. [Google Scholar] [CrossRef]
- Shen, J.; Yuan, L.; Zhang, J.; Li, H.; Bai, Z.; Chen, X.; Zhang, W.; Zhang, F. Phosphorus Dynamics: From Soil to Plant. Plant Physiol. 2011, 156, 997–1005. [Google Scholar] [CrossRef] [Green Version]
- Brown, L.K.; George, T.S.; Barrett, G.E.; Hubbard, S.F.; White, P.J. Interactions between root hair length and arbuscular mycorrhizal colonisation in phosphorus deficient barley (Hordeum vulgare). Plant Soil 2013, 372, 195–205. [Google Scholar] [CrossRef]
- Vance, C.P.; Uhde-Stone, C.; Allan, D.L. Phosphorus acquisition and use: Critical adaptations by plants for securing a nonrenewable resource. New Phytol. 2003, 157, 423–447. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Liao, H.; Lucas, W.J. Molecular mechanisms underlying phosphate sensing, signaling, and adaptation in plants. J. Integr. Plant Biol. 2014, 56, 192–220. [Google Scholar] [CrossRef]
- Yuan, H.; Liu, D. Signaling Components Involved in Plant Responses to Phosphate Starvation. J. Integr. Plant Biol. 2008, 50, 849–859. [Google Scholar] [CrossRef]
- Khan, G.A.; Vogiatzaki, E.; Glauser, G.; Poirier, Y. Phosphate Deficiency Induces the Jasmonate Pathway and Enhances Resistance to Insect Herbivory. Plant Physiol. 2016, 171, 632–644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Luo, D.; Ma, G.; Jia, L.; Xu, J.; Huang, H.; Tong, Z.; Lu, Y.-Q. Response of Chinese fir seedlings to low phosphorus stress and analysis of gene expression differences. J. For. Res. 2018, 30, 183–192. [Google Scholar] [CrossRef]
- Rosas, S.B.; Andrés, J.A.; Rovera, M.; Correa, N.S. Phosphate-solubilizing Pseudomonas putida can influence the rhizobia–legume symbiosis. Soil Biol. Biochem. 2006, 38, 3502–3505. [Google Scholar] [CrossRef]
- Fankem, H.; Nwaga, D.; Deubel, A.; Dieng, L.; Merbach, W.; Etoa, F.X. Occurrence and functioning of phosphate solubilizing microorganisms from oil palm tree (Elaeis guineensis) rhizosphere in Cameroon. Afr. J. Biotechnol. 2006, 5, 2450–2460. [Google Scholar]
- Kucey, R.M.N.; Janzen, H.H. Effects of VAM and reduced nutrient availability on growth and phosphorus and micronutrient uptake of wheat and field beans under greenhouse conditions. Plant Soil 1987, 104, 71–78. [Google Scholar] [CrossRef]
- Tao, G.-C.; Tian, S.-J.; Cai, M.-Y.; Xie, G.-H. Phosphate-Solubilizing and -Mineralizing Abilities of Bacteria Isolated from Soils. Pedosphere 2008, 18, 515–523. [Google Scholar] [CrossRef]
- Banik, S.; Datta, M. Effect of inoculation of a phosphate-solubilizing phytohormone producing Bacillus firmus on the growth and yield of soybean (Glycine max), grown in acid soil of Nagaland. Zent. Mikrobiol. 1988, 143, 139–147. [Google Scholar] [CrossRef]
- Richardson, A.E.; Simpson, R.J. Soil Microorganisms Mediating Phosphorus Availability Update on Microbial Phosphorus. Plant Physiol. 2011, 156, 989–996. [Google Scholar] [CrossRef] [Green Version]
- Long, X.-E.; Yao, H.; Huang, Y.; Wei, W.; Zhu, Y.-G. Phosphate levels influence the utilisation of rice rhizodeposition carbon and the phosphate-solubilising microbial community in a paddy soil. Soil Biol. Biochem. 2018, 118, 103–114. [Google Scholar] [CrossRef]
- Hinsinger, P.; Brauman, A.; Devau, N.; Gérard, F.; Jourdan, C.; Laclau, J.-P.; Le Cadre-Barthélémy, E.; Jaillard, B.; Plassard, C. Acquisition of phosphorus and other poorly mobile nutrients by roots. Where do plant nutrition models fail? Plant Soil 2011, 348, 29–61. [Google Scholar] [CrossRef]
