Effect of Citric Acid on Color Changes of Calcium Silicate-Based Cements an In Vitro Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Material Discoloration Measurement
2.3. Statistical Analysis
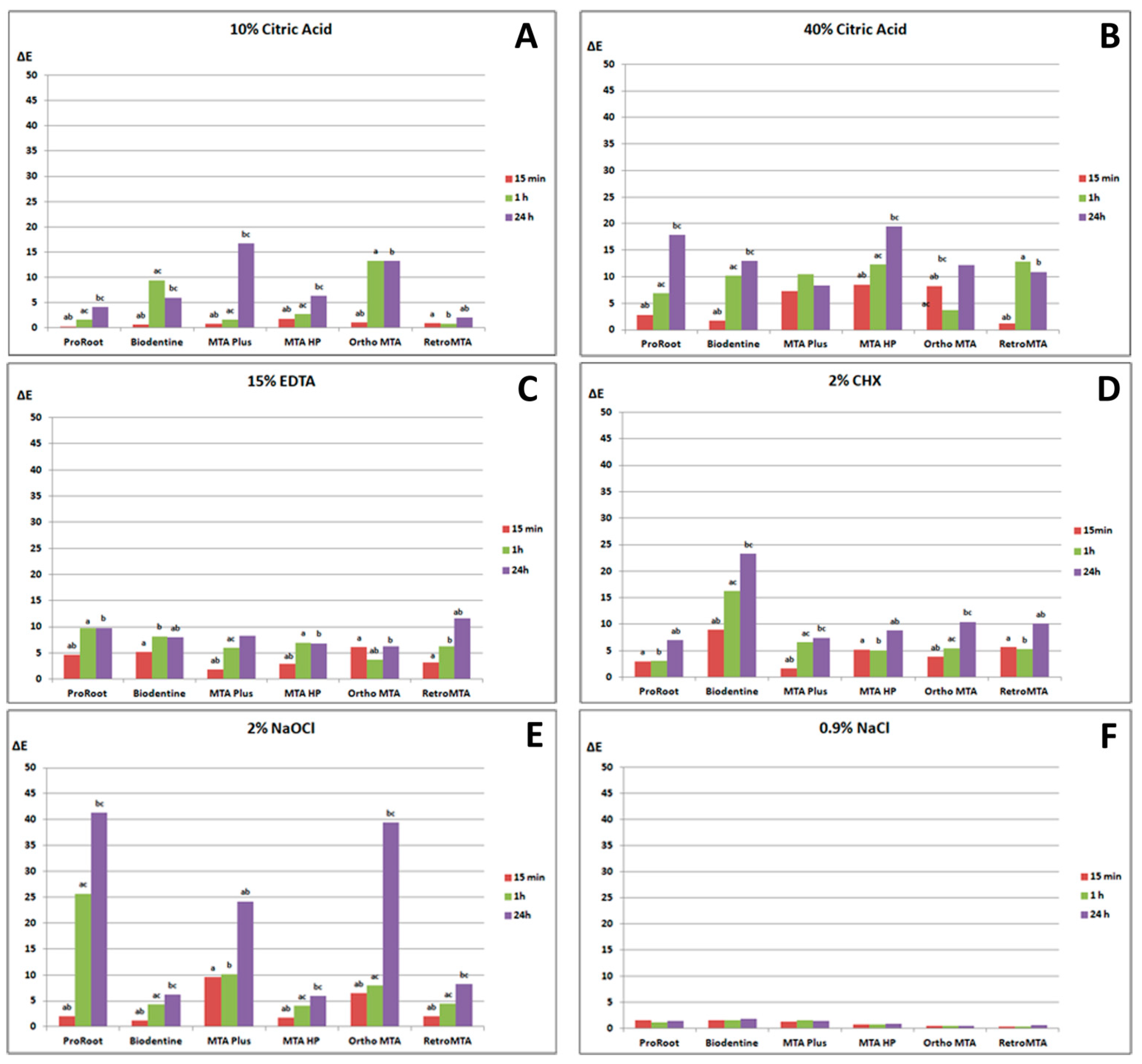
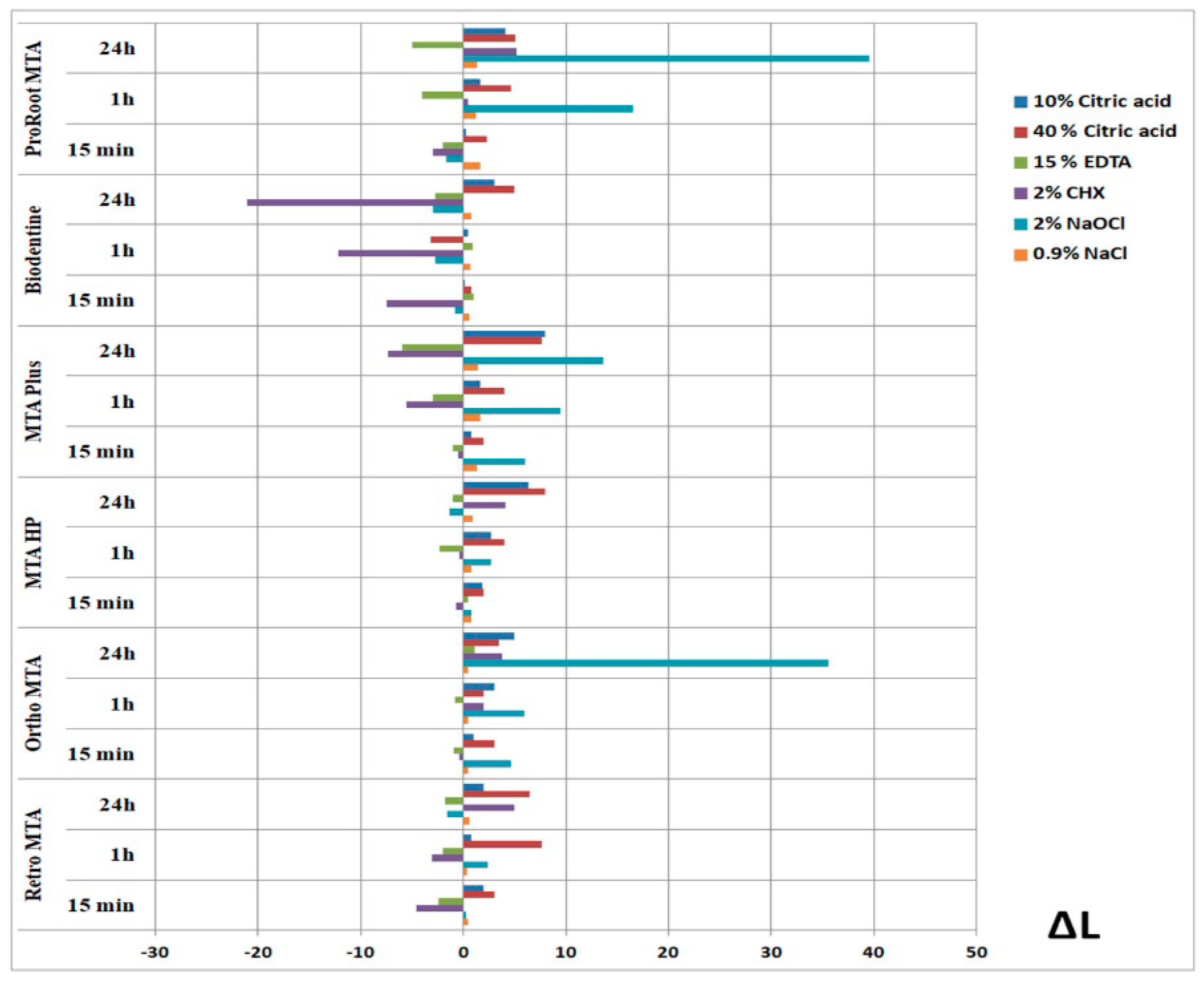
3. Results
3.1. Color Changes Caused by 10% and 40% CA
3.2. Color Changes Caused by 15% EDTA
3.3. Color Changes Caused by 2% CHX
3.4. Color Changes Caused by 2% NaOCl
3.5. Color Changes Caused by 0.9% NaCl
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paula, A.; Carrilho, E.; Laranjo, M.; Abrantes, A.M.; Casalta-Lopes, J.; Botelho, M.F.; Marto, C.M.; Ferreira, M.M. Direct pulp capping: Which is the most effective biomaterial? A retrospective clinical study. Materials 2019, 12, 3382. [Google Scholar] [CrossRef] [Green Version]
- Camilleri, J.; Formosa, L.; Damidot, D. The setting characteristics of MTA Plus in different environmental conditions. Int. Endod. J. 2013, 46, 831–840. [Google Scholar] [CrossRef]
- Reszka, P.; Nowicka, A.; Lipski, M.; Dura, W.; Droździk, A.; Woźniak, K. A comparative chemical study of calcium silicate-containing and epoxy resin-based root canal sealers. Biomed. Res. Int. 2016, 2016, 9808432. [Google Scholar] [CrossRef] [PubMed]
- Palma, P.J.; Martins, J.; Diogo, P.; Sequeira, D.; Ramos, J.C.; Diogenes, A.; Santos, J.M. Does Apical Papilla Survive and Develop in Apical Periodontitis Presence after Regenerative Endodontic Procedures? Appl. Sci. 2019, 9, 3942. [Google Scholar] [CrossRef] [Green Version]
- Dammaschke, T.; Nowicka, A.; Lipski, M.; Ricucci, D. Histological evaluation of hard tissue formation after direct pulp capping with a fast-setting mineral trioxide aggregate [RetroMTA] in humans. Clin. Oral. Investig. 2019, 23, 4289–4299. [Google Scholar] [CrossRef]
- Fagogeni, I.; Metlerska, J.; Lipski, M.; Falgowski, T.; Maciej, G.; Nowicka, A. Materials used in regenerative endodontic procedures and their impact on tooth discoloration. J. Oral. Sci. 2019, 61, 379–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Możyńska, J.; Metlerski, M.; Lipski, M.; Nowicka, A. Tooth discoloration induced by different calcium silicate-based cements: A systematic review of in vitro studies. J. Endod. 2017, 43, 1593–1601. [Google Scholar] [CrossRef]
