Immobilised Humic Substances as Low-Cost Sorbents for Emerging Contaminants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Immobilization of Humic Substances
2.1.1. Immobilization of Humic Substances by Polycondensation
2.1.2. Immobilization of Humic Substances with Iron Compounds
2.2. Characterization of Immobilized Humic Substances
2.3. Sorption Experiments
Statistical Data Processing
3. Results and Discussion
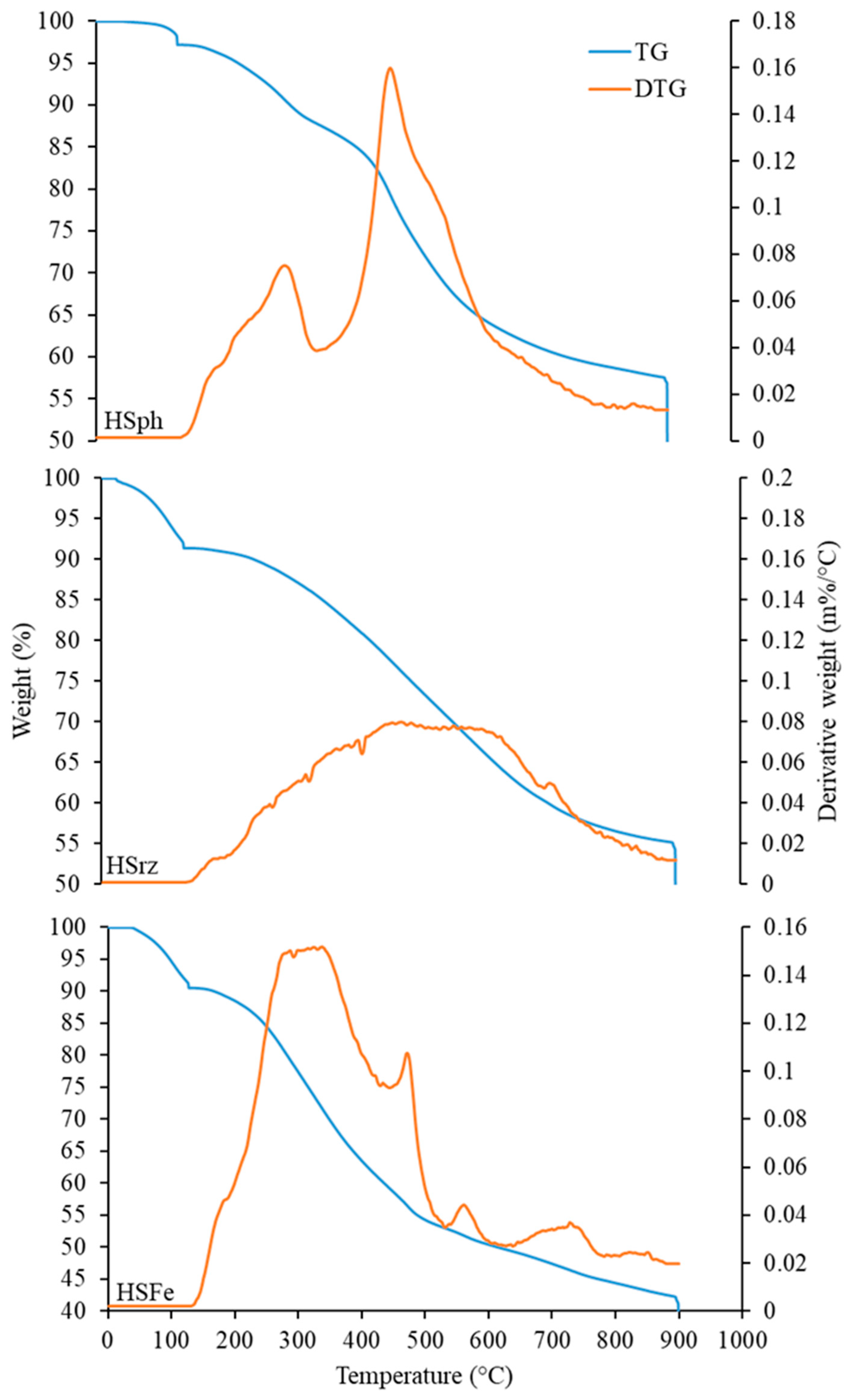
3.1. Characterization of Immobilized Humic Substances
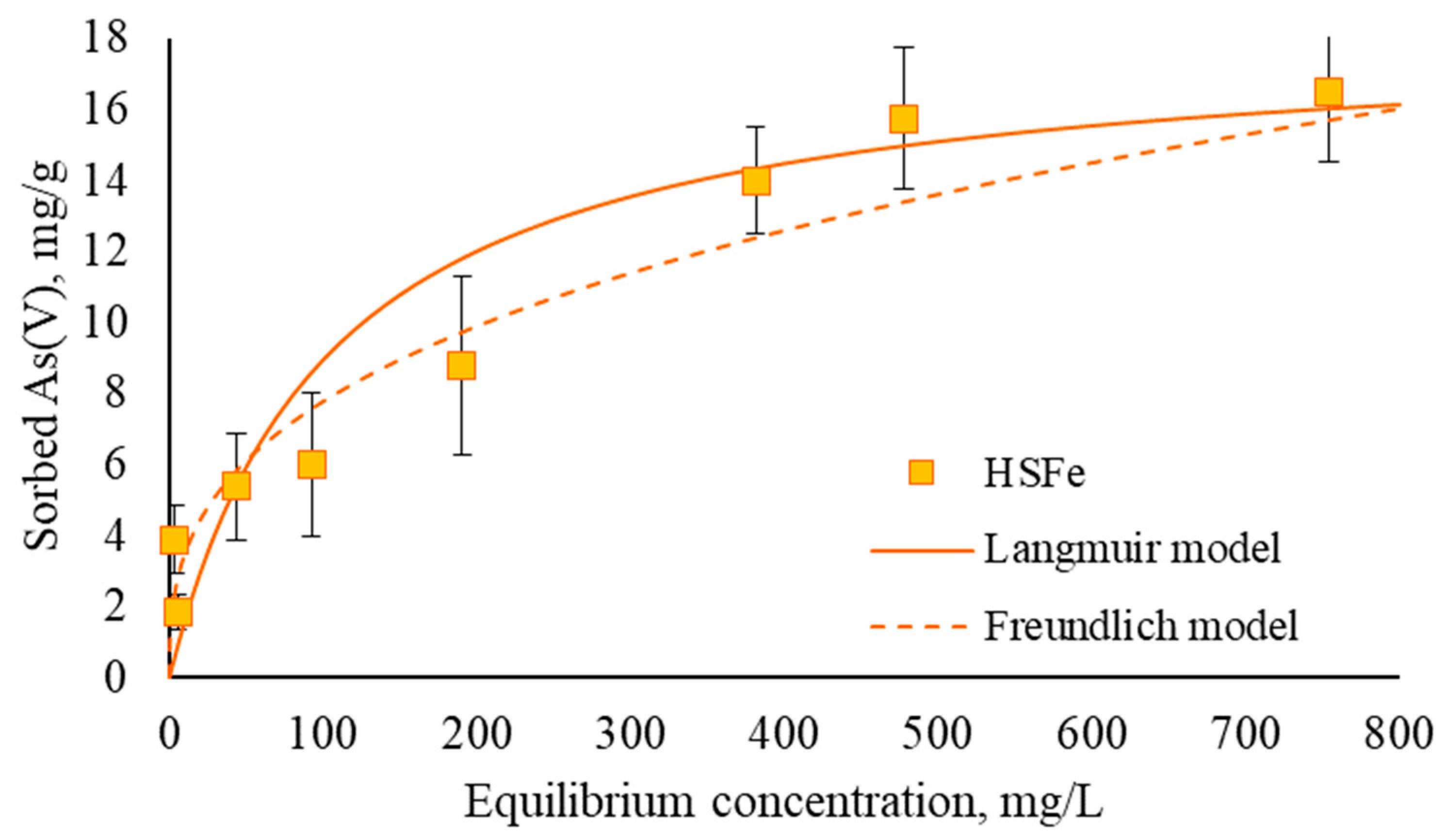
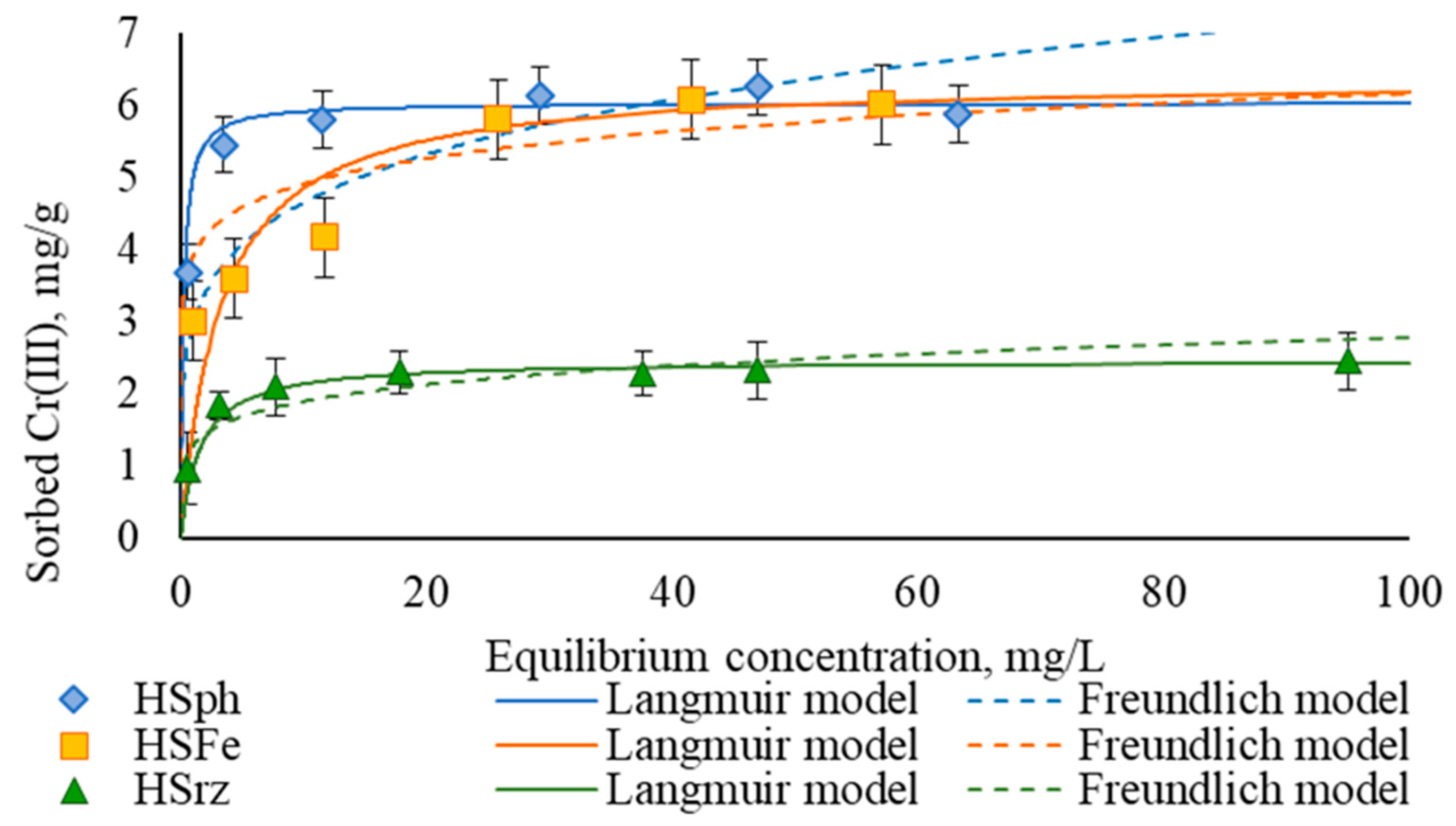
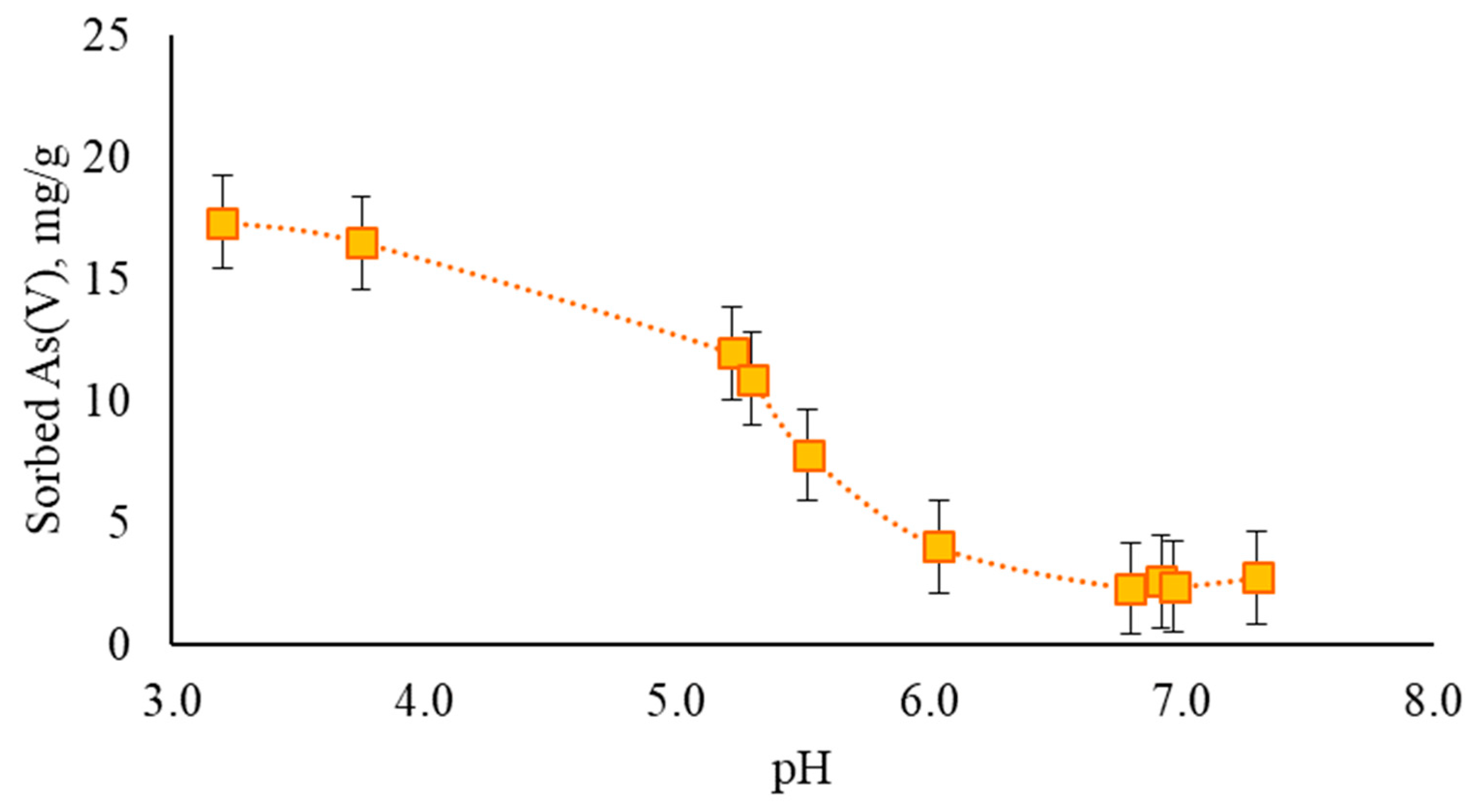