- Illmer, P.; Barbato, A.; Schinner, F. Solubilization of hardly-soluble AlPO4 with P-solubilizing microorganisms. Soil Biol. Biochem. 1995, 27, 265–270. [Google Scholar] [CrossRef]
- Richardson, A.E. Regulating the phosphorus nutrition of plants: Molecular biology meeting agronomic needs. Plant Soil 2009, 322, 17–24. [Google Scholar] [CrossRef]
- Dick, W.; Tabatabai, M. Activation of soil pyrophosphatase by metal ions. Soil Biol. Biochem. 1983, 15, 359–363. [Google Scholar] [CrossRef]
- Juma, N.G.; Tabatabai, M.A. Phosphatase activity in corn and soybean roots: Conditions for assay and effects of metals. Plant Soil 1988, 107, 39–47. [Google Scholar] [CrossRef]
- Nannipieri, P.; Giagnoni, L.; Landi, L.; Renella, G. Role of phosphatase enzymes in soil. In Phosphorus in Action. Soil Biology 26; Bünemann, E.K., Oberson, A., Frossard, E., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 215–243. [Google Scholar] [CrossRef]
- Messa, V.R.; Savioli, M.R. Improving sustainable agriculture with arbuscular mycorrhizae. Rhizosphere 2021, 19, 100412. [Google Scholar] [CrossRef]
- Bilyera, N.; Blagodatskaya, E.; Yevdokimov, I.; Kuzyakov, Y. Towards a conversion factor for soil microbial phosphorus. Eur. J. Soil Biol. 2018, 87, 1–8. [Google Scholar] [CrossRef]
- Zhu, Z.; Ge, T.; Luo, Y.; Liu, S.; Xu, X.; Tong, C.; Shibistova, O.; Guggenberger, G.; Wu, J. Microbial stoichiometric flexibility regulates rice straw mineralization and its priming effect in paddy soil. Soil Biol. Biochem. 2018, 121, 67–76. [Google Scholar] [CrossRef]
- Seneweera, S.P.; Conroy, J.P. Growth, grain yield and quality of rice (Oryza sativa L.) in response to elevated CO2 and phosphorus nutrition. Soil Sci. Plant Nutr. 1997, 43, 1131–1136. [Google Scholar] [CrossRef]
- Rogers, G.S.; Payne, L.; Milham, P.; Conroy, J. Nitrogen and phosphorus requirements of cotton and wheat under changing atmospheric CO2 concentrations. Plant Soil 1993, 155-156, 231–234. [Google Scholar] [CrossRef]
- Field, C.B.; Jackson, R.B.; Mooney, H.A. Stomatal responses to increased CO2: Implications from the plant to the global scale. Plant Cell Environ. 1995, 18, 1214–1225. [Google Scholar] [CrossRef]
- Hou, H.; Chen, H.; Cai, H.; Yang, F.; Li, D.; Wang, F. CO2 and N2O emissions from Lou soils of greenhouse tomato fields under aerated irrigation. Atmos. Environ. 2016, 132, 69–76. [Google Scholar] [CrossRef]
- Tian, H.; Drijber, R.; Zhang, J.; Li, X. Impact of long-term nitrogen fertilization and rotation with soybean on the diversity and phosphorus metabolism of indigenous arbuscular mycorrhizal fungi within the roots of maize (Zea mays L.). Agric. Ecosyst. Environ. 2013, 164, 53–61. [Google Scholar] [CrossRef]
- Hidayati, N.A.; Yamada-Oshima, Y.; Iwai, M.; Yamano, T.; Kajikawa, M.; Sakurai, N.; Suda, K.; Sesoko, K.; Hori, K.; Obayashi, T.; et al. Lipid remodeling regulator 1 (LRL 1) is differently involved in the phosphorus-depletion response from PSR 1 in Chlamydomonas reinhardtii. Plant J. 2019, 100, 610–626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, K.; Wu, Y. Rhizosphere calcareous soil P-extraction at the expense of organic carbon from root-exuded organic acids induced by phosphorus deficiency in several plant species. Soil Sci. Plant Nutr. 2014, 60, 640–650. [Google Scholar] [CrossRef]
- IFA International Fertilizer Association. Fertilizer Outlook 2018–2022, Proceedings of the IFA Annual Conference—19–20 June 2018 Berlin (Germany); IFA Report “Medium-Term Outlook for World Agriculture and Fertilizer Demand: 2017/18-2022/23; IFA International Fertilizer Association: Paris, France.