- Palma, P.J.; Marques, J.A.; Falacho, R.I.; Correia, E.; Vinagre, A.; Santos, J.M.; Ramos, J.C. Six-Month Color Stability Assessment of Two Calcium Silicate-Based Cements Used in Regenerative Endodontic Procedures. J. Funct. Biomater. 2019, 10, 14. [Google Scholar] [CrossRef] [Green Version]
- Palma, P.J.; Marques, J.A.; Santos, J.; Falacho, R.I.; Sequeira, D.; Diogo, P.; Caramelo, F.; Ramos, J.C.; Santos, J.M. Tooth Discoloration after Regenerative Endodontic Procedures with Calcium Silicate-Based Cements—An Ex Vivo Study. Appl. Sci. 2020, 10, 5793. [Google Scholar] [CrossRef]
- Lenherr, P.; Allgayer, N.; Weiger, R.; Filippi, A.; Attin, T.; Krastl, G. Tooth discoloration induced by endodontic materials: A laboratory study. Int. Endod. J. 2012, 45, 942–949. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, J.; Borg, J.; Damidot, D.; Salvadori, E.; Pilecki, P.; Zaslansky, P.; Darvell, B.W. Colour and chemical stability of bismuth oxide in dental materials with solutions used in routine clinical practice. PLoS ONE 2020, 11, 15–e0240634. [Google Scholar] [CrossRef]
- Ahmed, H.M.; Abbott, P.V. Discolouration potential of endodontic procedures and materials: A review. Int. Endod. J. 2012, 45, 883–897. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.H.; Shin, Y.S.; Lee, H.S.; Kim, S.O.; Shin, Y.; Jung, I.Y.; Song, J.S. Color changes of teeth after treatment with various mineral trioxide aggregate–based materials: An ex vivo study. J. Endod. 2015, 41, 737–741. [Google Scholar] [CrossRef] [PubMed]
- Vallés, M.; Mercadé, M.; Duran-Sindreu, F.; Bourdelande, J.L.; Roig, M. Color stability of white mineral trioxide aggregate. Clin. Oral. Investig. 2013, 17, 1155–1159. [Google Scholar] [CrossRef] [PubMed]
- Marciano, M.A.; Duarte, M.A.; Camilleri, J. Dental discoloration caused by bismuth oxide in MTA in the presence of sodium hypochlorite. Clin. Oral. Investig. 2015, 19, 2201–2209. [Google Scholar] [CrossRef] [PubMed]