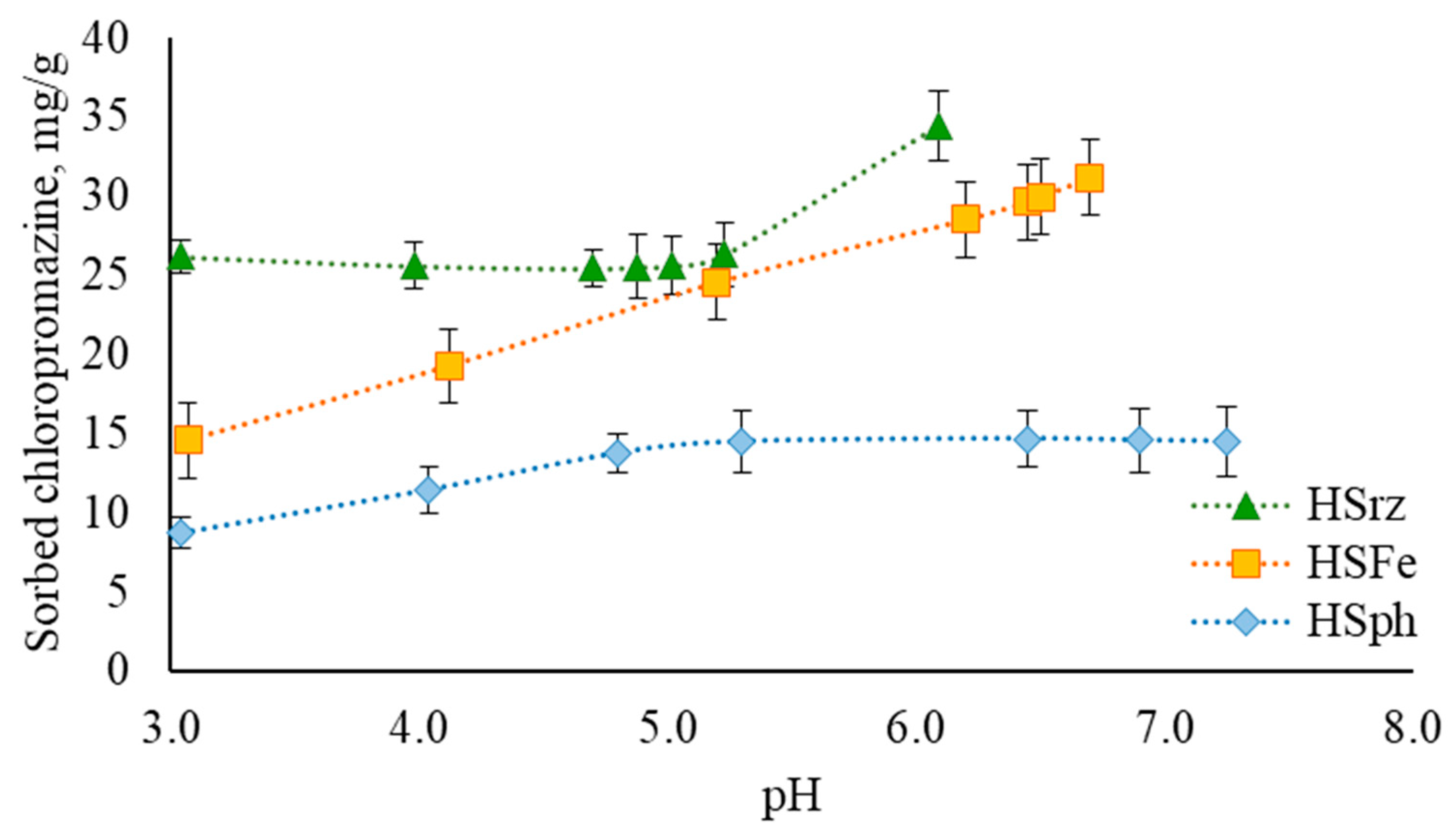
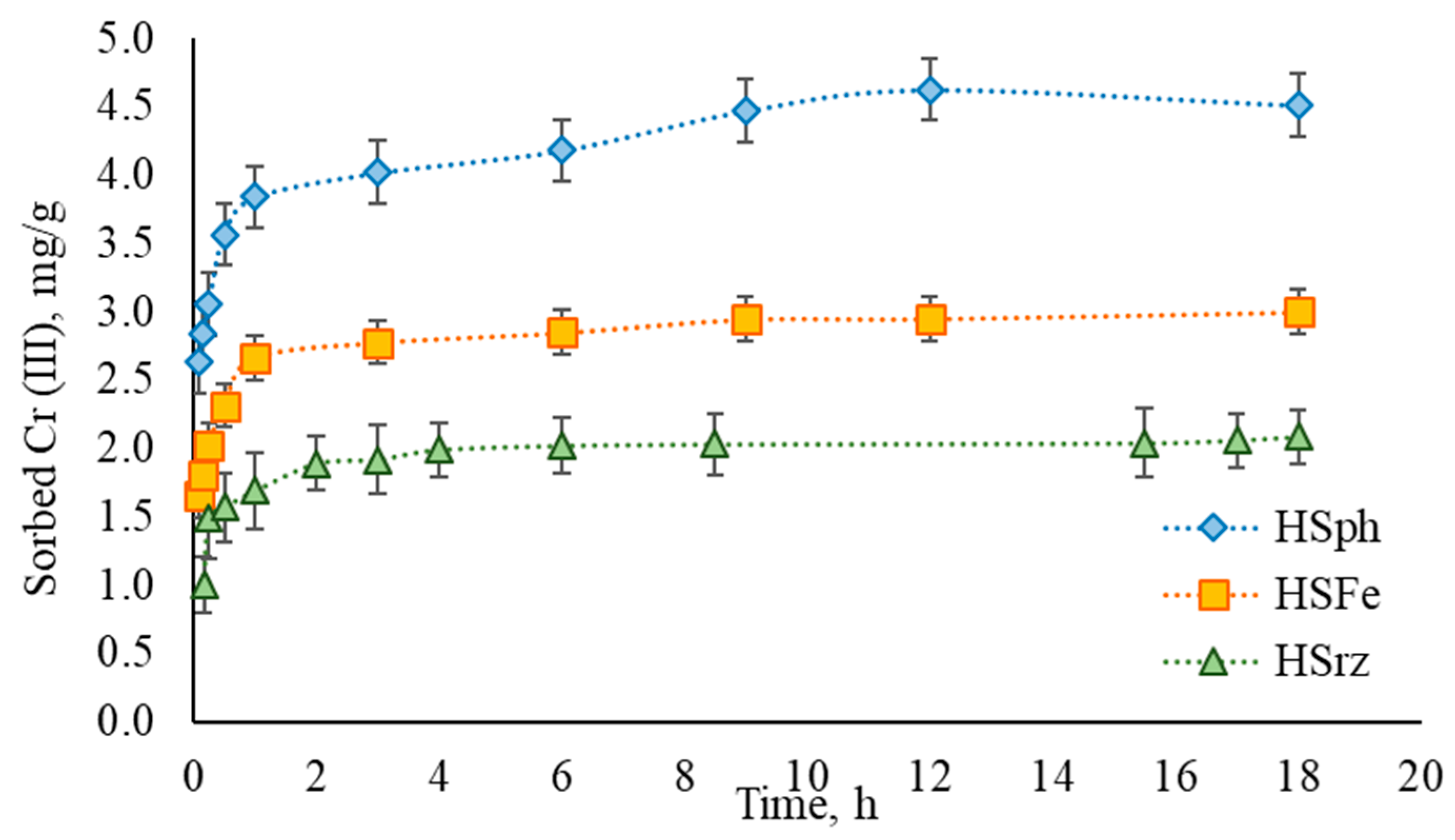
3.2. Sorption Efficiency
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Krzeminski, P.; Tomei, M.C.; Karaolia, P.; Langenhoff, A.; Almeida, C.M.R.; Felis, E.; Gritten, F.; Andersen, H.R.; Fernandes, T.; Manaia, C.M.; et al. Performance of secondary wastewater treatment methods for the removal of contaminants of emerging concern implicated in crop uptake and antibiotic resistance spread: A review. Sci. Total Environ. 2019, 648, 1052–1081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaheen, S.M.; Khan Niazi, N.; Hassan, N.E.E.; Bibi, I.; Wang, H.; Tsang, D.C.W.; Sik, O.Y.; Bolan, N.; Rinklebe, J. Wood-based biochar for the removal of potentially toxic elements in water and wastewater: A critical review. Int. Mater. Rev. 2018, 216–247. [Google Scholar] [CrossRef]
- WHO. Water, Health and Ecosystems. 2020. Available online: https://www.who.int/heli/risks/water/water/en/ (accessed on 2 August 2020).