- Schoumans, O.F.; Bouraoui, F.; Kabbe, C.; Oenema, O.; Van Dijk, K.C. Phosphorus management in Europe in a changing world. Ambio 2015, 44, 180–192. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.I.; Gwon, H.S.; Alam, M.A.; Song, H.J.; Das, S.; Kim, P.J. Short term effects of different green manure amendments on the composition of main microbial groups and microbial activity of a submerged rice cropping system. Appl. Soil Ecol. 2020, 147, 103400. [Google Scholar] [CrossRef]
- Garzón, J.E.; Cárdenas, E.A. Emisiones antropogénicas de amoniaco, nitratos y óxido nitroso: Compuestos nitrogenados que afectan el medio ambiente en el sector agropecuario colombiano. Rev. Fac. Med. Vet. Zootec. 2013, 60, 121–138. [Google Scholar]
- Jiang, J.; Wang, Y.; Yu, M.; Cao, N.; Yan, J. Soil organic matter is important for acid buffering and reducing aluminum leaching from acidic forest soils. Chem. Geol. 2018, 501, 86–94. [Google Scholar] [CrossRef]
- Adams, F. Interactions of Phosphorus with Other Elements in Soils and in Plants. In Soil Health Series; Wiley: Hoboken, NJ, USA, 2015; pp. 655–680. [Google Scholar]
- Nash, D.M.; McDowell, R.W.; Condron, L.M.; McLaughlin, M.J. Direct Exports of Phosphorus from Fertilizers Applied to Grazed Pastures. J. Environ. Qual. 2019, 48, 1380–1396. [Google Scholar] [CrossRef]
- Eghball, B.; Sander, D.H.; Skopp, J. Diffusion, Adsorption, and Predicted Longevity of Banded Phosphorus Fertilizer in Three Soils. Soil Sci. Soc. Am. J. 1990, 54, 1161–1165. [Google Scholar] [CrossRef]
- Demand, D.; Schack-Kirchner, H.; Lang, F. Assessment of diffusive phosphate supply in soils by microdialysis. J. Plant Nutr. Soil Sci. 2017, 180, 220–230. [Google Scholar] [CrossRef]
- Li, P.; Weng, J.; Zhang, Q.; Yu, L.; Yao, Q.; Chang, L.; Niu, Q. Physiological and Biochemical Responses of Cucumis melo L. Chloroplasts to Low-Phosphate Stress. Front. Plant Sci. 2018, 9, 1525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, H.; Huang, Y.; Wang, G.; Zhang, X.; Shang, M.; Feng, L.; Dong, J.; Shan, K.; Wu, D.; Zhou, B.; et al. Water eutrophication evaluation based on rough set and petri nets: A case study in Xiangxi-River, Three Gorges Reservoir. Ecol. Indic. 2016, 69, 463–472. [Google Scholar] [CrossRef]
- Barrow, N.; Shaw, T. Sodium bicarbonate as an extractant for soil phosphate, I. Separation of the factors affecting the amount of phosphate displaced from soil from those affecting secondary adsorption. Geoderma 1976, 16, 91–107. [Google Scholar] [CrossRef]
- Bolland, M.D.A.; Gilkes, R.; O’Hara, G. The Chemistry and Agronomic Effectiveness of Phosphate Fertilizers. J. Crop. Prod. 1998, 1, 139–163. [Google Scholar] [CrossRef]
- Zhong, K.; Lin, Z.; Zheng, X.-L.; Jiang, G.-B.; Fang, Y.-S.; Mao, X.-Y.; Liao, Z.-W. Starch derivative-based superabsorbent with integration of water-retaining and controlled-release fertilizers. Carbohydr. Polym. 2013, 92, 1367–1376. [Google Scholar] [CrossRef]
- Jin, S.G.; Yousaf, A.M.; Kim, K.S.; Kim, D.W.; Kim, J.K.; Yong, C.S.; Youn, Y.S.; Kim, J.O.; Choi, H.-G. Influence of hydrophilic polymers on functional properties and wound healing efficacy of hydrocolloid based wound dressings. Int. J. Pharm. 2016, 501, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Tomaszewska, M.; Jarosiewicz, A.; Karakulski, K. Physical and chemical characteristics of polymer coatings in CRF formulation. Desalination 2002, 146, 319–323. [Google Scholar] [CrossRef]