- Lipski, M.; Nowicka, A.; Kot, K.; Postek-Stefańska, L.; Wysoczańska-Jankowicz, I.; Borkowski, L.; Andersz, P.; Jarząbek, A.; Grocholewicz, K.; Sobolewska, E.; et al. Factors affecting the outcomes of direct pulp capping using Biodentine. Clin. Oral. Investig. 2018, 22, 2021–2029. [Google Scholar] [CrossRef] [Green Version]
- Estrela, C.; Holland, R.; de Estrela, C.R.A.; Alencar, A.H.G.; Sousa-Neto, M.D.; Pecora, J.D. Characterization of successful root canal treatment. Braz. Dent. J. 2014, 25, 3–11. [Google Scholar] [CrossRef]
- Darcey, J.; Jawad, S.; Taylor, C.; Roudsari, R.V.; Hunter, M. Modern Endodontic Principles Part 4: Irrigation. Dent. Update 2016, 43, 20–33. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.K.; Desmeules, M.; Cielecki, M.; Dabbagh, B.; Ferraz Dos Santos, B. Longitudinal cohort study of regenerative endodontic treatment for immature necro permanent teeth. J. Endod. 2017, 43, 395–400. [Google Scholar] [CrossRef]
- Kim, S.G.; Malek, M.; Sigurdsson, A.; Lin, L.M.; Kahler, B. Regenerative endodontics: A comprehensive review. Int. Endod. J. 2018, 51, 1367–1388. [Google Scholar] [CrossRef] [PubMed]
- Ivica, A.; Zehnder, M.; Mateos, J.M.; Ghayor, C.; Weber, F.E. Biomimetic conditioning of human dentin using citric acid. J. Endod. 2019, 45, 45–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chae, Y.; Yang, M.; Kim, J. Release of TGF-β1 into root canals with various final irrigants in regenerative endodontics: An in vitro analysis. Int. Endod. J. 2018, 51, 1389–1397. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Arepally, G.M. Anticoagulation techniques in apheresis: From heparin to citrate and beyond. J. Clin. Apher. 2012, 27, 117–125. [Google Scholar] [CrossRef] [Green Version]
- Martínez, A.; Vargas, R.; Galano, A. Citric acid: A promising copper scavenger. Comput. Theor. Chem. 2018, 1133, 47–50. [Google Scholar] [CrossRef]
- Eldeniz, A.U.; Erdemir, A.; Belli, S. Effect of EDTA and citric acid solutions on the microhardness and the roughness of human root canal dentin. J. Endod. 2005, 31, 107–110. [Google Scholar] [CrossRef]