- Olkowska, E.; Polkowska, Z.; Namiesnik, J. Analytics of surfactants in the environment: Problems and Challenges. Chem. Rev. 2011, 1115667–1115700. [Google Scholar] [CrossRef]
- Jardak, K.; Drogui, P.; Daghrir, R. Surfactants in aquatic and terrestrial environment: Occurrence, behavior, and treatment processes. Environ. Sci. Pollut. Res. 2016, 23, 3195–3216. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, W.; Song, W.; Guo, M. Remediation techniques for heavy metal—Contaminated soils: Principles and applicability. Sci. Total Environ. 2018, 633, 206–219. [Google Scholar] [CrossRef]
- Ramanayaka, S.; Tsang, D.C.W.; Hou, D.; Sik, O.Y.; Vithanage, M. Green synthesis of graphitic nanobiochar for the removal of emerging contaminants in aqueous media. Sci. Total Environ. 2020, 706, 135725. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.H.; Reinhard, M.; Gin, K.Y.-H. Occurrence and fate of emerging contaminants in municipal wastewater treatment plants from different geographical regions—A review. Water Res. 2018, 133, 182–207. [Google Scholar] [CrossRef] [PubMed]
- Petrie, B.; Barden, R.; Kasprzyk-Hordern, B. A review on emerging contaminants in wastewaters and the environment: Current knowledge, understudied areas and recommendations for future monitoring. Water Res. 2015, 72, 3–27. [Google Scholar] [CrossRef]
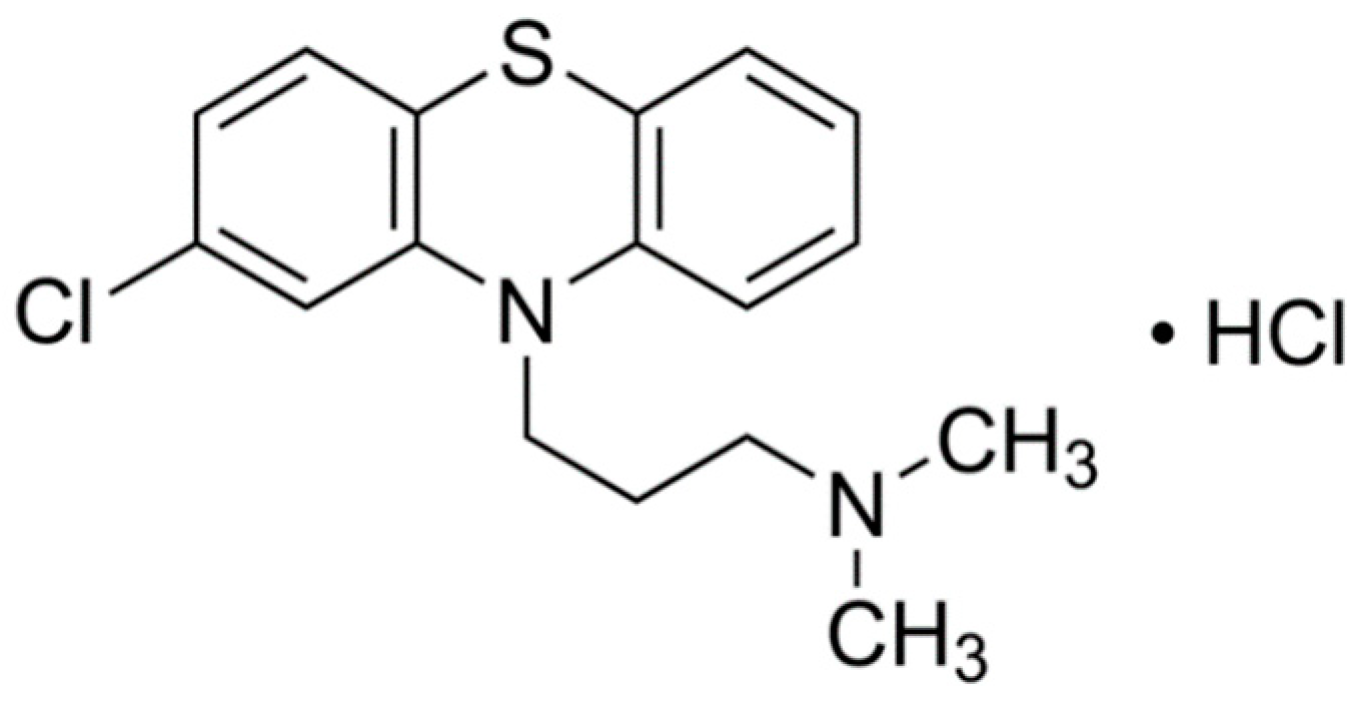
- Reichert, F.J.; Souza, D.M.; Martins, A.F. Antipsychotic drugs in hospital wastewater and a preliminary risk assessment. Ecotoxicol. Environ. Saf. 2019, 170, 559–567. [Google Scholar] [CrossRef]
- Parga, J.R.; Vazquez, V.; Moreno, H. Thermodynamic studies of the arsenic adsorption on iron species generated by electrocoagulation. J. Metall. 2009. [Google Scholar] [CrossRef] [Green Version]
- Xi, J.; He, M.; Lin, C. Adsorption of antimony(V) on kaolinite as a function of pH, ionic strength and humic acid. Environ. Earth Sci. 2010, 60, 715–722. [Google Scholar] [CrossRef]
- Uluozlu, O.D.; Sari, A.; Tuzen, M. Biosorption of antimony from aqueous solution by lichen (Physcia tribacia) biomass. Chem. Eng. J. 2010, 163, 382–388. [Google Scholar] [CrossRef]
- Klucakova, M.; Pavlikova, M. Lignitic humic acids as environmentally-friendly adsorbent for heavy metals. J. Chem. (Hindawi) 2017. [Google Scholar] [CrossRef]
- Ansone, L.; Klavins, M.; Jankevica, M.; Viksna, A. Biomass sorbents for metalloid removal. Adsorption 2014, 20, 275–286. [Google Scholar] [CrossRef]
- Janos, P. Sorption of basic dyes onto iron humate. Environ. Sci. Technol. 2003, 37, 5792–5798. [Google Scholar] [CrossRef]