- Wu, L.; Liu, M. Preparation and properties of chitosan-coated NPK compound fertilizer with controlled-release and water-retention. Carbohydr. Polym. 2008, 72, 240–247. [Google Scholar] [CrossRef]
- Ma, J.; Xu, Y.; Zhang, Q.; Zha, L.; Liang, B. Preparation and characterization of pH- and temperature-responsive semi-IPN hydrogels of carboxymethyl chitosan with poly (N-isopropyl acrylamide) crosslinked by clay. Colloid Polym. Sci. 2006, 285, 479–484. [Google Scholar] [CrossRef]
- Le Corre, K.S.; Valsami-Jones, E.; Hobbs, P.; Parsons, S.A. Phosphorus Recovery from Wastewater by Struvite Crystallization: A Review. Crit. Rev. Environ. Sci. Technol. 2009, 39, 433–477. [Google Scholar] [CrossRef] [Green Version]
- Britton, A.; Koch, A.F.; Mavinic, D.S.; Adnan, A.; Oldham, W.K.; Udala, B. Pilot-scale struvite recovery from anaerobic digester supernatant at an enhanced biological phosphorus removal wastewater treatment plant. J. Environ. Eng. Sci. 2005, 4, 265–277. [Google Scholar] [CrossRef] [Green Version]
- Chimenos, J.M.; Fernandez, A.I.; Villalba, G.; Segarra, M.; Urruticoechea, A.; Artaza, B.; Espiell, F. Removal of ammonium and phosphates from wastewater resulting from the process of cochineal extraction using MgO-containing by-product. Water Res. 2003, 37, 1601–1607. [Google Scholar] [CrossRef]
- Capdevielle, A.; Sýkorová, E.; Biscans, B.; Béline, F.; Daumer, M.-L. Optimization of struvite precipitation in synthetic biologically treated swine wastewater—Determination of the optimal process parameters. J. Hazard. Mater. 2013, 244-245, 357–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mattiello, E.M.; Filho, I.D.R.; Barreto, M.S.; Soares, A.R.; da Silva, I.R.; Vergütz, L.; Melo, L.C.; Soares, E.M. Soluble phosphate fertilizer production using acid effluent from metallurgical industry. J. Environ. Manag. 2016, 166, 140–146. [Google Scholar] [CrossRef]
- Santos, W.O.; Hesterberg, D.; Mattiello, E.; Vergutz, L.; Barreto, M.S.C.; Silva, I.R.; Filho, L.F.S.S. Increasing Soluble Phosphate Species by Treatment of Phosphate Rocks with Acidic Waste. J. Environ. Qual. 2016, 45, 1988–1997. [Google Scholar] [CrossRef] [Green Version]
- Mauter, M.S.; Elimelech, M. Environmental Applications of Carbon-Based Nanomaterials. Environ. Sci. Technol. 2008, 42, 5843–5859. [Google Scholar] [CrossRef]
- Lehmann, J.; Gaunt, J.; Rondon, M.B. Bio-char Sequestration in Terrestrial Ecosystems-A Review. Mitig. Adapt. Strat. Glob. Chang. 2006, 11, 403–427. [Google Scholar] [CrossRef]
- Subedi, R.; Taupe, N.; Ikoyi, I.; Bertora, C.; Zavattaro, L.; Schmalenberger, A.; Leahy, J.J.; Grignani, C. Chemically and biologically-mediated fertilizing value of manure-derived biochar. Sci. Total Environ. 2016, 550, 924–933. [Google Scholar] [CrossRef]
- Chen, L.; Chen, X.L.; Zhou, C.H.; Yang, H.M.; Ji, S.F.; Tong, D.S.; Zhong, Z.K.; Yu, W.H.; Chu, M.Q. Environmental-friendly montmorillonite-biochar composites: Facile production and tunable adsorption-release of ammonium and phosphate. J. Clean. Prod. 2017, 156, 648–659. [Google Scholar] [CrossRef]
- Marshall, J.A.; Morton, B.J.; Muhlack, R.; Chittleborough, D.; Kwong, C.W. Recovery of phosphate from calcium-containing aqueous solution resulting from biochar-induced calcium phosphate precipitation. J. Clean. Prod. 2017, 165, 27–35. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [Green Version]