- Camilleri, J. Color stability of white mineral trioxide aggregate in contact with hypochlorite solution. J. Endod. 2014, 40, 436–440. [Google Scholar] [CrossRef] [PubMed]
- Keskin, C.; Demiryurek, E.O.; Ozyurek, T. Color stabilities of calcium silicate-based materials in contact with different irrigation solutions. J. Endod. 2015, 41, 409–411. [Google Scholar] [CrossRef]
- Felman, D.; Parashos, P. Coronal tooth discoloration and white mineral trioxide aggregate. J. Endod. 2013, 39, 484–487. [Google Scholar] [CrossRef] [PubMed]
- International Commission on Illumination. Recommendations on Uniform Color Spaces, Color–Difference Equations, Psychometric Color Terms; Bureau Central de la CIE: Paris, France, 1978; Volume 1913. [Google Scholar]
- Kastiukas, G.; Zhou, X.; Castro-Gomes, J.; Huang, S.; Saafi, M. Effects of lactic and citric acid on early-age engineering properties of Portland/calcium aluminate blended cements. Constr. Build. Mater. 2015, 101, 389–395. [Google Scholar] [CrossRef] [Green Version]
- Agrafioti, A.; Tzimpoulas, N.; Chatzitheodoridis, E.; Kontakiotis, E.G. Compartive evaluation of sealing ability and microstructure of MTA and Biodentine after exposure to different environments. Clin. Oral. Investig. 2016, 20, 1535–1540. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.V.; da Silva, G.R.; Souza, G.L.; Magalhães, T.E.A.; Barbosa, G.L.R.; Turrioni, A.P.; Moura, C.C.G. A laboratory evaluation of cell viability, radiopacity and tooth discoloration induced by regenerative endodontic materials. Int. Endod. J. 2020, 53, 1140–1152. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.Y.; Seo, M.S. Comparative evaluation of volumetric changes of three different retrograde calcium silicate materials placed under different pH condititions. BMC Oral Health 2020, 20, 330. [Google Scholar] [CrossRef] [PubMed]