- Klavins, M.; Eglite, L. Immobilisation of humic substances. Colloids Surf. A Physicochem. Eng. Asp. 2002, 203, 47–54. [Google Scholar] [CrossRef]
- Tan, K.H. Soil Sampling, Preparation, and Analysis, 2nd ed.; Taylor & Francis: New York, NY, USA, 2005. [Google Scholar]
- Stevenson, F.J. Humus Chemistry: Genesis, Composition, Reactions, 2nd ed.; Wiley: New York, NY, USA, 1994. [Google Scholar]
- Erny, G.L.; Goncalves, B.M.; Esteves, V.I. Immobilised humic substances and immobilised aggregates of humic substances as sorbent for solid phase extraction. J. Chromatogr. A 2013, 1306, 104–108. [Google Scholar] [CrossRef]
- Cihlar, Z.; Vojtova, L.; Conte, P.; Nasir, S.; Kucerik, J. Hydration and water holding properties of cross-linked lignite humic acids. Geoderma 2014, 230–231, 151–160. [Google Scholar] [CrossRef] [Green Version]
- Speltini, A.; Merlo, F.; Maraschi, F.; Sturini, M.; Contini, M.; Calisi, N.; Profumo, A. Thermally condensed humic acids onto silica as SPE for effective enrichment of glucocorticoids from environmental waters followed by HPLC-HESI-MS/MS. J. Chromatogr. A 2018, 1540, 38–46. [Google Scholar] [CrossRef]
- Stathi, P.; Deligiannakis, Y. Humic acid-inspired hybrid materials as heavy metal adsorbents. J. Colloid Interface Sci. 2010, 351, 239–247. [Google Scholar] [CrossRef]
- Yang, Y.-H.; Koopal, L.K. Immobilisation of humic acids and binding of nitrophenol to immobilised humics. Colloids Surf. A Physicochem. Eng. Asp. 1999, 151, 201–212. [Google Scholar] [CrossRef]
- Fiol, N.; Villaescusa, I. Determination of sorbent point zero charge: Usefulness in sorption studies. Environ. Chem. Lett. 2009, 7, 79–84. [Google Scholar] [CrossRef]
- Esteves, V.I.; Duarte, A.C. Thermogravimetric properties of aquatic humic substances. Mar. Chem. 1999, 63, 225–233. [Google Scholar] [CrossRef]
- Francioso, O.; Montecchio, D.; Gioacchini, P.; Ciavatta, C. Thermal analysis (TG-DTA) and isotopic characterization of humic acids from different origins. Appl. Geochem. 2005, 20, 537–544. [Google Scholar] [CrossRef]
- Ghimire, K.N.; Inoue, K.; Makino, K.; Miyajima, T. Adsorptive removal of arsenic using orange juice residue. Sep. Sci. Technol. 2002, 37, 2785–2799. [Google Scholar] [CrossRef]
- Guo, H.; Stuben, D.; Berner, Z. Arsenic removal from water using natural iron mineral-quartz sand columns. Sci. Total Environ. 2007, 377, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Thirunavukkarasu, O.S.; Viraraghavan, T.; Subramanian, K.S. Removal of arsenic in drinking water by iron oxide-coated sand and ferrihydrite –batch studies. Water Qual. Res. J. 2001, 36, 55–70. [Google Scholar] [CrossRef]