- Kuhn, L.; Gorji, N.E. Review on the graphene/nanotube application in thin film solar cells. Mater. Lett. 2016, 171, 323–326. [Google Scholar] [CrossRef]
- Kiew, S.F.; Kiew, L.V.; Lee, H.B.; Imae, T.; Chung, L.Y. Assessing biocompatibility of graphene oxide-based nanocarriers: A review. J. Control. Release 2016, 226, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Tang, J.; Gooding, J.J. Strategies for chemical modification of graphene and applications of chemically modified graphene. J. Mater. Chem. 2012, 22, 12435–12452. [Google Scholar] [CrossRef]
- Xu, J.; Wang, Y.; Hu, S. Nanocomposites of graphene and graphene oxides: Synthesis, molecular functionalization and application in electrochemical sensors and biosensors. A review. Microchim. Acta 2017, 184, 807–814. [Google Scholar] [CrossRef]
- Jiang, Y.; Biswas, P.; Fortner, J.D. A review of recent developments in graphene-enabled membranes for water treatment. Environ. Sci. Water Res. Technol. 2016, 2, 915–922. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Fal′ko, V.I.; Colombo, L.; Gellert, P.R.; Schwab, M.G.; Kim, K. A roadmap for graphene. Nature 2012, 490, 192–200. [Google Scholar] [CrossRef]



| P Forms | Area | Factors | P Activation Response on Soil or Plant | Crops | Reference |
|---|---|---|---|---|---|
| C:P:N | Stoichiometric relationships | Physiological, cellular, morphological, and molecular factors | Oryza sativa Leymus chinensis Panicum maximum Bouteloua gracilis Populus deltoides | Dias Filho et al., 1992; Elser et al., 2010; Bell et al., 2014; Li et al., 2016; Shang et al., 2018; Zhu et al., 2018. | |
| P deficiency—H2PO4− | Radicle and proteoid root emission | Physiological, cellular, morphological, and molecular factors | mRNA and ethylene synthesis | Arabidiopsis sp. | Ramaekers et al., 2010; Haling et al., 2013; Neumann, 2016. |
| Alteration of biochemical processes | Biosynthesis of secondary metabolites, radicle emission (proteomic hairs) | Anthocyanines | Arabidiopsis sp. | Wang et al., 2018 | |
| Effect of global change on the P dynamics | P dynamics with temperature, water stress, greenhouse gases. | CO2 N2O O3 | Photosynthesis, metabolic expenditure, transpiration, microbial activity | Zea maiz Triticum vulgare Orysa sativa | Goufo et al., 2014 Wang et al., 2014 Hidayati et al., 2019 |
| Environmental impact of phosphatized fertilizers | Coating of the P molecule with different materials from different sources | Loss of soil, runoff, leaching, eutrophication | Zea maiz Triticum vulgare | Shaviv and Mikkelsen, 1993; Novoselov et al., 2012; Andelkovic et al., 2018 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lizcano-Toledo, R.; Reyes-Martín, M.P.; Celi, L.; Fernández-Ondoño, E. Phosphorus Dynamics in the Soil–Plant–Environment Relationship in Cropping Systems: A Review. Appl. Sci. 2021, 11, 11133. https://0-doi-org.brum.beds.ac.uk/10.3390/app112311133
Lizcano-Toledo R, Reyes-Martín MP, Celi L, Fernández-Ondoño E. Phosphorus Dynamics in the Soil–Plant–Environment Relationship in Cropping Systems: A Review. Applied Sciences. 2021; 11(23):11133. https://0-doi-org.brum.beds.ac.uk/10.3390/app112311133
Chicago/Turabian StyleLizcano-Toledo, Rodolfo, Marino Pedro Reyes-Martín, Luisella Celi, and Emilia Fernández-Ondoño. 2021. "Phosphorus Dynamics in the Soil–Plant–Environment Relationship in Cropping Systems: A Review" Applied Sciences 11, no. 23: 11133. https://0-doi-org.brum.beds.ac.uk/10.3390/app112311133