- Ashofteh Yazdi, K.; Ghabraei, S.; Bolhari, B.; Kafili, M.; Meraji, N.; Nekoofar, M.H.; Dummer, P.M.H. Microstructure and chemical analysis of four calcium silicate-based cements in different environmental conditions. Clin. Oral Invest. 2019, 23, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.J.; Carvalho, N.K.; Zanon, M.; Senna, P.M.; DE-Deus, G.; Zuolo, M.L.; Zaia, A.A. Push-out bond strength of MTA HP, a new high-plasticity calcium silicate-based cement. Braz. Oral Res. 2016, 30, e84. [Google Scholar] [CrossRef] [Green Version]
- Aguiar, B.A.; Frota, L.M.A.; Taguatinga, D.T.; Vivan, R.R.; Camilleri, J.; Duarte, M.A.H.; de Vasconcelos, B.C. Influence of ultrasonic agitation on bond strength, marginal adaptation, and tooth discoloration provided by three coronary barrier endodontic materials. Clin. Oral. Investig. 2019, 23, 4113–4122. [Google Scholar] [CrossRef] [PubMed]
- Duarte, M.A.H.; Minotti, P.G.; Rodrigues, C.T.; Zapata, R.O.; Bramante, C.M.; Tanomaru Filho, M.; Vivan, R.R.; Gomes de Moraes, I.; Bombarda de Andrade, F. Effect of different radiopacifying agents on the physicochemical properties of white Portland cement and white mineral trioxide aggregate. J. Endod. 2012, 38, 394–397. [Google Scholar] [CrossRef]
- Amoroso-Silva, P.A.; Marciano, M.A.; Guimaraes, B.M.; Duarte, M.A.H.; Sanson, A.F.; de Moraes, I.G. Apical adaptation, sealing ability and push–out bond strength of five root–end filling materials. Braz. Oral Res. 2014, 28, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cintra, L.T.A.; Benetti, F.; de Azevedo Queiroz, Í.O.; de Araújo Lopes, J.M.; Penha de Oliveira, S.H.; Sivieri Araújo, G.; Gomes-Filho, J.E. Cytotoxicity, biocompatibility, and biomineralization of the new high-plasticity MTA material. J. Endod. 2017, 43, 774–778. [Google Scholar] [CrossRef] [Green Version]
- Camilleri, J. Staining potential of Neo MTA Plus, MTA Plus, and Biodentine used for pulpotomy procedures. J. Endod. 2015, 41, 1139–1145. [Google Scholar] [CrossRef] [PubMed]
- Voveraityte, V.; Gleizniene, S.; Lodiene, G.; Grabliauskiene, Z.; Machiulskiene, V. Spectrophotometric analysis of tooth discolouration induced by mineral trioxide aggregate after final irrigation with sodium hypochlorite: An in vitro study. Aust. Endod. J. 2017, 43, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Sobhnamayan, F.; Adl, A.; Ghanbaran, S. Effect of different irrigation solutions on the colour stability of three calcium silicate–based materials. J. Dent. Biomater. 2017, 4, 373–378. [Google Scholar]