- Ansone, L.; Klavins, M.; Viksna, A. Arsenic removal using natural biomaterial-based sorbents. Environ. Geochem. Health. 2013, 35, 633–642. [Google Scholar] [CrossRef]
- Kuriakose, S.; Singh, T.S.; Pant, K.K. Adsorption of As(III) from Aqueous Solution onto Iron Oxide Impregnated Activated Alumina. Wat. Qual. Res. J. Can. 2004, 39, 258–266. [Google Scholar] [CrossRef]
- Ho, Y.-S.; Ofomaja, A.E. Kinetics and thermodynamics of lead ion sorption on palm kernel fibre from aqueous solution. Process Biochem. 2005, 40, 3455–3461. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U. Arsenic removal from water/ wastewater using adsorbents-A critical review. J. Haz. Mat. 2007, 142, 1–53. [Google Scholar] [CrossRef] [PubMed]
- Visual MINTEQ 3.1. Available online: https://vminteq.lwr.kth.se/ (accessed on 2 August 2020).
- National Library of Medicine. National Center of Biotechnology Information. PubChem. Chlorpromazine. Available online: https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3033#section=Interactions-(Complete) (accessed on 2 August 2020).
- Hai, J.; Bai, B.; Ding, C.; Wang, H.; Suo, Y. Removal of oil from water surface by novel composite NSM-g-P (MMA-co-BA) Super Oil absorption Resin. Polym. Compos. 2016. [Google Scholar] [CrossRef]





| Immobilized Humic Substances | Elemental Content (wt.%) | Phenolic Groups (meq/g) | Carboxylic Groups (meq/g) | Total Acidity (meq/g) | Content of Fe (mg/g) | Surface Area (m2/g) | |||
|---|---|---|---|---|---|---|---|---|---|
| C | H | N | O | ||||||
| HSph | 65.92 | 5.15 | 0.47 | 28.46 | 8.8 | 2.2 | 11 | 4.1 | 0.550 |
| HSrz | 61.13 | 4.60 | 0.43 | 33.84 | - | - | - | - | 0.120 |
| HSFe | 41.42 | 3.60 | 0.99 | 53.99 | 3.9 | 1 | 4.9 | 133.3 | 0.035 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ansone-Bertina, L.; Upska, K.; Dobkevica, L.; Kviesis, J.; Meija, R.; Klavins, M. Immobilised Humic Substances as Low-Cost Sorbents for Emerging Contaminants. Appl. Sci. 2021, 11, 3021. https://0-doi-org.brum.beds.ac.uk/10.3390/app11073021
Ansone-Bertina L, Upska K, Dobkevica L, Kviesis J, Meija R, Klavins M. Immobilised Humic Substances as Low-Cost Sorbents for Emerging Contaminants. Applied Sciences. 2021; 11(7):3021. https://0-doi-org.brum.beds.ac.uk/10.3390/app11073021
Chicago/Turabian StyleAnsone-Bertina, Linda, Karina Upska, Linda Dobkevica, Jorens Kviesis, Raimonds Meija, and Maris Klavins. 2021. "Immobilised Humic Substances as Low-Cost Sorbents for Emerging Contaminants" Applied Sciences 11, no. 7: 3021. https://0-doi-org.brum.beds.ac.uk/10.3390/app11073021