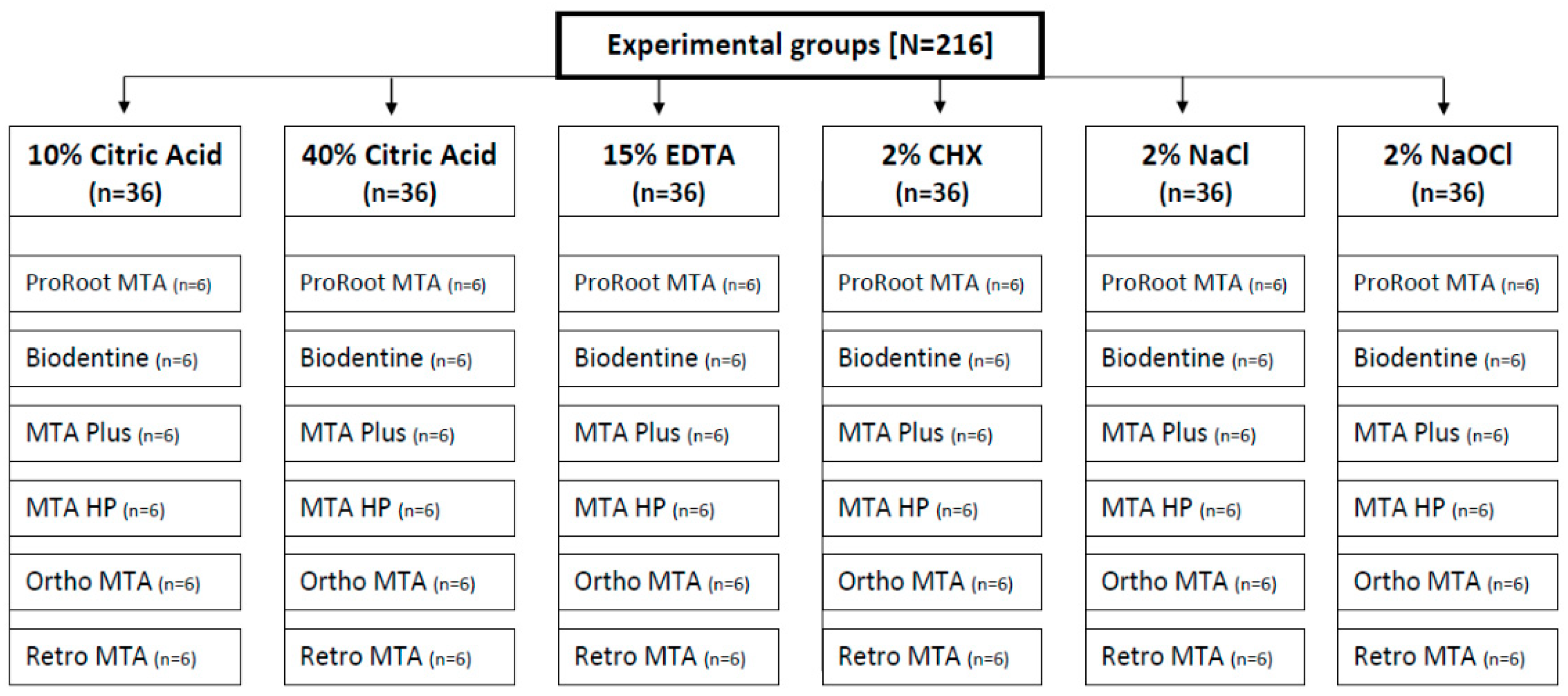


| Material | Manufacturer | Ingredients | Preparation Procedure |
|---|---|---|---|
| ProRoot MTA | Dentsply, Tulsa, OK, USA | tricalcium silicate, dicalcium silicate, tricalcium aluminate, tetracalciumaluminoferrite, free calcium oxide, and bismuth oxide | Mix powder + liquid ratio 1:3 (mix manually) |
| Biodentine | Septodont, Saint Maur-des-Fossés, France | powder: tricalcium silicate, calcium carbonate and oxide filler, iron oxide shade, and zirconium oxide liquid: calcium chloride as accelerator, hydrosoluble polymer water-reducing agent, water | 0.7 g capsule of powder + 5 drops of liquid mix 30 s; 4000–4200 rpm (mixing device) |
| MTA Plus | Avalon Biomed Inc, by Prevest Denpro Limited, Jammu, India | powder: tricalcium silicate, dicalcium silicate, bismuth oxide, calcium sulfate, and silica liquid: hydrated polymer gel | Mix powder + liquid ratio 1:1 (mix manually) |
| MTA Repair HP | Angelus, Londrina, PR, Brazil | powder: tricalcium silicate, dicalcium silicate, tricalcium aluminate, calcium oxide, and calcium tungstate liquid: water and plasticizer | 0.085 g capsules of powder + 12 drops of liquid (mix manually) |
| Ortho MTA | BioMTA, Seoul, Korea | calcium carbonate, silicon dioxide, aluminium oxide, and dibismuth trioxide | 0.2 g pouches of powder + 2 drops of water (mix manually) |
| Retro MTA | BioMTA, Seoul, Korea | calcium carbonate, silicon dioxide, aluminium oxide, and calcium zirconia complex | 0.3 g pouches of powder + 3 drops of water (mix manually) |
| Material | Irrigant | |||||||
|---|---|---|---|---|---|---|---|---|
| Scale | 10% CA | 40% CA | 15% EDTA | 2% CHX | 2% NaOCl | 0.9% NaCl | ||
| ProRoot MTA | color | 0 | 6 | 6 | 6 | 6 | 0 | 6 |
| 1 | 0 | 0 | 0 | 0 | 6 | 0 | ||
| 2 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| structure | a | 6 | 6 | 6 | 6 | 6 | 6 | |
| b | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Biodentine | color | 0 | 6 | 6 | 6 | 4 | 6 | 6 |
| 1 | 0 | 0 | 0 | 2 | 0 | 0 | ||
| 2 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| structure | a | 0 | 1 | 6 | 6 | 6 | 6 | |
| b | 6 | 5 | 0 | 0 | 0 | 0 | ||
| MTA Plus | color | 0 | 6 | 6 | 6 | 6 | 4 | 6 |
| 1 | 0 | 0 | 0 | 0 | 2 | 0 | ||
| 2 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| structure | a | 6 | 6 | 6 | 6 | 6 | 6 | |
| b | 0 | 0 | 0 | 0 | 0 | 0 | ||
| MTA Repair HP | color | 0 | 1 | 0 | 6 | 6 | 6 | 6 |
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 2 | 5 | 6 | 0 | 0 | 0 | 0 | ||
| structure | a | 0 | 0 | 6 | 6 | 6 | 6 | |
| b | 6 | 6 | 0 | 0 | 0 | 0 | ||
| Ortho MTA | color | 0 | 6 | 6 | 6 | 6 | 0 | 6 |
| 1 | 0 | 0 | 0 | 0 | 6 | 0 | ||
| 2 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| structure | a | 6 | 6 | 6 | 6 | 6 | 6 | |
| b | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Retro MTA | color | 0 | 6 | 6 | 6 | 6 | 6 | 6 |
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 2 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| structure | a | 0 | 0 | 6 | 6 | 6 | 6 | |
| b | 6 | 6 | 0 | 0 | 0 | 0 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Metlerska, J.; Dammaschke, T.; Lipski, M.; Fagogeni, I.; Machoy-Mokrzyńska, A.; Nowicka, A. Effect of Citric Acid on Color Changes of Calcium Silicate-Based Cements an In Vitro Study. Appl. Sci. 2021, 11, 2339. https://0-doi-org.brum.beds.ac.uk/10.3390/app11052339
Metlerska J, Dammaschke T, Lipski M, Fagogeni I, Machoy-Mokrzyńska A, Nowicka A. Effect of Citric Acid on Color Changes of Calcium Silicate-Based Cements an In Vitro Study. Applied Sciences. 2021; 11(5):2339. https://0-doi-org.brum.beds.ac.uk/10.3390/app11052339
Chicago/Turabian StyleMetlerska, Joanna, Till Dammaschke, Mariusz Lipski, Irini Fagogeni, Anna Machoy-Mokrzyńska, and Alicja Nowicka. 2021. "Effect of Citric Acid on Color Changes of Calcium Silicate-Based Cements an In Vitro Study" Applied Sciences 11, no. 5: 2339. https://0-doi-org.brum.beds.ac.uk/10.3390/app11052339






